Abstract
As a class of important endogenous small noncoding RNAs that regulate gene expression at the posttranscriptional level, microRNAs (miRNAs) play a critical role in many physiological and pathological processes. It is believed that miRNAs contribute to the development, differentiation, and synaptic plasticity of the neurons, and their dysregulation has been linked to a series of diseases. MiRNAs exist in the tissues and as circulating miRNAs in several body fluids, including plasma or serum, cerebrospinal fluid, urine, and saliva. There are significant differences between the circulating miRNA expression profiles of healthy individuals and those of patients. Consequently, circulating miRNAs are likely to become a novel class of noninvasive and sensitive biomarkers. Although little is known about the origin and functions of circulating miRNAs at present, their roles in the clinical diagnosis and prognosis of diseases make them attractive markers, particularly for tumors and cardiovascular diseases. Until now, however, there have been limited data regarding the roles of circulating miRNAs in central nervous system (CNS) diseases. This review focuses on the characteristics of circulating miRNAs and their values as potential biomarkers in CNS diseases, particularly in Alzheimer’s disease, Huntington’s disease, multiple sclerosis, schizophrenia, and bipolar disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
MicroRNAs (miRNAs) are ~22 nucleotides long, single-stranded, nonprotein-coding RNA molecules that regulate protein expression levels either by promoting mRNA degradation or by attenuating protein translation at the posttranscriptional level. Since the first miRNA (lin-4) was found in 1993 (Lee et al. 1993), miRNAs have elicited worldwide interest because of their characteristic functions and modes of action. Specific miRNAs have been revealed to be engaged in a variety of biological processes, such as cell development, proliferation, differentiation, and apoptosis (Li et al. 2010). In human beings, it has been estimated that there are >1,000 miRNAs in the genome that regulate ~60 % of all protein-coding genes (Friedman et al. 2009; Siomi and Siomi 2010). Most mRNA targets contain multiple miRNA binding sites, and each miRNA can regulate multiple genes. Therefore, the dysregulation of miRNA levels might perturb the expression of many genes, thereby playing a key role in the occurrence of diseases.
One of the main challenges in modern medicine is treating central nervous system (CNS) disorders which are characterized by complex mechanisms and are difficult to cure. Common CNS disorders include neurodevelopmental disorders, neurodegenerative disorders, neuroimmunological disorders, neuro-oncological disorders and other psychiatric disorders, such as schizophrenia, bipolar disorder (BD), major depression and autism spectrum disorders (Qureshi et al. 2010). It is difficult to cure these CNS diseases primarily because of the complexity of their pathomechanisms and the difficulty of achieving an accurate diagnosis at an early stage. For example, once patients are diagnosed with Parkinson’s disease because of the presence of dyskinesia, their dopaminergic neurons have already degenerated by over 60 %. Therefore, finding biomarkers that allow for an accurate diagnosis at an early stage is crucial for curing CNS disorders. For example, certain specific proteins in the cerebrospinal fluid (CSF) have been identified in recent years as potential biomarkers for both the early diagnosis and prognosis of Alzheimer’s disease (AD). It has been shown that Aβ42 and tau protein levels in the CSF, as well as the ratios of tau/Aβ42 and p-tau/Aβ42, can be used not only in differential diagnosis but also to predict conversion and the rate of progression from cognitive normalcy to mild dementia and severe impairment (Holtzman 2011; Fagan and Perrin 2012).
Although the detailed etiologies of many CNS disorders are still poorly understood, the defects of epigenetic mechanism may be revealed as risk factors for the occurrence of many diseases. In particular, the critical effects of miRNAs in the CNS have been recognized, and the expression patterns of characteristic miRNA profiles have been implicated in modulating various neurologic- disease states, which could identify whether the tissue is in a normal condition or in various disease stages (Hebert and De Strooper 2009). As a subset of miRNAs, special circulating miRNAs that are found in body fluids have been implicated in diagnosing and prognosing various disorders. In this review, we discuss the potential functions of miRNAs and summarize the current progress regarding using circulating miRNAs as potential biomarkers for the diagnosing and prognosing several CNS diseases. Therefore, this review will provide a comprehensive and wide review of the literatures of biomarker studies in CNS diseases.
Sources and Potential Functions of Circulating MicroRNAs
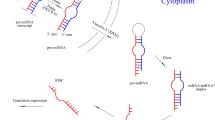
MicroRNAs control various important biological processes by regulating gene expression. Recently, a number of miRNAs have been detected in extracellular human body fluids, including plasma or serum, urine, saliva, and other body fluids (Cogswell et al. 2008; Mitchell et al. 2008; Park et al. 2009; Hanke et al. 2010; Zubakov et al. 2010). In 2008, Mitchell et al. demonstrated the existence of endogenous miRNAs in human plasma; since then, the putative biological functions of cell-free miRNAs in the peripheral blood have been studied and have become the focus of the translational research. In particular, the characteristic changes of miRNAs in the serum and plasma are in accordance with changes that have been observed in targeted tissues under certain pathological status, including cancer, cardiovascular disease, injuries, etc. Recently, studies have suggested that the miRNAs in the plasma and serum might be derived from circulating blood cells under healthy conditions, but might be released from pathological tissues during an illness (Chen et al. 2008; Fichtlscherer et al. 2010). The strong correlation between circulating miRNAs and tissue miRNAs indicates that circulating miRNAs might serve as biomarkers for various diseases.
Although miRNAs have been detected in body fluids, the sources of circulating miRNAs are largely unclear. Some hypotheses have proposed that these miRNAs are passively leaked or actively released from cells into the circulatory system. Recent studies have suggested that circulating miRNAs are the by-products of necrotic or apoptotic cells. For example, cardiocyte-spesific miR-208a is leaked into the circulating blood from apoptotic cells after an acute myocardial infarction (Corsten et al. 2010). This passive leakage of miRNAs has been observed during tumor metastasis and chronic inflammation. The leaked miRNAs have been found to be combined with argonaute proteins or in apoptotic bodies, which protect miRNAs against RNase degradations (Wang et al. 2010; Arroyo et al. 2011). However, multiple lines of evidence support the idea that cellular miRNAs can also be actively secreted into the circulating blood. The actively secreted miRNAs are observed to reside in exosomes and microparticles or are bound to lipoproteins (Valadi et al. 2007; Gibbings et al. 2009; Vickers et al. 2011). Similar to hormones and cytokines, the secreted miRNAs might serve as novel signaling molecules of cell-to-cell communication as well (Valadi et al. 2007). Several excellent reviews have discussed this topic in depth.
Advantages of and Research Strategy for Circulating MiRNA as Biomarkers
To be classified as an ideal biomarker, it is crucial that miRNAs show satisfactory predictability and the ability to be supervised and inspected during disease and prognosis. The ease of obtaining and detecting the biomarker from clinical sample is also important. Recently, neuroimaging and protein-based biochemical markers in CSF or plasma/serum have been identified as attractive biomarker candidates for accurately diagnosing early stage CNS diseases. However, neuroimaging is not a suitable first screening step during the diagnostic process because it requires costly equipment. In addition, another major obstacle in the development of imaging measures as effective biomarkers is the paucity of longitudinal studies. For protein-based biomarkers, the diversity of posttranslational modifications, the sequence variations among various tissues and species, and the difficulties in developing suitable high-affinity and high-sensitivity assay methods complicate the clinical diagnostic process. Circulating miRNAs have become new and promising forms of potential biomarkers in diagnosing CNS diseases because of the limitations of other methods and the following advantages: (1) unlike other nucleic acid molecules that are unable to exist in an extracellular environment without being damaged, circulating miRNAs exhibit an unexpected stability in various body fluids. MiRNAs can remain stable not only in the RNase-rich blood environment, but also in extreme conditions, including boiling temperatures, high or low pH, extended storage, and freeze–thaw cycles (Chen et al. 2008; Mitchell et al. 2008); (2) the sequences of most miRNAs are conserved among various species and have been identified in various tissue and cell types, and miRNAs can easily be detected using quantitative reverse transcription-polymerase chain reaction (qRT-PCR); (3) miRNAs expression is tissue- or cell-specific and is altered during the pathophysiologic processes. Most importantly, the changes of miRNAs levels in circulation reflect the changes in diseased tissues; (4) obtaining clinical samples that contain circulating miRNAs is a noninvasive and simple process; (5) compared with previous studied biomarkers, miRNAs have a lower complexity which facilitates exploration. These advantages indicate that circulating miRNAs have the potential to be useful candidates for diagnosis and other clinical applications in human diseases. However, there are limitations for this potential biomarker. Because one miRNA can target many proteins and one protein can be regulated by many miRNAs, there is an inherent complexity in the relationship between a single miRNA and a pathogenic protein. Therefore, assaying combinations of multiple miRNAs, rather than a single miRNA, may improve the sensitivity and specificity of the diagnosis. In addition, because of the small amount of circulating miRNAs and the large amount of proteins, extracting miRNA from serum or plasma is technically challenging. Because no suitable endogenous controls can be endorsed, data normalization is another critical issue. Consequently, it is imperative to develop standardization methods for quantifying the circulating miRNA. Despite these limitations, the application value of circulating miRNAs in various diseases is gradually being uncovered.
At present, the strategy for discovering aberrant expressed circulating miRNAs has taken two forms: one is screening and detecting differential circulating miRNAs from the plasma or serum of patients or pathological animals directly, and the other is distinguishing the differential expression of miRNAs from diseased tissues and then verifying the miRNA targets in the body fluids. qRT-PCR is the most widely used method for quantifying circulating miRNA levels. This method offers the advantages of simplicity and high sensitivity and reproducibility for detecting low levels of abundant circulating miRNAs. Microarray-based expression analysis is another method that is frequently used to screen the differential expression of miRNAs. Compared with qRT-PCR, microarray analysis requires a large amount of RNA and has lower reproducibility. Deep sequencing technology is an excellent method to discover unknown miRNAs and identify disease-specific miRNA fingerprints. However, this method is unsuitable for routine analysis because of the expensive equipment that is required. Regardless of which quantifying method is used, the miRNA extraction from plasma or serum is the greatest challenge because of the small amount of circulating miRNAs and the large amount of proteins. Because the efficiency of RNA extraction can be affected by the variability in protein and lipid contents of plasma or serum among individuals, Kroh et al. used C. elegans synthetic miRNAs (without any sequence similarity to known human, rat, and mouse miRNAs) during the extraction process to control for technical variations and normalize the data (Kroh et al. 2010).
Aberrant levels of circulating miRNAs have now been detected in a variety of disorders and have attracted more attention as candidate biomarkers, particularly in tumor and cardiovascular diseases. Many studies have shown that a certain amount of miRNAs have potential significance for the diagnosis, metastasis, progression, treatment, and prognosis in a variety of malignant tumors (Zhou et al. 2012). Circulating miRNAs have been implicated as a novel biomarker and have been identified as being involved in >12 cancer disorders (Brase et al. 2010; Wittmann and Jack 2010). Cardiovascular disease is the largest health problem worldwide, and research on the interaction between miRNAs and this disorder has become increasingly important. A series of miRNAs associated with heart disorders display interesting features in the tissues and circulation; for example, cardiac-restricted miR-208 showed both high sensitivity and specificity in of the plasma levels in acute myocardial infarction (Ji et al. 2009). Characteristic changes of miRNAs have been observed in many cardiovascular disorders, including coronary artery disease, acute myocardial infarction, heart failure, essential hypertension, and viral myocarditis (Gupta et al. 2010; Xu et al. 2012). Circulating miRNAs have been closely associated with multiple disorders, and their transcendent properties in biomarker studies indicated that they have great potential in the clinical setting. Therefore, circulating miRNAs are regarded not only as a signal for diagnosing and prognosing diseases, but they also may offer the potential for new insights into miRNA-based intervention therapy.
The Key Role of MiRNA in CNS Functions and Disorders
The CNS receives external incoming signals and responds with related brain activity processes through its sophisticated neural network connectivity and accurate synaptic-based recognition. MiRNAs are abundant and are expressed in a spatially and temporally controlled manner in the CNS, and numerous data indicate that miRNAs mediate neurobiological processes, including neurogenesis, neural differentiation, and synaptic plasticity, as well as neurologic- and psychiatric diseases.
A close association between miRNA and CNS development and differentiation has been revealed. For example, Dicer is pivotal in the biogenesis of miRNAs, and Dicer mutants show severe defects in neural tube morphogenesis. The symptoms of this malformation can be partially reversed by introducing miR-430 into zebrafish. Progressive cell death was observed when Dicer was postnatally inactivated in the cerebellum (Schaefer et al. 2007) or in the dopaminergic neurons in the forebrain (Kim et al. 2007). In addition, miR-9, miR-124, miR-125, and miR-137 have been demonstrated to play crucial roles in neurogenesis and differentiation (Rajasethupathy et al. 2009; Zhao et al. 2009; Edbauer et al. 2010; Smrt et al. 2010).
In the mature CNS, miRNAs are expected to affect the level of mRNAs in dendrites and to regulate local protein synthesis during synaptic plasticity. For example, miR-134 has been identified in mammalian hippocampus neurons and can restrict the dendritic spine size by antagonizing the translation of limk1 mRNA, which can be prevented by brain-derived neurotrophic factor (BDNF) under synaptic stimulation conditions. In addition, miR-138 has been proposed to negatively regulate the dendritic spine size in rat hippocampal neurons through the local suppression of acyl-protein thioesterase 1 (Siegel et al. 2009), and miR-132 induces activity-dependent dendritic growth by down-regulating p250GAP, which plays an important role in the synaptic structure and function (Klein et al. 2007).
In addition to maintaining the normal physiological functions of the CNS, specific miRNAs are closely related to the presence and development of CNS disorders. Table 1 shows the aberrant expression patterns of miRNAs in certain CNS diseases. For example, among these aberrantly expressed miRNAs, miR-107 is remarkably reduced in early onset in AD patients and has been suggested to accelerate the process of disease by increasing the expression of the β-side amyloid (Aβ) precursor protein cleaving enzyme 1 (BACE1) (Geekiyanage and Chan 2011). Multiple studies have indicated that the Fragile X syndrome pathogenesis may be caused by miRNA-mediated fragile X mental retardation 1 gene transcriptional inactivation (Barbato et al. 2008). In multiple sclerosis (MS) patients, the levels of miR-155 or miR-326 are obviously elevated in regions of brain injury. Knocking-out or silencing one of these two miRNAs in vivo can alleviate the symptoms in an MS animal model (Du et al. 2009; Junker et al. 2009). Because of their potential ability to target regulatory factors (including cyclin D2, PTPN1 and JUN), miR-206 and miR-198 have been correlated with schizophrenia (Hansen et al. 2007). Understanding the roles of miRNAs will provide new insights for diagnosing and treating CNS disorders. Multiple reviews have addressed the role of miRNAs in CNS development and differentiation and in CNS diseases, including AD, PD, and MS, etc. Herein, we discuss this topic briefly.
Circulating MiRNA as Potential Biomarkers for the Diagnosis and Evaluation of Therapy in CNS Disorders
Given the crucial role of miRNAs in physiological and pathological processes of the CNS, an increasing number of studies have investigated the potential of circulating miRNAs toward clinical diagnosis and therapy assessments in various CNS disorders, particularly AD, Huntington’s disease (HD), MS, schizophrenia, and BD.
Alzheimer’s disease is an age-related neurodegenerative disorder that is characterized by progressive memory loss and deteriorated higher cognitive functions (Heese and Akatsu 2006). The Aβ peptide and tau protein are thought to play principal roles in AD development, which may lead to neurodegeneration, as well as synaptic and neuronal loss (Alves et al. 2012). Geekiyanage and Chan (2011) reported that the levels of ceramides were increased concomitantly with an increase in serine palmitoyltransferase long chain-1 (SPTLC1) and SPTLC2 in the brain cortex in a subgroup of sporadic AD patients. Furthermore, the SPTLC1/2 mRNA levels in these AD patients did not differ significantly from those of the control samples, but the negative correlations between the expression of miR-137/-181c and SPTLC1, as well as between miR-9/-29a/b and SPTLC2 protein, were detected in autopsy brain samples. In addition, gain- and loss-of-function experiments confirmed the inhibitory effect of these special miRNAs on SPTLC1/2 and revealed their ability to repress Aβ protein expression. Consequently, the levels of miR-137, -181c, -9, -29a, and -29b in the serum of patients with probable AD and mild cognitive impairment (MCI)/probable early AD were all down-regulated compared with healthy controls (Geekiyanage et al. 2012). Other groups have also reported that miR-9 was decreased in both blood and brain tissue, including cortex and hippocampus, of AD patients (Chan and Kocerha 2012). The consistency of the aberrant expression of miRNAs in the brain and serum indicates that the circulating miRNAs are released from the brain and reflect the pathological processes. Members of the miR-9 and miR-29 family have also been shown to directly regulate BACE1. The down-regulation of miR-9 and miR-29 in AD patients can increase BACE1 expression, thereby inducing an accumulation of Aβ (Hebert et al. 2008). Moreover, members of the miR-29 family were proved to be down-regulated in the gray matter during early AD progression and associated with the density of diffuse amyloid plaques (Wang et al. 2011), supporting the argument that this miRNA may be one of the neuropathologic hallmarks of AD. Consequently, the aberrant expression patterns of circulating miRNAs may be closely associated with the corresponding miRNAs and the causative proteins that they regulate in the brain, which offers strong evidence to recommend them as candidate biomarkers. In addition to miRNAs, neuroimaging and some specific proteins in the CSF might offer other promising potential biomarkers in AD. Positron emission tomography (PET) imaging of β-amyloid protein deposition and magnetic resonance imaging (MRI) of the hippocampal volumes have reportedly detected the conversion of MCI to AD (Barber 2010). However, these imaging methods can only be used in the validation step and not a first screen step of the diagnostic process. CSF Aβ42 and tau protein, particularly the ratios of tau/Aβ42 and p-tau/Aβ42, are also useful to predict the risk of progression from MCI/very mild dementia to AD (Holtzman 2011; Fagan and Perrin 2012). Unfortunately, the changes in these candidate biomarkers in the plasma do not well match those in the CSF. Therefore, developing a plasma-based screening test remains a major challenge in increasing the efficiency and accuracy of AD diagnoses.
Huntington’s disease is an incurable neurodegenerative disease caused by abnormal CAG expansion in the gene encoding the protein Huntington (HTT) (The Huntington’s Disease Collaborative Research Group 1993). This dominantly inherited disorder is characterized by widespread mRNA dysregulation in striatal and cortical neurons. As a hereditary disease, HD patients can be identified using predictive genetic testing for the CAG expansion within the HTT gene before they develop symptoms. However, specific biomarkers are needed to track the disease progression and to assess the efficacy of therapeutic interventions. Structural imaging (striatal atrophy measurement and cortical volume loss) and functional and metabolic imaging (abnormal cortical activation and progressive dopamine D2 receptor loss) biomarkers have been studied. Brain-specific compounds in the CSF, such as neurofilament proteins, 24S-hydroxy cholesterol, and homovanillic acid, as well as stress-related hormones and immuno-related cytokines in the plasma, represent other appealing candidates (Weir et al. 2011). Recently, assays of circulating miRNAs have been investigated in HD as a novel approach to identify new biomarkers. The expression of several miRNAs in neurons has changed in pathological conditions of HD, and these changes have been correlated with related factors at the molecular level. In two transgenic mouse models of HD (YAC128 and R6/2), there were some commonly dysregulated miRNAs. Especially at the age when the mice present HD phenotypes, YAC128 mice showed reduced Dicer expression, and R6/2 mice showed reduced Drosha expression, suggesting altered miRNA biogenesis in these mice (Lee et al. 2011). Brain-specific miR-9/9*, miR-124a, and other miRNAs, including miR-29b and miR-132, are significantly misregulated in the cortices of HD patients. In particularly, miR-9/9* is down-regulated in HD and has been determined to regulate RE1-silencing transcription factor (REST), the abnormal expression of which is considered to be one of the major mechanisms of HD (Packer et al. 2008). In addition, other types of transcriptional dysregulation may also contribute to occurrence of this disease. For example, the function of p53 has been identified as being important in HD and is targeted by miR-34 (He et al. 2007). Importantly, circulating miR-34b has been evaluated in the plasma of HD patients before the onset of symptom; this circulating miRNA has been suggested to be a novel biomarker for the neurodegenerative disorder. Moreover, miR-34b can be induced using mutant Huntington in neuronally differentiated human cells and in human plasma (Gaughwin et al. 2011). Therefore, comprehensive analysis of miRNAs in the brain and circulatory system may offer new sight into the etiological pathways and preliminary diagnosis.
Multiple sclerosis is a chronic inflammatory autoimmune demyelinating disease that is found among young adults. As an autoimmune disease of the CNS, MS might be closely linked to the aberrant alterations of the miRNAs that regulate immune cell functions. Recently, many studies have reported that certain miRNAs that highly expressed in immune cells mediate MS. MiR-22 was detected to be increased 2.6-fold in the regulatory T cell of MS patients (De Santis et al. 2010). Moreover, miR-22 may influence the susceptibility to MS because of its inverted tendency to be associated with B cell translocation gene-1 in B cell during the morbid period (Mandel et al. 2004). Correspondingly, an up-regulation of circulating miR-22 was observed in the plasma of MS patients. In addition, miR-422a has been correlated with inflammatory demyelinating diseases by modulating cholesterol 7alpha-hydroxylase and has been found to be increased in both the plasma and peripheral blood cells of MS patients (Song et al. 2010). In addition to miR-22 and miR-422a, there were five additional miRNAs (miR-616, miR-648, miR-572, miR-1826, and miR-1979) have been reported to be altered in the plasma of MS patients. MiR-614 affected neuronal differentiation and immune function by modulating relative transcription factor, and miR-648 targets myelin-associated oligodendrocyte basic protein (MOBP), a relatively abundant CNS-specific myelin protein, to stabilize the myelin sheath in the CNS (Kaushansky et al. 2010; Banerjee 2011). Both of these circulating miRNAs, together with miR-572 and miR-1826, are increased in the plasma of patients, whereas miR-1979 is decreased (Siegel et al. 2012). Furthermore, the up-regulation of both miR-18b and miR-599 in peripheral blood mononuclear cells indicated which MS patients were in relapse status, whereas a tendency toward up-regulation of miR-96 indicated that the patients were in remission (Otaegui et al. 2009). Although no evidence has shown that these three miRNAs can reflect prognosis, these miRNAs are good candidates in future biomarker studies in MS, and at least miR-18b and miR-599 have the potential to be good markers to characterize the relapse status. In addition to the miRNAs in immune cells or plasma mentioned above, a series of miRNAs in white matter, the main damaged region in MS patients, have been observed to change, which might reflect the presence of CNS lesions (Junker et al. 2009). Although several differentially expressed circulating miRNAs have been detected, further studies are needed to explore their values as potential biomarkers in MS. As in AD and HD, several other promising biomarker candidates, including structural and functional neuroimaging (the upper cervical cord area atrophy, whole brain atrophy, black holes, and retinal nerve fiber layer thickness), neurophysiology (visual or motor evoked potentials and multimodal evoked potential scores), inflammatory and neurodegenerative-based CSF markers (chemokine CXCL13, multiple interleukins, interferon gamma, tumor necrosis factor alpha, MOBP, neurofilament, S100B acidic calcium-binding protein, glial fibrillary acidic protein, etc.), and serum autoantibodies against myelin (e.g., anti-MOBP, anti-myelin oligodendrocyte glycoprotein and anti-N-glycosylated peptide) have also been developed and evaluated (Ziemann et al. 2011). Although no biomarker is currently established, these candidates, including miRNAs, provide a perspective that may change the diagnosis of this formidable neurodegenerative disorder from the clinical end-point to sensitive and reliable biomarkers.
Schizophrenia is a severe psychiatric disorder and its typical clinical symptoms include hallucinations, delusions, anhedonia, and social withdrawal (Oertel-Knochel et al. 2011). Environmental influences and related genetic vulnerability are widely considered to be the critical factors in the pathogenesis of this mental illness (van Os et al. 2008). Several altered expression patterns of miRNAs have been detected in schizophrenia patients compared with healthy controls; these expressions may arise from the pathophysiology of the disease. After analyzing the data from the literatures and gene database using bioinformatic methods, Shi et al. quantified a number of circulating miRNAs in the serum of patients suffering from schizophrenia and that of healthy individuals. The up-regulation of circulating miR-181b, miR-219-2-3p, miR-1308, and let-7g and the down-regulation of miR-195 were observed (Shi et al. 2012). The authors concluded that these changes in the serum level of miR-181b, miR-219-2-3p, miR-1308, and let-7g and miR-195 can reflect schizophrenia status and may be used as candidate biomarkers for diagnosing schizophrenia. MiR-181b and miR-195 are relatively highly expressed in the brain. Similar to serum miR-195, brain miR-195 is down-regulated in the prefrontal cortex of schizophrenia patients according to study that employed a postmortem case–control design (Perkins et al. 2007; Mellios et al. 2009). It also has been reported that miR-195 could regulate BDNF and alter the expressions of neuropeptide Y and somatostatin in the gamma-aminobutyric acid (GABA)ergic neurons of the prefrontal cortex in schizophrenia (Mellios et al. 2009). The up-regulation of miRNA-181b in the temporal cortex of schizophrenia patients has also been observed, and this up-regulation inhibits the expression of α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) glutamate receptor subunit 2 (GRIA2) (Beveridge et al. 2008). MiR-219 shows specific expression in the human brain and in the cerebral cortex of mouse (Coyle 2009; Beveridge et al. 2010). It may regulate N-methyl-d-aspartate (NMDA) receptor-related function by controlling the translation of a calcium/calmodulin-dependent protein kinase II subunit, whereas the expression of miR-219 in the mouse prefrontal cortex is reduced by applying an NMDA receptor antagonist. In addition, miR-219 has been observed to be relevant to behavioral deficits in schizophrenia and miR-219-mediated NMDA receptor dysfunction may affect the occurrence of schizophrenia (Kocerha et al. 2009). In addition to circulating miRNAs, molecular genetics, CSF-based biochemical candidates (VGF [nerve growth factor inducible]-derived peptide], transthyretin, fatty acid binding protein 7, apolipoprotein A1, etc.), structural neuroimaging (cortical thickness and ventricle size), and functional neuroimaging (prefrontal hyperactivation and imaging based on altered striatal and cortical dopaminergic neurotransmissions) markers offer other avenues for schizophrenia biomarker research (Oertel-Knochel et al. 2011). Because schizophrenia is one of the most complex mental disorders, the combination of heterogeneous, multifaceted, and multifactorial markers is a promising strategy to capture the subtle and intricate pathological characteristics of the disease.
Bipolar disorder is characterized by repeated episodes of mania and depression. Growing evidence shows that BD might arise from abnormalities of the synaptic plasticity; miRNA-134 localizes in the dendrites of rat hippocampal neurons and controls the size of dendritic spines and modulates the excitatory synaptic transmission by inhibiting lim-domain containing protein kinase 1 (LIMK1) translation. BDNF was able to relieve miR-134-induced inhibition of LIMK1 translation. This miR-134-induced signal regulation plays a critical role in the synaptic development, maturation, and plasticity (Schratt et al. 2006). Rong et al. observed the plasma miRNA levels obtained from BD patients who were presenting manic symptoms (Rong et al. 2011). They found that circulating miR-134 in drug-free patients with bipolar mania was significantly lower than that in normal groups. After extended treatment, the plasma miR-134 level increases gradually. Moreover, a marked negative relationship between severity of manic symptoms (BRMS scores) and the plasma miR-134 level is observed in BD patients before and after various durations of drug treatments. The correlation between miRNA level and the typical symptoms of this disease implies that miR-134 has potential value in diagnosing BD patients and evaluating their therapy. Besides miRNAs, neuroimaging markers (decreased activation and diminution of gray matter and increased activation in ventral limbic brain regions, including the parahippocampal gyrus extending to the amygdala, the thalamus, and the caudate nucleus) and some peripheral biochemical compounds (such as BDNF, oxidative stress-related compounds, cytokines, etc.) are other appealing candidates (Berk et al. 2009; Houenou et al. 2011). In particular, the BDNF level has been observed to be decreased in both mania and depression when compared to controls, and an increase in BDNF levels following the treatment for acute mania (Fernandes et al. 2011). Because of the interaction between BDNF and miR-134, peripheral BDNF and miR-134 could be used as a biomarker of mood states and disease progression for BD.
Although considerable miRNAs with aberrant expression patterns in the brain have been reported in many CNS diseases, and the mechanisms have been explored gradually, the data regarding the circulating miRNAs in these diseases remain limited. At present, it is still difficult to establish the correlation between certain circulating miRNAs and diseases diagnoses. Therefore, further studies are required. In addition, although these three types of novel potential biomarkers: neuroimaging, miRNA- and protein-based biochemical markers each has respective advantages and limitations, we think that panels of various biomarkers should be combined and used in succession to improve the efficacy and accuracy of the early detection of CNS disorders. For example, blood-based biochemical markers, such as particular miRNAs and proteins, should be used to screen possible patients and then neuroimaging or CNS-based biomarkers can be used to validate the disease.
The discovery of circulating miRNAs opens a new field for diagnosis and therapy assessment in various diseases. In recent years, increasing numbers of aberrant expression patterns of circulating miRNAs have been detected and appear to have potential as noninvasive biomarkers for neuropsychiatric disorders. However, the research on circulating miRNA is in its infancy, and numerous technical problems must be solved, such as establishing standardization procedures for sample preparation, developing unified methodologies for detecting and quantifying the circulating miRNAs and optimizing the results interpretation. With the development of new techniques and further research investigations, circulating miRNAs undoubtedly exhibit a promising perspective in diagnosing CNS disorders and assessing related therapies.
Abbreviations
- Aβ:
-
β-Side amyloid
- AD:
-
Alzheimer’s disease
- AMPA:
-
α-Amino-3-hydroxyl-5-methyl-4-isoxazole-propionate
- BACE1:
-
β-Side amyloid precursor protein cleaving enzyme 1
- BD:
-
Bipolar disorder
- BDNF:
-
Brain-derived neurotrophic factor
- CNS:
-
Central nervous system
- GAP:
-
GTPase-activating protein
- GRIA:
-
AMPA glutamate receptor subunit
- HD:
-
Huntington’s disease
- HTT:
-
Protein Huntington
- JUN:
-
Jun proto-oncogene
- LIMK1:
-
Lim-domain containing protein kinase 1
- miRNA:
-
MicroRNA
- MS:
-
Multiple sclerosis
- NMDA:
-
N-methyl-d-aspartate
- PTPN1:
-
Protein tyrosine phosphates, nonreceptor type 1
- qRT-PCR:
-
Quantitative reverse transcription-polymerase chain reaction
- REST:
-
RE1-silencing transcription factor
- SPTLC:
-
Serine palmitoyltransferase long chain
References
Alves L, Correia AS, Miguel R, Alegria P, Bugalho P (2012) Alzheimer’s disease: a clinical practice-oriented review. Front Neurol 3:63. doi:10.3389/fneur.2012.00063
Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, Mitchell PS, Bennett CF, Pogosova-Agadjanyan EL, Stirewalt DL, Tait JF, Tewari M (2011) Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci USA 108:5003–5008
Banerjee D (2011) Recent advances in the pathobiology of Hodgkin’s lymphoma: potential impact on diagnostic, predictive, and therapeutic strategies. Adv Hematol 2011:439–456
Barbato C, Giorgi C, Catalanotto C, Cogoni C (2008) Thinking about RNA? MicroRNAs in the brain. Mamm Genome 19:541–551
Barber RC (2010) Biomarkers for early detection of Alzheimer disease. J Am Osteopath Assoc 110:S10–S15
Berk M, Malhi GS, Hallam K, Gama CS, Dodd S, Andreazza AC, Frey BN, Kapczinski F (2009) Early intervention in bipolar disorders: clinical, biochemical and neuroimaging imperatives. J Affect Disord 114:1–13
Beveridge NJ, Tooney PA, Carroll AP, Gardiner E, Bowden N, Scott RJ, Tran N, Dedova I, Cairns MJ (2008) Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet 17:1156–1168
Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ (2010) Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry 15:1176–1189
Brase JC, Wuttig D, Kuner R, Sultmann H (2010) Serum microRNAs as non-invasive biomarkers for cancer. Mol Cancer 9:306. doi:10.1186/1476-4598-9-306
Chan AW, Kocerha J (2012) The path to microRNA therapeutics in psychiatric and neurodegenerative disorders. Front Genet 3:82. doi:10.3389/fgene.2012.00082
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K, Guo J, Zhang Y, Chen J, Guo X, Li Q, Li X, Wang W, Wang J, Jiang X, Xiang Y, Xu C, Zheng P, Zhang J, Li R, Zhang H, Shang X, Gong T, Ning G, Zen K, Zhang CY (2008) Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res 18:997–1006
Cogswell JP, Ward J, Taylor IA, Waters M, Shi Y, Cannon B, Kelnar K, Kemppainen J, Brown D, Chen C, Prinjha RK, Richardson JC, Saunders AM, Roses AD, Richards CA (2008) Identification of miRNA changes in Alzheimer’s disease brain and CSF yields putative biomarkers and insights into disease pathways. J Alzheimers Dis 14:27–41
Corsten MF, Dennert R, Jochems S, Kuznetsova T, Devaux Y, Hofstra L, Wagner DR, Staessen JA, Heymans S, Schroen B (2010) Circulating microRNA-208b and microRNA-499 reflect myocardial damage in cardiovascular disease. Circ Cardiovasc Genet 3:499–506
Coyle JT (2009) MicroRNAs suggest a new mechanism for altered brain gene expression in schizophrenia. Proc Natl Acad Sci USA 106:2975–2976
De Santis G, Ferracin M, Biondani A, Caniatti L, Rosaria Tola M, Castellazzi M, Zagatti B, Battistini L, Borsellino G, Fainardi E, Gavioli R, Negrini M, Furlan R, Granieri E (2010) Altered miRNA expression in T regulatory cells in course of multiple sclerosis. J Neuroimmunol 226:165–171
Du C, Liu C, Kang J, Zhao G, Ye Z, Huang S, Li Z, Wu Z, Pei G (2009) MicroRNA miR-326 regulates TH-17 differentiation and is associated with the pathogenesis of multiple sclerosis. Nat Immunol 10:1252–1259
Edbauer D, Neilson JR, Foster KA, Wang CF, Seeburg DP, Batterton MN, Tada T, Dolan BM, Sharp PA, Sheng M (2010) Regulation of synaptic structure and function by FMRP-associated microRNAs miR-125b and miR-132. Neuron 65:373–384
Fagan AM, Perrin RJ (2012) Upcoming candidate cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med 6:455–476
Fernandes BS, Gama CS, Cereser KM, Yatham LN, Fries GR, Colpo G, de Lucena D, Kunz M, Gomes FA, Kapczinski F (2011) Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res 45:995–1004
Fichtlscherer S, De Rosa S, Fox H, Schwietz T, Fischer A, Liebetrau C, Weber M, Hamm CW, Roxe T, Muller-Ardogan M, Bonauer A, Zeiher AM, Dimmeler S (2010) Circulating microRNAs in patients with coronary artery disease. Circ Res 107:677–684
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Gaughwin PM, Ciesla M, Lahiri N, Tabrizi SJ, Brundin P, Bjorkqvist M (2011) Hsa-miR-34b is a plasma-stable microRNA that is elevated in pre-manifest Huntington’s disease. Hum Mol Genet 20:2225–2237
Geekiyanage H, Chan C (2011) MicroRNA-137/181c regulates serine palmitoyltransferase and in turn amyloid beta, novel targets in sporadic Alzheimer’s disease. J Neurosci 31:14820–14830
Geekiyanage H, Jicha GA, Nelson PT, Chan C (2012) Blood serum miRNA: non-invasive biomarkers for Alzheimer’s disease. Exp Neurol 235:491–496
Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O (2009) Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol 11:1143–1149
Gupta SK, Bang C, Thum T (2010) Circulating microRNAs as biomarkers and potential paracrine mediators of cardiovascular disease. Circ Cardiovasc Genet 3:484–488
Hanke M, Hoefig K, Merz H, Feller AC, Kausch I, Jocham D, Warnecke JM, Sczakiel G (2010) A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urol Oncol 28:655–661
Hansen T, Olsen L, Lindow M, Jakobsen KD, Ullum H, Jonsson E, Andreassen OA, Djurovic S, Melle I, Agartz I, Hall H, Timm S, Wang AG, Werge T (2007) Brain expressed microRNAs implicated in schizophrenia etiology. PLoS ONE 2:e873
He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, Jackson AL, Linsley PS, Chen C, Lowe SW, Cleary MA, Hannon GJ (2007) A microRNA component of the p53 tumour suppressor network. Nature 447:1130–1134
Hebert SS, De Strooper B (2009) Alterations of the microRNA network cause neurodegenerative disease. Trends Neurosci 32:199–206
Hebert SS, Horre K, Nicolai L, Papadopoulou AS, Mandemakers W, Silahtaroglu AN, Kauppinen S, Delacourte A, De Strooper B (2008) Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer’s disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci USA 105:6415–6420
Heese K, Akatsu H (2006) Alzheimer’s disease: an interactive perspective. Curr Alzheimer Res 3:109–121
Holtzman DM (2011) CSF biomarkers for Alzheimer’s disease: current utility and potential future use. Neurobiol Aging 32:S4–S9
Houenou J, Frommberger J, Carde S, Glasbrenner M, Diener C, Leboyer M, Wessa M (2011) Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord 132:344–355
Ji X, Takahashi R, Hiura Y, Hirokawa G, Fukushima Y, Iwai N (2009) Plasma miR-208 as a biomarker of myocardial injury. Clin Chem 55:1944–1949
Junker A, Krumbholz M, Eisele S, Mohan H, Augstein F, Bittner R, Lassmann H, Wekerle H, Hohlfeld R, Meinl E (2009) MicroRNA profiling of multiple sclerosis lesions identifies modulators of the regulatory protein CD47. Brain 132:3342–3352
Kaushansky N, Eisenstein M, Zilkha-Falb R, Ben-Nun A (2010) The myelin-associated oligodendrocytic basic protein (MOBP) as a relevant primary target autoantigen in multiple sclerosis. Autoimmun Rev 9:233–236
Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A (2007) A microRNA feedback circuit in midbrain dopamine neurons. Science 317:1220–1224
Klein ME, Lioy DT, Ma L, Impey S, Mandel G, Goodman RH (2007) Homeostatic regulation of MeCP2 expression by a CREB-induced microRNA. Nat Neurosci 10:1513–1514
Kocerha J, Faghihi MA, Lopez-Toledano MA, Huang J, Ramsey AJ, Caron MG, Sales N, Willoughby D, Elmen J, Hansen HF, Orum H, Kauppinen S, Kenny PJ, Wahlestedt C (2009) MicroRNA-219 modulates NMDA receptor-mediated neurobehavioral dysfunction. Proc Natl Acad Sci USA 106:3507–3512
Kroh EM, Parkin RK, Mitchell PS, Tewari M (2010) Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR). Methods 50:298–301
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Lee ST, Chu K, Im WS, Yoon HJ, Im JY, Park JE, Park KH, Jung KH, Lee SK, Kim M, Roh JK (2011) Altered microRNA regulation in Huntington’s disease models. Exp Neurol 227:172–179
Li M, Li J, Ding X, He M, Cheng SY (2010) MicroRNA and cancer. AAPS J 12:309–317
Mandel M, Gurevich M, Pauzner R, Kaminski N, Achiron A (2004) Autoimmunity gene expression portrait: specific signature that intersects or differentiates between multiple sclerosis and systemic lupus erythematosus. Clin Exp Immunol 138:164–170
Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S (2009) Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry 65:1006–1014
Mitchell PS, Parkin RK, Kroh EM, Fritz BR, Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O’Briant KC, Allen A, Lin DW, Urban N, Drescher CW, Knudsen BS, Stirewalt DL, Gentleman R, Vessella RL, Nelson PS, Martin DB, Tewari M (2008) Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci USA 105:10513–10518
Oertel-Knochel V, Bittner RA, Knochel C, Prvulovic D, Hampel H (2011) Discovery and development of integrative biological markers for schizophrenia. Prog Neurobiol 95:686–702
Otaegui D, Baranzini SE, Armananzas R, Calvo B, Munoz-Culla M, Khankhanian P, Inza I, Lozano JA, Castillo-Trivino T, Asensio A, Olaskoaga J, Lopez de Munain A (2009) Differential microRNA expression in PBMC from multiple sclerosis patients. PLoS ONE 4:e6309
Packer AN, Xing Y, Harper SQ, Jones L, Davidson BL (2008) The bifunctional microRNA miR-9/miR-9* regulates REST and CoREST and is downregulated in Huntington’s disease. J Neurosci 28:14341–14346
Park NJ, Zhou H, Elashoff D, Henson BS, Kastratovic DA, Abemayor E, Wong DT (2009) Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clin Cancer Res 15:5473–5477
Perkins DO, Jeffries CD, Jarskog LF, Thomson JM, Woods K, Newman MA, Parker JS, Jin J, Hammond SM (2007) MicroRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol 8:R27
Qureshi IA, Mattick JS, Mehler MF (2010) Long non-coding RNAs in nervous system function and disease. Brain Res 1338:20–35
Rajasethupathy P, Fiumara F, Sheridan R, Betel D, Puthanveettil SV, Russo JJ, Sander C, Tuschl T, Kandel E (2009) Characterization of small RNAs in Aplysia reveals a role for miR-124 in constraining synaptic plasticity through CREB. Neuron 63:803–817
Rong H, Liu TB, Yang KJ, Yang HC, Wu DH, Liao CP, Hong F, Yang HZ, Wan F, Ye XY, Xu D, Zhang X, Chao CA, Shen QJ (2011) MicroRNA-134 plasma levels before and after treatment for bipolar mania. J Psychiatr Res 45:92–95
Schaefer A, O’Carroll D, Tan CL, Hillman D, Sugimori M, Llinas R, Greengard P (2007) Cerebellar neurodegeneration in the absence of microRNAs. J Exp Med 204:1553–1558
Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain-specific microRNA regulates dendritic spine development. Nature 439:283–289
Shi W, Du J, Qi Y, Liang G, Wang T, Li S, Xie S, Zeshan B, Xiao Z (2012) Aberrant expression of serum miRNAs in schizophrenia. J Psychiatr Res 46:198–204
Siegel G, Obernosterer G, Fiore R, Oehmen M, Bicker S, Christensen M, Khudayberdiev S, Leuschner PF, Busch CJ, Kane C, Hubel K, Dekker F, Hedberg C, Rengarajan B, Drepper C, Waldmann H, Kauppinen S, Greenberg ME, Draguhn A, Rehmsmeier M, Martinez J, Schratt GM (2009) A functional screen implicates microRNA-138-dependent regulation of the depalmitoylation enzyme APT1 in dendritic spine morphogenesis. Nat Cell Biol 11:705–716
Siegel SR, Mackenzie J, Chaplin G, Jablonski NG, Griffiths L (2012) Circulating microRNAs involved in multiple sclerosis. Mol Biol Rep 39:6219–6225
Siomi H, Siomi MC (2010) Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell 38:323–332
Smrt RD, Szulwach KE, Pfeiffer RL, Li X, Guo W, Pathania M, Teng ZQ, Luo Y, Peng J, Bordey A, Jin P, Zhao X (2010) MicroRNA miR-137 regulates neuronal maturation by targeting ubiquitin ligase mind bomb-1. Stem Cells 28:1060–1070
Song KH, Li T, Owsley E, Chiang JY (2010) A putative role of microRNA in regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes. J Lipid Res 51:2223–2233
The Huntington’s Disease Collaborative Research Group (1993) A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. Cell 72:971–983
Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol 9:654–659
van Os J, Rutten BP, Poulton R (2008) Gene-environment interactions in schizophrenia: review of epidemiological findings and future directions. Schizophr Bull 34:1066–1082
Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT (2011) MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol 13:423–433
Wang K, Zhang S, Weber J, Baxter D, Galas DJ (2010) Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res 38:7248–7259
Wang WX, Huang Q, Hu Y, Stromberg AJ, Nelson PT (2011) Patterns of microRNA expression in normal and early Alzheimer’s disease human temporal cortex: white matter versus gray matter. Acta Neuropathol 121:193–205
Weir DW, Sturrock A, Leavitt BR (2011) Development of biomarkers for Huntington’s disease. Lancet Neurol 10:573–590
Wittmann J, Jack HM (2010) Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta 1806:200–207
Xu J, Zhao J, Evan G, Xiao C, Cheng Y, Xiao J (2012) Circulating microRNAs: novel biomarkers for cardiovascular diseases. J Mol Med (Berl) 90:865–875
Zhao C, Sun G, Li S, Shi Y (2009) A feedback regulatory loop involving microRNA-9 and nuclear receptor TLX in neural stem cell fate determination. Nat Struct Mol Biol 16:365–371
Zhou L, Zhao YP, Liu WJ, Dong J, Chen WY, Zhang TP, Chen G, Shu H (2012) Circulating microRNAs in cancer: diagnostic and prognostic significance. Expert Rev Anticancer Ther 12:283–288
Ziemann U, Wah M, Hattingen E, Tumani H (2011) Development of biomarkers for multiple sclerosis as a neurodegenerative disorder. Prog Neurobiol 95:670–685
Zubakov D, Boersma AW, Choi Y, van Kuijk PF, Wiemer EA, Kayser M (2010) MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Legal Med 124:217–226
Acknowledgments
This study was supported by Project of National Science and Technology Support Program in China (2012BAI01B07).
Author information
Authors and Affiliations
Corresponding author
Additional information
Xue-Feng Jin and Ning Wu contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, XF., Wu, N., Wang, L. et al. Circulating MicroRNAs: A Novel Class of Potential Biomarkers for Diagnosing and Prognosing Central Nervous System Diseases. Cell Mol Neurobiol 33, 601–613 (2013). https://doi.org/10.1007/s10571-013-9940-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10571-013-9940-9