Abstract
The cerebrospinal fluid (CSF) signature of reduced amyloid beta 1–42 (Aβ42), elevated total tau (t-tau), and phosphorylated tau181 (p-tau) is important for the early diagnosis of Alzheimer’s disease (AD). Aβ42, t-tau, and p-tau have been reported in numerous studies to contribute to predicting cognitive impairment in Parkinson’s disease (PDCI). However, no consistent conclusion can be drawn so far. Literatures regarding Aβ42, t-tau, and p-tau in CSF were systematically reviewed, and a meta-analysis was thus performed to evaluate the changes of these biomarkers in PDCI patients, including PD with mild cognitive impairment (PDMCI) and PD dementia (PDD) patients, relative to PD with normal cognition (PDNC) patients. Databases of “PubMed,” “EBSCO,” and “Springer” were retrieved for articles concerning Aβ42, t-tau, and p-tau in PDCI patients relative to those in PDNC patients published from January 1, 2000 to February 1, 2017. The following keywords were set, namely, “dementia” or “cognitive impairment” or “mild cognitive impairment” and “cerebrospinal fluid” and “Parkinson*.” Sixteen articles comprising 590 PDCI patients and 1182 PDNC patients were included. The results showed that CSF Aβ42 level in PDCI cohort was lower than that in PDNC cohort (pooled Std.MD = −0.44, 95% CI [−0.61, −0.26], p < 0.00001). Reduced Aβ42 (pooled Std.MD = −0.60, 95% CI [−0.75, −0.45], p < 0.00001) as well as elevated t-tau (pooled Std.MD = 0.21, 95% CI [0.06, 0.35], p = 0.006) and p-tau (pooled Std.MD = 0.36, 95% CI [0.02, 0.69], p = 0.04) could be observed in PDD cohort compared with PDNC cohort. Therefore, amyloid pathology and tauopathy may participate in the development of PDD, which is similar to AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mild cognitive impairment (PDMCI) and dementia (PDD) in Parkinson’s disease (PD) have been recognized as the two phenotypes of cognitive impairment (PDCI) [1]. Moreover, PDMCI is often considered to be an intermediate condition between PD with normal cognition (PDNC) and PDD [2]. PDMCI progresses accompanied by age, less education, disease duration, symptom profile, and disease severity [2, 3], which is an independent risk factor for subsequent development of early PDD [4].
Three pathologic subgroups of PDD, including predominant synucleinopathy, predominant synucleinopathy with amyloid-β (Aβ) deposition but minimal or no cortical tauopathy, and synucleinopathy and Aβ deposition with at least moderate neocortical tauopathy, have been identified, based on aggregation of α-synuclein, Aβ, and tau protein in the brain [5]. The pathological accumulation of α-synuclein in the brain represents the primary pathological hallmark of PD, which is characterized by the formation of abundant α-synuclein neuronal inclusions, such as Lewy bodies and Lewy neurites [6]. This has resulted in progressive dopaminergic nigrostriatal neurodegeneration [6]. However, depositions of Aβ and tau protein have been suggested to be correlated with PDCI [5, 7].
Amyloid beta 1–42 (Aβ42), total tau (t-tau), and phosphorylated tau181 (p-tau) are core cerebrospinal fluid (CSF) biomarkers for the early diagnosis of Alzheimer’s disease (AD). Meanwhile, the CSF signature of reduced Aβ42 as well as elevated t-tau and p-tau is closely related to both AD-induced mild cognitive impairment (MCI) and dementia [8]. A 2-year prospective study has been conducted recently, in which decreasing CSF Aβ42 level has been reported to play a role in predicting early PDCI among the newly diagnosed PD patients with mild motor symptoms [9]. Moreover, Bäckström DC et al. discovered in their 5–9-year follow-up study that high levels of neurofilament light chain protein and heart fatty acid-binding protein while low Aβ42 level in CSF of PD patients were linked with PDD development [10]. On the other hand, Liu C et al. suggested in their prospective cohort research that signature of CSF biomarkers, such as the levels of Aβ42, t-tau, p-tau, p-tau/t-tau, t-tau/Aβ42, and p-tau/Aβ42, had no relationship with the development of PDCI in the newly diagnosed drug-naive PD patients [11]. In the meantime, higher CSF levels of p-tau and p-tau/Aβ42 had played a role in predicting the subsequent cognitive reduction upon the initiation of levodopa treatment [11]. But CSF level of Aβ42 was not correlated with the development of PDCI [11]. The changes of CSF core biomarkers, such as Aβ42, t-tau, and p-tau, in PDMCI and/or PDD patients relative to PDNC have been extensively investigated [9,10,11]. However, no consistent findings can be gained so far.
A meta-analysis enrolling the maximum studies was thereby conducted to assess the changes of CSF Aβ42, t-tau, and p-tau levels in PDMCI and/or PDD cohorts relative to PDNC cohorts. Hopefully, those inconsistent findings in previous studies could be understood in this meta-analysis and the possible heterogeneity sources could be found out.
Methods
Search strategy
Databases of “PubMed,” “EBSCO,” and “Springer” were retrieved for published articles. The search was limited to articles published from January 1, 2000 to February 1, 2017 with language restriction as “English.” In addition, bibliographic references of retrieved articles and related reviews were conducted a hand search to identify potential studies. The following combined keywords, such as “dementia” or “cognitive impairment” or “mild cognitive impairment” and “cerebrospinal fluid” and “Parkinson*,” were employed.
Inclusion and exclusion criteria
Inclusion criteria were listed as follows: (1) retrospective or prospective cohort or cross-sectional study designs; (2) including a group of patients (the number is more than 5) who were clearly diagnosed with PDNC; (3) including a group of patients (the number is more than 5) who were clearly diagnosed by PDCI including PDMCI and/or PDD; and (4) the CSF Aβ42, t-tau, and p-tau levels in patients with PDNC or PDCI were detected. Furthermore, exclusion criteria included (1) non-human studies or non-original studies; (2) studies concerning children, adolescents, and pregnant women; and (3) studies in which the mean value and standard deviation (SD) of CSF Aβ42, t-tau, and p-tau cannot be quantified.
Data extraction and quality assessment
Two investigators reviewed the retrieved articles to determine eligibility and extract study data independently. Methodological quality was assessed by the Newcastle-Ottawa Scale (NOS) criteria [12], which included the selection (0–4 scores), comparability (0–2 scores), and exposure (0–3 scores) categories. Besides, studies were low-quality methodology in accordance with NOS scores lower than 6. Discrepancies were resolved through discussion and consultation of a third author when necessary.
Statistical analysis
Heterogeneity test was conducted by the I 2 statistic. Standard mean difference (Std.MD), 95% confidence interval (CI), and overall effect from individual studies were calculated by weighted fixed-effect model when the heterogeneity test was p ≥ 0.05. Accordingly, random-effect model was applied when the heterogeneity test was p < 0.05. The data of CSF Aβ42, t-tau, and p-tau in PDCI patients and PDNC patients were extracted and pooled for separate meta-analysis. To deal with heterogeneity, subgroup analysis was chosen. Besides, publication bias was assessed by funnel plots. Additionally, heterogeneity, Std.MD, overall effect, and sensitivity analysis were calculated by Review Manager version 5.3 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Funnel plot was charted by Review Manager version 5.3 when the heterogeneity test was p ≥ 0.05. Meanwhile, funnel plot was charted by Comprehensive Meta-Analysis 2 (Biostat, Englewood, NJ, USA) when the heterogeneity test was p < 0.05.
Results
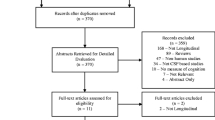
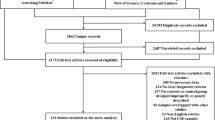
Literature retrieval strategy and characteristics of the studies involved
A total of 1389 reports were identified through systematic retrieval, and 585 of them were selected after removing duplicates and reports with irrelevant topics. Sixteen cohort studies [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28] were selected finally in accordance with the inclusion and exclusion criteria, including 10 retrospective cohort studies [13,14,15,16,17,18, 20, 22, 23, 26], 4 prospective cohort studies [19, 21, 25, 28], and 2 cross-sectional studies [24, 27].
The general characteristics of the included studies were listed in Table 1. Altogether 17 groups were pooled, involving 1182 PDNC patients and 590 PDCI patients. The mean age of PDNC and PDCI patients at lumbar puncture ranged from 56 to 72 and 61.1 to 78 years, respectively. Differences in age and gender were selected to investigate the comparability categories of NOS criteria [12]. Studies by Montine TJ et al. and Nutu M et al. [16, 20] had suggested that the age of PDD patients was obviously elder than that of PDNC patients. But difference in gender was not mentioned in study by Montine TJ et al. [16], while significant difference in gender was observed in study by Nutu M [20]. Therefore, zero star was given to the comparability category of those two studies when comparing the CSF core biomarkers in PDD patients with those in PDNC patients [16, 20].
CSF levels of core biomarkers of AD in PDCI relative to PDNC
Significant heterogeneity (I 2 = 58%, p = 0.001) could be seen within the pooled 17 groups (Fig. 1) when comparing CSF Aβ42 level in PDCI cohorts with that in PDNC cohorts. Moreover, CSF Aβ42 level in PDCI patients was lower than that in PDNC patients (pooled Std.MD = −0.44, 95% CI [−0.61, −0.26], p < 0.00001) (Fig. 1). Meanwhile, that in PDD patients was lower than that in PDNC patients (pooled Std.MD = −0.60, 95% CI [−0.75, −0.45], p < 0.00001) (Table 2). Besides, CSF levels of t-tau (pooled Std.MD = 0.21, 95% CI [0.06, 0.35], p = 0.006) (Fig. 2) and p-tau (pooled Std.MD = 0.36, 95% CI [0.02, 0.69], p = 0.04) (Fig. 3) in PDD patients were higher than those in PDNC patients. However, differences in CSF levels of t-tau (pooled Std.MD = 0.17, 95% CI [−0.00, 0.34], p = 0.05) and p-tau (pooled Std.MD = 0.15, 95% CI [−0.08, 0.37], p = 0.20) between PDCI patients and PDNC patients were not statistically significant (Table 2). In addition, differences in CSF levels of Aβ42 (pooled Std.MD = −0.05, 95% CI [−0.21, 0.11], p = 0.55), t-tau (pooled Std.MD = −0.16, 95% CI [−0.58, 0.26], p = 0.45), and p-tau (pooled Std.MD = 0.06, 95% CI [−0.10, 0.22], p = 0.47) between PDMCI patients and PDNC patients showed no statistical significance (Table 2).
Cerebrospinal fluid (CSF) levels of amyloid beta 1–42 (Aβ 42) in Parkinson’s disease with cognitive impairment (PDCI) cohorts were lower than that in Parkinson’s disease with normal cognition (PDNC) cohorts. SD standard deviation, CI confidence interval. Diamonds standard (Std.) mean difference estimates from inverse-variance (IV) weighted random-effect model. Unit of cerebrospinal fluid Aβ 42 level pg/ml
Cerebrospinal fluid (CSF) levels of total tau (t-tau) in Parkinson’s disease dementia (PDD) cohorts raised relative to those in Parkinson’s disease with normal cognition (PDNC) cohorts. SD standard deviation, CI confidence interval. Diamonds standard (Std.) mean difference estimates from inverse-variance (IV) weighted fixed-effect model. Unit of cerebrospinal fluid t-tau level pg/ml
Cerebrospinal fluid (CSF) levels of phosphorylated tau181 (p-tau) in Parkinson’s disease dementia (PDD) cohorts raised relative to those in Parkinson’s disease with normal cognition (PDNC) cohorts. SD standard deviation, CI confidence interval. Diamonds standard (Std.) mean difference estimates from inverse-variance (IV) weighted random-effect model. Unit of cerebrospinal fluid p-tau level pg/ml
Sensitivity analysis
Conditions such as PDMCI and PDD were selected, and all details were listed in Table 2. There was no heterogeneity within studies in terms of CSF Aβ42 level when comparing PDMCI (I 2 = 0%, p = 0.47) or PDD (I 2 = 0%, p = 0.46) patients with PDNC patients. Low heterogeneity could be observed within studies when CSF t-tau levels in PDD patients were compared with that in PDNC patients (I 2 = 43%, p = 0.07). No further heterogeneity could be seen in CSF p-tau level within studies when comparing PDMCI patients with PDNC patients (I 2 = 0%, p = 0.45). Furthermore, there was no heterogeneity between prospective cohort studies and cross-sectional studies when CSF p-tau levels in PDD patients were compared with that in PDNC patients (I 2 = 0%, p = 0.89). Low heterogeneity was observed within studies when comparing CSF p-tau level in PDD patients with that in PDNC patients. In the meantime, there was low heterogeneity within studies in which zero or one star was given to the comparability category of NOS criteria (I 2 = 46%, p = 0.12).
Significant heterogeneity could be observed within studies when comparing CSF t-tau level in PDMCI patients with that in PDNC patients (I 2 = 71%, p = 0.02). At the same time, marked heterogeneity was found within studies when comparing changes of CSF p-tau in PDD patients with that in PDNC patients (I 2 = 69%, p = 0.002). There was remarkable heterogeneity within retrospective cohort studies, in which CSF p-tau levels in PDD patients were compared with that in PDNC patients (I 2 = 59%, p = 0.04). Moreover, significant heterogeneity was observed within studies in which CSF p-tau level in PDD patients was compared with that in PDNC patients. Two stars were given to the comparability category of NOS criteria (I 2 = 82%, p = 0.004).
Publication bias
Publication bias was assessed by visual inspection of funnel plot when comparing levels of CSF Aβ42, t-tau, and p-tau in PDCI patients or PDD patients with those in PDNC patients. Symmetrical distribution in the funnel plot could be observed when CSF Aβ42 level in PDCI cohorts (Fig. 4a), as well as Aβ42 (Fig. 4b) and p-tau (Fig. 4d) levels in PDD cohorts, was compared with those in PDNC cohorts. It suggested low risk of publication bias in the separate meta-analysis. Moreover, an asymmetry was present in the funnel plot on t-tau level in PDD cohorts relative to PDNC cohorts (Fig. 4c), suggesting a publication bias.
Funnel plot for assessment of publication bias. a Cerebrospinal fluid (CSF) amyloid beta 1–42 (Aβ 42) in Parkinson’s disease with cognitive impairment (PDCI) cohorts relative to that in Parkinson’s disease with normal cognition (PDNC) cohorts. CSF Aβ42 (b), total tau (t-tau) (c), and phosphorylated tau181 (p-tau) (d) in Parkinson’s disease dementia (PDD) cohorts relative to that in PDNC cohorts. Funnel plot a, d were charted by comprehensive meta-analysis 2. Funnel plot b, c were charted by Review Manager version 5.3
Discussion
CSF Aβ42 level in PDCI cohorts is lower than that in PDNC patients as can be seen from the current meta-analysis. Meanwhile, the CSF signature of reduced Aβ42, as well as elevated t-tau and p-tau can also be observed in PDD cohorts relative to PDNC cohorts. CSF Aβ42 level in PDCI cohorts is reduced compared with that in PDNC cohorts. In contrast, the decreasing amplitude is more pronounced when CSF Aβ42 level in PDD cohorts is compared with that in PDNC cohorts. However, there are no differences in CSF levels of Aβ42, t-tau, and p-tau between PDMCI cohorts and PDNC cohorts. There is obvious heterogeneity within the originally included studies when comparing CSF levels of Aβ42, t-tau, and p-tau in PDCI patients with those in PDNC patients. The major sources of heterogeneity are found out by sensitivity analysis. Besides, the current meta-analysis is the first to reveal that CSF Aβ42, t-tau, and p-tau levels are associated with PDD, rather than PDMCI.
Subgroup analysis is employed to evaluate the influence of discrepant severity of PDCI, such as PDMCI and PDD on heterogeneity. Firstly, no heterogeneity can be observed within the included studies when pooling studies comparing CSF Aβ42 level in PDMCI or PDD patients with that in PDNC patients. It is indicated that variations of PDCI severity account for the main source of heterogeneity when investigating the difference in CSF Aβ42 level between PDCI cohorts and PDNC cohorts. Secondly, low heterogeneity is observed within studies when comparing CSF t-tau level in PDD patients with that in PDNC patients. In addition, there is no heterogeneity within studies when CSF p-tau level in PDMCI patients is compared with that in PDNC patients. Therefore, it is suggested that variations of PDCI severity also contributes to the main source of heterogeneity when investigating the differences in CSF t-tau or p-tau levels between PDCI patients and PDNC patients. Thirdly, no heterogeneity can be seen between prospective cohort studies and cross-sectional studies when comparing CSF p-tau level in PDD cohorts with that in PDNC cohorts. Meanwhile, low heterogeneity can be observed within studies that are given zero or one star to the comparability category of NOS criteria. The study design method and comparability category of NOS criteria have contributed to one of the sources of heterogeneity when comparing CSF p-tau level in PDD patients with that in PDNC patients.
Reduced CSF Aβ42 level is associated with increased cortical Aβ deposition, as is measured by [11C] PiB positron emission tomography (PET) [29]. It represents the amyloid status and retention of Aβ tracers in the brain of AD patients [29]. Two major pathological subgroups, namely, neocortical synucleinopathy (38%) and neocortical synucleinopathy with Aβ deposition (59%), have been identified based on the distribution and severity of pathological proteins including α-synuclein, Aβ, and tau deposition in the brains of 32 consecutive autopsied patients [5]. Therefore, reduced CSF Aβ42 level in PDD patients relative to PDNC patients indicates much more pathological Aβ deposition in the brains of PDD patients than in PDNC patients (Table 2). Moreover, a large amount of Aβ deposition, together with α-synuclein, can better predict cognitive decline in PD than Aβ42 alone [30].
Nine articles are reviewed to assess whether levels of specific CSF protein biomarkers, such as t-tau and p-tau, are predictors of progression to cognitive impairment in PD patients [31]. However, the viewpoint that levels of CSF t-tau or p-tau are useful predictors of future PDCI is poorly supported. It has been strongly supported by results in the current meta-analysis that levels of CSF t-tau or p-tau are useful biomarkers to distinguish PDD patients from PDNC patients. This is different from findings in Leaver K et al.’s review [31]. Unlike global tauopathy in diverse brain regions of AD patients, overexpression of p-tau in the brains of PD and PDD patients is probably restricted within dopaminergic neurons of the nigrostriatal region. This is also shown by a study on postmortem striata and inferior frontal gyri from patients with PD and PDD [32]. Moreover, progressive and diffuse accumulation of p-tau has been confirmed in the striata of the 11-month-old transgenic PD mice with overexpression of α-synuclein [33].
Recently, in a prospectively study for 3 years [34], lower CSF Aβ42 as well as dopamine deficiency, global atrophy, and genetic factors independently contributed to prediction cognitive impairment in de novo Parkinson disease. However, in this multi-center and large-scale collaborative study [34], α-synuclein, t-tau, and p-tau cannot be recognized as baseline biomarkers to predict the development of PDCI. To some extent, these views are confirmed by present meta-analysis (Fig. 1 and Table 2). What is different and significant is that CSF signature of reduced Aβ42 as well as elevated t-tau and p-tau may predict the development of PDD, suggesting a concomitant AD pathology.
Nevertheless, this meta-analysis is also associated with several limitations. Firstly, most of the included original studies are observational cohort studies, in which patients are selected from hospital, except for three reports [19, 21, 23]. Undisputedly, this will result in selective bias, such as admission bias. Secondly, according to diagnostic criteria of PDMCI and PDD, methods of neuropsychological assessment are diversified, including Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), and Clinical Dementia Rating (CDR). This will lead to diagnostic bias and clinical heterogeneity of PDCI. Thirdly, distinctly elevated CSF tau level may be related to extensive neuronal damage due to tauopathy [31]. However, tauopathy in PDD patients may be restricted within dopaminergic neurons of the nigrostriatal region [32]. Thus, t-tau and p-tau levels may not be remarkably increased until the presence of crucial damage of dopaminergic neurons in nigrostriatal region. Unfortunately, the correlation of CSF tau with the severity of dopaminergic neuronal injury in the nigrostriatal region has not been reported so far. Fourthly, levels of Aβ42, t-tau, and p-tau in blood would be more useful than lumbar puncture in CSF for PD patients, since the latter is taken in less frequency. Nevertheless, no included studies have examined the changes of blood Aβ42, t-tau, and p-tau in PDCI patients relative to those in PDNC patients. Fifthly, there is a publication bias on t-tau level in PDD cohorts relative to PDNC cohorts (Fig. 4c) because articles only published in English are included in the meta-analysis. Moreover, some negative results of their clinical researches may be unpublished.
In conclusion, CSF Aβ42 level in PDCI cohorts is obviously lower than that in PDNC cohorts. Meanwhile, CSF signature of reduced Aβ42, as well as elevated t-tau and p-tau in PDD cohorts relative to PDNC cohorts, suggests that amyloid pathology and tauopathy may be involved in the development of PDD. As is shown by subgroup analysis, variations of severity of cognitive impairment account for the main source of heterogeneity when CSF Aβ42, t-tau, and p-tau in PDCI patients are compared with those in PDNC patients. The results of this meta-analysis support the hypothesis that the synergies of different proteins participate in the development of PDD. This has suggested a larger application in clinical practice and has indicated the research purpose.
References
Santangelo G, Vitale C, Picillo M, Moccia M, Cuoco S, Longo K et al (2015) Mild cognitive impairment in newly diagnosed Parkinson’s disease: a longitudinal prospective study. Parkinsonism Relat Disord 21:1219–1226
Garcia-Ptacek S, Kramberger MG (2016) Parkinson disease and dementia. J Geriatr Psychiatry Neurol 29:261–270
Pfeiffer HC, Løkkegaard A, Zoetmulder M, Friberg L, Werdelin L (2014) Cognitive impairment in early-stage non-demented Parkinson’s disease patients. Acta Neurol Scand 129:307–318
Pedersen KF, Larsen JP, Tysnes OB, Alves G (2013) Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest study. JAMA Neurol 70:580–586
Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD et al (2012) Pathologic accumulation of alpha-synuclein and Abeta in Parkinson disease patients with dementia. Arch Neurol 69:1326–1331
Recasens A, Dehay B, Bové J, Carballo-Carbajal I, Dovero S, Pérez-Villalba A et al (2014) Lewy body extracts from Parkinson disease brains trigger alpha-synuclein pathology and neurodegeneration in mice and monkeys. Ann Neurol 75:351–362
Zhang QS, Heng Y, Yuan YH, Chen NH (2017) Pathological alpha-synuclein exacerbates the progression of Parkinson’s disease through microglial activation. Toxicol Lett 265:30–37
Olsson B, Lautner R, Andreasson U, Öhrfelt A, Portelius E, Bjerke M et al (2016) CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol 15:673–684
Terrelonge M, Marder KS, Weintraub D, Alcalay RN (2016) CSF beta-amyloid 1-42 predicts progression to cognitive impairment in newly diagnosed Parkinson disease. J Mol Neurosci 58:88–92
Bäckström DC, Eriksson Domellöf M, Linder J, Olsson B, Öhrfelt A, Trupp M et al (2015) Cerebrospinal fluid patterns and the risk of future dementia in early, incident Parkinson disease. JAMA Neurol 72:1175–1182
Liu C, Cholerton B, Shi M, Ginghina C, Cain KC, Auinger P et al (2015) CSF tau and tau/Abeta42 predict cognitive decline in Parkinson’s disease. Parkinsonism Relat Disord 21:271–276
Stang A (2010) Critical evaluation of the Newcastle-Ottawa Scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25:603–605
Mollenhauer B, Trenkwalder C, von Ahsen N, Bibl M, Steinacker P, Brechlin P et al (2006) Beta-amlyoid 1-42 and tau-protein in cerebrospinal fluid of patients with Parkinson’s disease dementia. Dement Geriatr Cogn Disord 22:200–208
Parnetti L, Tiraboschi P, Lanari A, Peducci M, Padiglioni C, D’Amore C et al (2008) Cerebrospinal fluid biomarkers in Parkinson’s disease with dementia and dementia with Lewy bodies. Biol Psychiatry 64:850–855
Compta Y, Marti MJ, Ibarretxe-Bilbao N, Junque C, Valldeoriola F, Munoz E et al (2009) Cerebrospinal tau, phospho-tau, and beta-amyloid and neuropsychological functions in Parkinson’s disease. Mov Disord 24:2203–2210
Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C et al (2010) CSF Abeta(42) and tau in Parkinson’s disease with cognitive impairment. Mov Disord 25:2682–2685
Hall S, Öhrfelt A, Constantinescu R, Andreasson U, Surova Y, Bostrom F et al (2012) Accuracy of a panel of 5 cerebrospinal fluid biomarkers in the differential diagnosis of patients with dementia and/or Parkinsonian disorders. Arch Neurol 69:1445–1452
Maetzler W, Tian Y, Baur SM, Gauger T, Odoj B, Schmid B et al (2012) Serum and cerebrospinal fluid levels of transthyretin in Lewy body disorders with and without dementia. PLoS One 7:e48042
Beyer MK, Alves G, Hwang KS, Babakchanian S, Bronnick KS, Chou YY et al (2013) Cerebrospinal fluid Abeta levels correlate with structural brain changes in Parkinson’s disease. Mov Disord 28:302–310
Nutu M, Zetterberg H, Londos E, Minthon L, Nägga K, Blennow K et al (2013) Evaluation of the cerebrospinal fluid amyloid-beta1-42/amyloid-beta1-40 ratio measured by alpha-LISA to distinguish Alzheimer’s disease from other dementia disorders. Dement Geriatr Cogn Disord 36:99–110
Alves G, Lange J, Blennow K, Zetterberg H, Andreasson U, Førland MG et al (2014) CSF Abeta42 predicts early-onset dementia in Parkinson disease. Neurology 82:1784–1790
Vranová HP, Hényková E, Kaiserová M, Menšíková K, Vaštík M, Mareš J et al (2014) Tau protein, beta-amyloid(1)(−)(4)(2) and cluster in CSF levels in the differential diagnosis of Parkinsonian syndrome with dementia. J Neurol Sci 343:120–124
Yarnall AJ, Breen DP, Duncan GW, Khoo TK, Coleman SY, Firbank MJ et al (2014) Characterizing mild cognitive impairment in incident Parkinson disease: the ICICLE-PD study. Neurology 82:308–316
Yu SY, Zuo LJ, Wang F, Chen ZJ, Hu Y, Wang YJ et al (2014) Potential biomarkers relating pathological proteins, neuroinflammatory factors and free radicals in PD patients with cognitive impairment: a cross-sectional study. BMC Neurol 14:113. doi:10.1186/1471-2377-14-113
Skogseth RE, Bronnick K, Pereira JB, Mollenhauer B, Weintraub D, Fladby T et al (2015) Associations between cerebrospinal fluid biomarkers and cognition in early untreated Parkinson’s disease. J Parkinsons Dis 5:783–792
Compta Y, Buongiorno M, Bargalló N, Valldeoriola F, Muñoz E, Tolosa E et al (2016) White matter hyperintensities, cerebrospinal amyloid-beta and dementia in Parkinson’s disease. J Neurol Sci 367:284–290
Buongiorno M, Antonelli F, Compta Y, Fernandez Y, Pavia J, Lomeña F et al (2017) Cross-sectional and longitudinal cognitive correlates of FDDNP PET and CSF amyloid-beta and tau in Parkinson’s disease. J Alzheimers Dis 55:1261–1272
Schrag A, Siddiqui UF, Anastasiou Z, Weintraub D, Schott JM (2017) Clinical variables and biomarkers in prediction of cognitive impairment in patients with newly diagnosed Parkinson’s disease: a cohort study. Lancet Neurol 16:66–75
Lewczuk P, Matzen A, Blennow K, Parnetti L, Molinuevo JL, Eusebi P et al (2017) Cerebrospinal fluid Abeta42/40 corresponds better than Abeta42 to amyloid PET in Alzheimer’s disease. J Alzheimers Dis 55:813–822
Stewart T, Liu C, Ginghina C, Cain KC, Auinger P, Cholerton B et al (2014) Cerebrospinal fluid alpha-synuclein predicts cognitive decline in Parkinson disease progression in the DATATOP cohort. Am J Pathol 184:966–975
Leaver K, Poston KL (2015) Do CSF biomarkers predict progression to cognitive impairment in Parkinson’s disease patients? A systematic review. Neuropsychol Rev 25:411–423
Wills J, Jones J, Haggerty T, Duka V, Joyce JN, Sidhu A (2010) Elevated tauopathy and alpha-synuclein pathology in postmortem Parkinson’s disease brains with and without dementia. Exp Neurol 225:210–218
Haggerty T, Credle J, Rodriguez O, Wills J, Oaks AW, Masliah E et al (2011) Hyperphosphorylated tau in an alpha-synuclein-overexpressing transgenic model of Parkinson’s disease. Eur J Neurosci 33:1598–1610
Caspell-Garcia C, Simuni T, Tosun-Turgut D, Wu IW, Zhang Y, Nalls M et al (2017) Multiple modality biomarker prediction of cognitive impairment in prospectively followed de novo Parkinson disease. PLoS One 12:e0175674. doi:10.1371/journal.pone.0175674
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The research is supported by Jingzhou Science and Technology Bureau Foundation (grant number 2016073).
Rights and permissions
About this article
Cite this article
Hu, X., Yang, Y. & Gong, D. Changes of cerebrospinal fluid Aβ42, t-tau, and p-tau in Parkinson’s disease patients with cognitive impairment relative to those with normal cognition: a meta-analysis. Neurol Sci 38, 1953–1961 (2017). https://doi.org/10.1007/s10072-017-3088-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3088-1