Abstract
Amyloid-β (Aβ) as a crucial factor in pathogenesis of Alzheimer’s disease (AD) is derived from amyloid precursor protein (APP) through a proteolytic process catalyzing by β- and γ-secretase—in amyloidogenesis pathway. Products of α-secretase cleavage also have protective effects against Aβ toxicity. According to existing evidences, microRNAs (miRNAs) show a unique pattern of expression in AD. Moreover, miRNAs regulatory effects on expression of secretases and their main components have been demonstrated in AD. The miRNAs levels may be changed in preclinical conditions and may be considered as diagnostic biomarkers in AD. Therefore, in this paper, we review the miRNAs involved in APP cleavage pathways and the formation of Aβ in order to evaluate the potential diagnostic biomarkers in AD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Alzheimer’s disease (AD), as a destructive and progressive neurodegenerative condition, is the most prevalent dementia throughout the world which leads to death within 3 to 9 years after the emergence of clinical symptoms [1]. It is estimated that the prevalence of AD doubles every 20 years, which means that about 115 million people may be affected by 2050 [2]. In an AD brain, there is a gradual widespread synaptic and neuronal loss and that causes a progressive memory loss and other cognitive functions [1]. Histopathologically, AD is described by two main hallmarks: extracellular amyloid-β (Aβ) plaques and intracellular neurofibrillary tangles (NFTs) [2].
Aβ peptide is derived from an abnormal proteolytic processing of amyloid precursor protein (APP) through the function of β-secretase (BACE-1) and γ-secretase. Following the cleavage of APP, two peptide fragments, Aβ40 and Aβ42, are produced. The former fragment is more abundant and the latter is more pathogenic [3]. Aβ deposition in the brain of patient with AD may disrupt neuronal networks and synaptic activities, followed by neural death [1]. On the contrary, α-secretase cleavage generates a form of APP (APPsα), which has been reported to have neurotrophic and neuroprotective properties [4].
Increasing and accumulation of Aβ were proposed as the main factors in the disease incidence and development [1, 5]. Early and accurate diagnosis of AD has a vital role in the prevention of irreversible dementia. In clinical diagnosis, neuroimaging techniques such as magnetic resonance imaging (MRI) and functional MRI (fMRI) as well as biochemical evaluations such as cerebrospinal fluid (CSF) analysis are used. MRI-based measures are ideal options in AD prediction [6], but there are some reports that the sensitivity and accuracy of these imaging techniques are not satisfactory [7]. Positron emission tomography (PET) as an alternative imaging technique can demonstrate Aβ deposition or brain hypometabolism in AD patients [8]. Although PET has been associated with some success in the diagnosis of probable AD, nevertheless, wide fluctuation related to its reports, high costs, and unavailability are the main limitations for this method [9].
As biochemical index, reduced levels of Aβ42 in CSF of AD patients have been shown in preclinical phases of the disease [6]. However, CSF sampling is an invasive procedure and may have potential side effects [7].
Biomarkers are new indicators for AD diagnosis, especially in cases with atypical clinical representations. The ongoing development of new biomarkers based on less or non-invasive procedures will provide a great opportunity for extensive use to enhance the accuracy of diagnosis of cognitive impairments. In this regard, microRNAs (miRNAs) could represent a considerable potential. Since 1993, when the first miRNA was discovered, miRNAs have attracted extensive interests, because of their specific function [10]. miRNAs are ~ 22 nucleotides, single-stranded, non-protein-coding RNA molecules, characterized by the absence of open reading frames for translation. These oligonucleotides regulate the levels of gene expression, both by increasing mRNA degradation and by posttranscriptional suppression of protein translation [10].
miRNAs are involved in nearly all of the biological processes, including proliferation, development, apoptosis, and inflammation and their expression is extremely under regulation, either by stabilizer enzymes or epigenetic mechanisms such as DNA methylation and histone modification [5].
Recent evidence shows that increased expression of the APP may lead to increased levels of Aβ and raises the risk of AD. The 3′ untranslated regions (3′ UTRs) of APP and BACE1 mRNAs are potential targets for several miRNAs [11]. Dysregulation of these miRNAs might have a role in the incidence and progress of AD, as the change in the level of miRNAs is demonstrated in the blood or CSF of AD patients. We review potential application of the miRNAs involved in APP cleavage pathways and the formation of Aβ in AD as biomarker.
miRNA biology
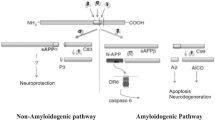
The miRNAs, as non-coding RNAs, are thought to negatively regulate messenger RNA (mRNA) translation through incomplete base pairing to the 3′ UTRs [12]. The primary miRNA transcripts (pri-miRNAs) are transcribed by RNA polymerase II and their length can be several thousand bases. Inside the nucleus, these transcripts are processed by the Drosha/DGCR8 complex to produce precursor miRNAs (pre-miRNAs), which are about 70 nucleotides oligomers and are transported to the cytoplasm by Exportin 5/Ran complex. After that, pre-miRNAs are cleaved in the cytoplasm by Dicer to form mature miRNAs which bind to argonaute (Argo) proteins in RNA-induced silencing complexes (RISC) to modulate mRNA translation [1, 5] (Fig. 1). The miRNAs has a critical regulatory role in various biological processes and their type and level of expression are mostly dysregulated in AD. So, miRNAs can be considered as potential biomarkers to predict the disease onset or progression [12].
Gamma-secretase
Because of the vital role of gamma-secretase (γ-secretase) in the production of Aβ, it is considered as a potential biomarker for AD. γ-secretase is a membrane-bound aspartic protease and includes 4 essential components: presenilin1 (PS1), nicastrin (NCSTN), anterior pharynx defective 1 homolog A (APH1A), and presenilin2 (PS2) [13]. In addition, there are some other proteins which affect γ-secretase activities. Gamma-secretase activating protein (GSAP) was described first by He et al. as a negative regulator of GSAP. The ability of this protein in reducing the accumulation of Aβ plaques in animal models has been proven [14]. Studies have shown that miRNAs can have direct regulatory effects on the components of the γ-secretase.
Presenilin1
Presenilin1 (PS1) is one of the subunits of γ-secretase complex which plays an important role in generation of Aβ from APP. It was found that miR-9 expression was specifically reduced in PS1 knocked out mice. So, miR-9 is likely essential for brain development and function and its reduced levels may lead to the brain impairment [15]. In AD, increased level of PS1 mRNA accelerates the production and accumulation of Aβ through sequential cleavage of APP [16]. Therefore, it is suggested that reduction of miR-9 could be associated with upregulation of PS1 in AD [15].
Presenilin2
Studies on presenilin2 (PS2) knocked out mice have showed that this protein may influence inflammatory signaling pathways, intrinsic immunity functions and neurodegeneration, probably by miRNAs regulation [17]. It was reported that miR146a and miR146b are downregulated in PS2-deficient animals. These findings demonstrate that miR146 level has an effect on protein levels of its target. Moreover, miR146 may be associated with PS2 dysfunction in aged mice [17]. Interestingly, the miR146 has been previously proposed as a circulating biomarker in AD [18].
Nicastrin
Nicastrin (NCSTN), a γ-secretase component, also plays an important role in the generation of Aβ in AD. A significant decline in γ-secretase activity has been shown when NCSTN was inhibited via RNA interference [19]. The existence of the target site for mirR-128, -27a, and -27b within the coding sequence of NCSTN gene is a possible cause of decreased expression of NCSTN in diseases [20]. Delay et al. introduced a number of brain-expressed miRNAs that regulate the expression of endogenous NCSTN and eventually Aβ peptide production. They reported that immediately after the expression of miR-24, miR-186, and miR-455, endogenous NCSTN was downregulated. So, they suggested that these miRNAs could be considered as a regulator for human NCSTN and decrease Aβ generation [21].
Parsi et al. have investigated the therapeutic efficacy of miR-16 in AD. They delivered miR-16 mimics into the mouse brain and reported a remarkable downregulation of NCSTN in the treated mice. They identified one miR-16 binding site located in 3′ UTR of NCSTN [22]. The other target of miR-16 is APP and decreased expression of miR-16, increases the level of APP in AD mice [23].
Anterior pharynx defective 1 homolog A
Another component of the γ-secretase complex is anterior pharynx defective 1 homolog A (APH1A) which is involved in Aβ generation and has altered expression in AD brain. It has been found that a specific site on 5′ UTR of protein which could match by miR-324-5p and regulates APH1A. Polymorphisms within this target region could disrupt the miRNA-APH1A interaction and leads to increase in expression of APH1A and thereby contributes in the disease [24].
Beta-site amyloid precursor protein cleaving enzyme 1
APP is initially cleaved by β-secretase in the amyloidogenic pathway. Studies have indicated that β-secretase cleaves only membrane-bound substrates and the enzyme is probably a membrane-bound aspartyl protease or is stoutly associated with membrane proteins [3]. This cleavage produces two fragments: (1) N-terminal soluble β-APP fragment which is released and (2) C-terminal fragment (C99) which is retained in the membrane, and is cleaved again by γ-secretase [3, 13].
β-secretase is active in most of the cells and tissues. However, greatest activity was reported in neural tissue and neuronal cells [13]. Studies have indicated that increased BACE1 expression raises risk of sporadic AD.
It has been found that reduction or absence of specific miRNAs can increase the levels of BACE1 and Aβ in AD. Although, some of the long non-coding RNAs (lncRNA), including an antisense lncRNA, BACE1-AS, involved in the regulation of BACE1. There are evidence that the elevated expression of BACE1-AS increases BACE1 protein and production of Aβ plaque in AD [25].
Hebert et al. found that miR-29a and -29b-1 are the main suppressors of BACE1 in the brain of a sporadic AD cases. They observed that increased BACE1 expression is followed by alternations in miR-29a/b-1 levels [11]. It was also reported a significant correlation between reduction of miR-29a, miR-29b-1 and increasing of BACE1 protein during normal brain development and AD [11]. Furthermore, miR-186 is a strong negative regulator of BACE1 in neuronal cells and it could be one of the molecular turning points within the process of aging and the heightened risk of AD [26]. A study on miR-29 family in the blood of AD patients showed a significant reduction in the expression level of miR-29c and increased level of BACE1 [27]. The miRTarbase alignment indicated that miR-29c is a target for the 3′ UTR of BACE1 in the brain of AD cases and directly downregulates the expression of BACE1 [28].
Muler et al. surveyed miR-27a, miR-29a, miR-29b, and miR-125b expression levels in cell-free CSF samples of AD patients and reported a significant increase in miR-29a level with good sensitivity and moderate specificity [29]. It has been reported that miR-125b is in correlation with Mini-Mental State Examination (MMSE) score that means more severe cognitive impairment is associated with higher miR-125b levels. Because of the high sensitivity/specificity of serum miR-125b (80.8%/68.3%), it was suggested that serum miR-125b may be considered as an appropriate non-invasive biomarker for AD [30]. In addition, miR-125b upregulation in the CSF and brain of AD patients has been reported [31]. A study in primary cultured SAMR1 mice hippocampal neurons demonstrated that upregulation of miR-29c decreases Aβ production via BACE1 regulation. So, it is proposed that miR-29c can be a peripheral biomarker for AD and may correlate with memory and cognition impairments in AD [32]. Furthermore, miR-29a may regulate BACE1 expression and serve as a CSF biomarker for AD [29]. Additionally, treatment with miR-29c could improve learning and memory in the SAMP8 mice through the secretase independent pathway. [27].
Zhu et al. discovered that miR-195 levels were decreased in SAMP8-aged mice. They reported that miR-195 likely affects the BACE1 mRNA translation by acting on the 3′ UTR binding site [33]. Moreover, downregulation of miR-195 is reported in the plasma of dementia patients [34].
Reducing the expression of APP and BACE1 and consequently, a significant reduction in Aβ level, following by a decreased miR-195 level suggesting APP and BACE1 as possible targets for this miRNA [35, 36]. So, exogenous forms of miR-195 may protect dementia-susceptible individuals through the inhibition of Aβ production [34].
Transfecting miR-124 mimics or inhibitors in cultured neuronal cells showed reduction and increase in the expression of BACE1 expression, respectively [37]. The miR-124 downregulation and involvement in the abnormal splicing of APP also are observed in AD brain [38]. After Aβ exposure, increased expression of BACE1 and reduction in miR-124 has been observed in damaged cells [37]. The miR-124 is the most abundant miRNA in the brain and also, it is the main negative regulator of neuronal gene expression in some neurological disorders.
The inhibition of miR-124 expression subsequently increases the expression of BACE1 and reduces the cell viability. Therefore, it is suggested that miR-124 may act as a regulatory factor in AD-related neurodegeneration [37].
The lower levels of miR-384 were found in the CSF and serum of AD patients and transgenic mice. In addition, miR-384 suppresses the expression of APP and BACE-1 and negatively correlates with Aβ42 in serum and CSF. In addition, there is evidence that Aβ42 may have a significant effect on downregulation of miR-384. Moreover, miR-384 is being suggested as a blood-based biomarker related to the progression of AD [39].
Alpha-secretase
In the non-amyloidogenic pathway in healthy subjects, APP is cleaved by α- and γ-secretase. Cleavage of APP by the α-secretase leads to the formation of sAPPα, a fragment with neurotrophic and neuroprotective effects, and C83, which subsequently is cleaved by γ-secretase and reduces the production of toxic Aβ peptides [40]. Evidence has demonstrated that a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) is the main α-secretase that catalyzes the cleavage of APP in the brain [4]. In most studies, it is mentioned that a reduction in the amount or activity of ADAM10 decreases the formation of sAPPα in AD [40] (Table 1).
Augustin et al. (2012) found three α-secretase-related miRNAs. miR-103, miR-107, and miR-1306 directly bind to human ADAM10 3′-UTR in AD and reduce this protein levels. Therefore, they have suggested that these three miRNAs are likely involved in AD by regulation of ADAM10′ [41].
Conclusion
Due to the vital role of BACE1 and γ-secretase in Aβ generation, these are two important diagnostic factors in AD. However, the existing knowledge about α-secretase is limited and needs more investigations.
Changes in the levels of secretase-related miRNAs have been proved during AD. This has led to the increasing interest in employing these alterations as reliable signals, though the other relevant biomolecules. Therefore, blood-based biomarkers, because of non-invasiveness, simplicity, cost- and time-effectiveness, and availability at routine examinations are preferred. The unique expression pattern of serum miRNAs made it a specific non-invasive biomarker for the diagnosis of AD. So, secretase-related miRNAs in serum can be considered as possible diagnostic biomarkers for AD. It should be noted that the use of miRNA biomarkers in the diagnosis of diseases is currently confronted with various limitations. First, despite equal number of miRNAs in blood and CSF, their blood levels are four times lower [36]. Second, the expression of genes can be influenced by several factors, including disease comorbidities and environmental conditions. Third, since most of the diseases are multifactorial and polygenic, each biomarker is just a fragment of the complex puzzle of each disease, and for a better diagnosis, a profile of multiple biomarkers should be taken into account. Fourth, the true value of biomarkers is their specific potential for diagnosing presence of the disease before the onset of clinical symptoms.
Therefore, numerous follow-up studies are needed to determine the unique, sensitive, and specific miRNA-based diagnostic profile of AD and advances in this field will help the normalization and standardization of circulating miRNA-based tests.
References
Schonrock N, Matamales M, Ittner LM, Gotz J (2012) MicroRNA networks surrounding APP and amyloid-β metabolism—implications for Alzheimer’s disease. Exp Neurol 235:447–454
Povova J, Ambroz P, Bar M, Pavulova V, Sery O, Tomaskova H, Janout V (2012) Epidemiological of and risk factor for Alzheimer’s disease: a review. Biomed Pep Med Fac Univ Palacky Olomouc Czech Repub 156(2):108–114
Haass C, Selkoe DJ (2007) Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8(2):101–112
Kuhn P, Wang H, Dislich B, Colombo A, Zeitschel U, Ellwart JW, Kremmer E, RoBner S, Lichtenthaler SF (2010) ADAM10 is the physiologically relevant, constitutive alpha-secretase of the amyloid precursor protein in primary neurons. EMBO J 29(17):3020–3032
Femminella GD, Ferrara N, Rengo G (2015) The emerging role of microRNAs in Alzheimer’s disease. Front Physiol 6(40)
Berti V, Polito C, Lombardi G, Ferrari C, Sorbi S, Pupi A (2016) Rethinking on the concept of biomarkers in preclinical Alzheimer’s disease. Neurol Sci 37:663–672
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Neurol Sci 235(2):491–496
During EH, Osorio RS, Elahi FM, Mosconi L, de Leon MJ (2011) The concept of FDG-PET endophenotype in Alzheimer’s disease. Neurol Sci 32:559–569
Toledo JB, Show LM, Trojanowski JQ (2013) Plasma amyloid beta measurements - a desired but elusive Alzheimer’s disease biomarker. Alzheimers res Ther 5(2):8
Jin XF, Wu N, Wang L, Li J (2013) Circulating microRNAs: a novel class of potential biomarkers for diagnosing and prognosing central nervous system diseases. Cell Mol Neurobiol 33(5):601–613
Hebert SS, Horré K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu NA, Kauppinen S, Delacourte A, Strooper BD (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/ -secretase expression. Proc Natl Acad Sci U S A 105(17):6415–6420
Rooij EV, Kauppinen S (2014) Development of microRNA therapeutics is coming of age. EMBO Mol Med 8(12):1361–1471
Haass C (2004) Take five-BACE and the gamma-secretase quartet conduct Alzheimer’s amyloid beta-peptide generation. EMBO J 23(3):483–488
He G, Luo W, Li P, Remmers C, Netzer WJ, Hendrick J, Bettayeb K, Flajolet M, Gorelick F, Greengard W, Greengard P (2010) Gamma-secretase activating protein is a therapeutictarget for Alzheimer’s disease. Nature 467:95–98
Krichevsky AM, King KS, Donahue CP, Khrapko K, Kosik KS (2003) A microRNA array reveals extensive regulation of microRNAs during brain development. RNA 9:1274–1281
Ikeda K, Urakami K, Arai H, Wada K, Ji Y, Adachi Y, Okada A, Kowa H, Sasaki H, Ohno K, Ohtsuka Y, Ishikawa Y, Nakashima K (2000) The expression of Presenilin 1 mRNA in skin fibroblasts and brains from sporadic Alzheimer’s disease. Dement Geriatr Cogn Disord 11:245–250
Jayadev S, Case A, Alajajian B, Moller ET, Garden GA (2013) Presenilin 2 influences miR146 level and activity in microglia. J Neurochem 127(5):592–599
Galimberti D, Villa C, Fenoglio C, Serpente M, Ghezzi L, Cioffi S, Arighi A, Fumagalli G, Scarpini E (2014) Circulating miRNAs as potential biomarkers in Alzheimer’s disease. J Alzheimers Dis 42(4):1261–1267
Sorbi S, Nacmias B, Forleo P, Latorraca S, Gobbini I, Bracco L, Piacentini S, Amaducci L (1994) ApoE allele frequencies in Italian sporadic and familial Alzheimer’s disease. Neurosci Lett 177(1–2):100–102
Orlacchio A, Kawarai T, Polidoro M, Stefani A, Orlacchio A, George-Hyslpo PH, Bernardi G (2002) Association analysis between Alzheimer’s disease and the Nicastrin gene polymorphisms. Neurosci Lett 333(2):115–118
Delay C, Dorval V, Fok A, Grenier-Boley LJC, Hsiung GY, Hebert SS (2014) MicroRNAs targeting Nicastrin regulate Aβ production and areaffected by target site polymorphisms. Front Mol Neurosci 7:67
Paris S, Smith PY, Goupil C, Dorval V, Hebert SS (2015) Preclinical evaluation of miR-15/107 family members as multifactorial drug targets for Alzheimer’s disease. Mol Ther Nucleic Acids 4(10):e256
Liu W, Liu C, Zhu J, Shu P, Yin B, Gong Y, Qiang B, Yuan J, Peng X (2012) MicroRNA-16 targets amyloid precursor protein to potentially modulate Alzheimer’s-associated pathogenesis in SAMP8 mice. Neurobiol Aging 33(3):522–534
Mallick B, Ghosh Z (2011) A complex crosstalk between polymorphic microRNA target sites and AD prognosis. RNA Biol 8(4):665–673
Fatica A, Bozzoni I (2014) Long non-coding RNAs: new players in cell differentiation and development. Nat Rev Neurol 15(1):7–21
Kim J, Yoon H, Chung D, Brown JL, Belmonte K, Kim J (2016) miR-186 is decreased in aged brain and suppresses BACE1 expression. J Neurochem 137(3):436–445
Yang G, Song Y, Zhou X, Deng Y, Liu T, Weng G, Yu D, Pan S (2015) MicroRNA-29c targets β-site amyloid precursor protein-cleaving enzyme 1 and has a neuroprotective role in vitro and in vivo. Mol Med Rep 12(2):3081–3088
Lei X, Lei L, Zhang Z, Zhang Z, Cheng Y (2015) Downregulated miR-29c correlates with increased BACE1 expression in sporadic Alzheimer’s disease. Int J Clin Exp Pathol 8(2):1565–1574
Muller M, Jakel L, Bruinsma IB, Claassen JA, Kuiperij HB, Verbeek MM (2016) MicroRNA-29a is a candidate biomarker for Alzheimer’s disease in cell-free cerebrospinal fluid. Mol Neurobiol 53(5):2894–2899
Tan L, Yu JT, Liu QY, Tan MS, Zhang W, Hu N, Wang YL, Sun L, Jiang T, Tan L (2014) Circulating miR-125b as a biomarker of Alzheimer’s disease. J Neurol Sci 336(1–2):52–56
Dangla-Valls A, Molinuevo JL, Altirriba J, Sanchez-Valle R, Alcolea D, Fortea J, Rami L, Balasa M, Munoz-Garcia C, Ezquerra M, Fernandez-Santiago R, Lleo A, Antonell A (2016) CSF microRNA profiling in Alzheimer’s disease: a screening and validation study. Mol Neurobiol:1–8
Chen Y, Huang X, Zhang YW, Rockenstein E, Bu G, Golde TE, Masliah E, Xu H (2012) Alzheimer’s β-secretase (BACE1) regulates the cAMP/PKA/CREB pathway independently of β-amyloid. J Neurosci 32(33):11390–11395
Zhu HC, Wang LM, Wang M, Song B, Tan S, Teng JF, Duan DX (2012) MicroRNA-195 downregulates Alzheimer’s disease amyloid-β production by targeting BACE1. Brain Res Bull 88(6):596–601
Ai J, Sun LH, Che H, Zhang R, Zhang TZ, Wu WC, Su XL, Chen X, Yang G, Li K, Wang N, Ban T, Bao YN, Guo F, Niu HF, Zhu YL, Zhu XY, Zhao SG, Yang BF (2013) MicroRNA-195 protects against dementia induced by chronic brain hypoperfusion via its anti-amyloidogenic effect in rats. J Neurosci 33(9):3989–4001
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol 235(2):491–496
Sørensen SS, Nygaard AB, Christensen T (2016) miRNA expression profiles in cerebrospinal fluid and blood of patients with Alzheimer’s disease and other types of dementia - an exploratory study. Transl Neurodegener 5:6
Fang M, Wang J, Zhang X, Geng Y, Hu Z, Rudd JA, Ling S, Chen W, Hun S (2012) The miR-124 regulates the expression of BACE1/ -secretase correlated with cell death in Alzheimer’s disease. Toxicol Lett 209(1):94–105
Smith P, Hashimi AA, Girard J, Delay C, Hebert SS (2010) In vivo regulation of amyloid precursor protein neuronal splicing by microRNAs. J Neurochem 116(2):240–247
Liu CG, Wang JL, Li L, Wang PC (2014) MicroRNA-384 regulates both amyloid precursor protein and β-secretase expression and is a potential biomarker for Alzheimer’s disease. Int J Mol Med 34(1):160–166
Endres K, Fahrenholz F (2012) Regulation of alpha-secretase ADAM10 expression and activity. Exp Brain Res 217(3):343–352
Augustin R, Endres K, Reinhardt S, Kuhn P, Lichtenthaler SF, Hansen J, Wurst W, Trumbach D (2012) Computational identification and experimental validation of microRNAs binding to the Alzheimer-related gene ADAM10. BMC Med Genet 13(1):35–46
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hajjari, S.N., Mehdizadeh, M., Sadigh-Eteghad, S. et al. Secretases-related miRNAs in Alzheimer’s disease: new approach for biomarker discovery. Neurol Sci 38, 1921–1926 (2017). https://doi.org/10.1007/s10072-017-3086-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3086-3