Abstract
The main objectives of this study are to evaluate 28 selected pro-angiogenic and anti-angiogenic microRNA (miRNA) expressions in plasma of acute ischemic stroke (AIS) patients and controls and to assess the correlations of these miRNAs with risk and severity of AIS. In the exploring stage, 10 AIS patients and 10 controls with vascular risk factors were enrolled. And in the validating stage, 106 AIS patients and 110 controls with the same eligibility were recruited. Blood samples were collected from participants within 24 h post the onset of symptoms, and plasma levels of miRNAs were evaluated by the qPCR method. In the exploring stage, 11 differentially expressed miRNAs (DEM) were identified and included into the validating stage. In the validating stage, the expression of miR-126, miR-130a, and miR-378 in plasma declined in the AIS patients; however, miR-222, miR-218, and miR-185 plasma levels were elevated. Univariate and multivariate logistic regression analysis disclosed that miR-126, miR-130a, miR-222, miR-218, and miR-185 were independent predicting factors for AIS. When these five DEMs were combined together, they presented a good diagnostic value with an area under curve (AUC) value of 0.767 (95% CI 0.705–0.829), sensitivity of 87.7%, and specificity of 54.5% at best cutoff point. Additionally, miR-126, miR-378, miR-101, miR-222, miR-218, and miR-206 were associated with National Institutes of Health Stroke Scale (NIHSS) score. Circulating miR-126, miR-130a, miR-222, miR-218, and miR-185 could be served as promising and independent biomarkers for risk of AIS, and miR-126, miR-378, miR-222, miR-101, miR-218, and miR-206 could be used for disease severity management of AIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the increasing incidence of stroke-related deaths worldwide, intense attention has been paid to acute ischemic stroke (AIS), a specific type of stroke triggered by cerebral ischemia [1]. Brain tissue deficits, focal neurological deficits, and disability occurred in patients post AIS, causing burdens to patients as well as a large amount of money to society every year [2]. Non-contrast computerized tomography (CT), diffusion-weighted magnetic resonance image (MRI), and gradient echo T2-weighted susceptibility MRI are used for stroke diagnosis and are of good accuracy; however, the diagnosis becomes critically difficult for strokes that are in initial hours or when the atypical symptoms occurred [3].
MicroRNAs (miRNAs) are a class of 20–25-nt single-stranded non-coding RNAs that regulate cellular functions through mediating messenger RNA (mRNA) degradation and repressing translation of mRNAs [4]. MiRNAs could regulate cell proliferation and apoptosis, and accumulating published data have showed a correlation of miRNA with stroke pathogenesis [5, 6]. Moreover, multiple miRNAs were found to have the potential of being a biomarker for diagnosis and prognosis of AIS [7]. Through RNA profiling, aberrantly expressed miRNAs have been identified in ischemic stroke patients compared to healthy individuals [8]. For instance, miR-290 is upregulated while miR-30a and miR-126 are downregulated following ischemic strokes [9,10,11]. Furthermore, in the pathological processes of AIS, miRNAs participate in multiple mechanisms such as excitotoxicity, ischemic oxidative stress, and post ischemic inflammation [12,13,14]. Several miRNAs were reported to modulate vascular growth, which is vital in the etiology of stroke, and may serve as pro-angiogenesis or anti-angiogenesis miRNAs [15, 16]. For instance, miR-454 inhibits angiogenesis in pancreatic ductal adenocarcinoma through targeting LRP6 [15]. And miR-182 induced by hypoxia targets RASA1 and thus promotes angiogenesis in hepatocellular carcinoma [16]. However, the roles of these angiogenesis-related miRNAs in AIS as well as their associations with severity of AIS were still elusive.
Therefore, we selected 28 pro-angiogenesis and anti-angiogenesis miRNAs to evaluate their expressions in plasma of AIS patients as well as controls and to assess the correlations of these miRNAs with risk and severity of AIS.
Material and methods
Participants
A hundred and six patients with AIS admitted to the Department of Emergency at Cangzhou Central Hospital, from May 2013 to Oct 2016, were consecutively recruited in this study. The inclusion criteria were as follows: within 24 h post the onset of symptom, diagnosed with AIS according to patient history, laboratory and neurological examination, CT scan, magnetic resonance imaging (MRI), and/or magnetic resonance angiography (MRA). Patients with infection, renal or hepatic failure, hematological malignancies, solid tumors, immunosuppressive therapy, or treatment with thrombolytic therapy were excluded from the study. A hundred and ten age- and gender-matched controls with vascular risk factors from Cangzhou Central Hospital were enrolled in the same duration as well. Controls with a history of stroke, myocardial infarction or peripheral vascular disease, severe infection, renal or hepatic dysfunction, and experiencing non-specific dizziness and non-organic headaches were excluded.
The Ethics Committee of Cangzhou Central Hospital approved this study, and all participants or direct family members signed the informed consents.
Study design
This study was divided into two parts: exploring stage and validating stage (Fig. 1). In the exploring stage, 28 candidate miRNAs in plasma were detected in 10 AIS patients and 10 controls; the differentially expressed miRNAs (DEMs) were subsequently included in the validating stage with 106 patients and 110 controls. And the correlation of DEMs in the validating stage with disease severity by National Institutes of Health Stroke Scale (NIHSS) score was further analyzed.
Candidate miRNA selection
Twenty-eight pro-angiogenic and anti-angiogenic miRNAs were selected based on the analysis of previous studies [17,18,19,20,21,22,23,24,25], among which 14 were reported to be pro-angiogenic while other 14 were revealed to be anti-angiogenic miRNAs.
Samples
Within 24 h post onset of symptoms, 5-ml blood samples were collected from the participants and stored at ethylene diamine tetraacetic acid (EDTA)-2k tubes immediately. Then, the blood samples were centrifuged at 1500g for 20 min at room temperature; after that, a centrifugation of 12,000 rpm for 10 min at 4 °C was performed to get the plasma of the blood samples. After centrifugation, the plasma was stored at −8 °C for RNA extraction.
MiRNA detection
Total RNA was extracted from the plasma using TRIzol solution (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol; the concentration and purity of RNA were evaluated by an enzyme-linked immunosorbent assay (ELISA). Subsequently, RNA was reversely transcribed by TransScript First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China). Real-time qPCR was performed using the SYBR Premix Ex Taq kit (Takara, Dalian, China) to assess the relative quantity of miRNAs, and U6 was used as an internal reference for normalization of miRNA quantity. Then, the results were calculated by the 2−ΔΔt formula.
Statistics
Statistical analysis was performed by SPSS 21.0 (SPSS, Chicago, Illinois, USA). Data were presented as mean ± standard division, count (%), and median (25th–75th quarter). The comparison between the two groups was determined by the Student test, chi-squared test, and Mann-Whitney test. Univariate and multivariate logistic analysis was conducted to assess the risk factors for AIS. Receiver operating characteristic (ROC) curve analysis was performed to evaluate the ability of DEMs to distinguish AIS patients from controls. In addition, the Spearman two-way test was used to assess the associations of DEMs with NIHSS scores. p < 0.05 was considered significant.
Results
Characteristics of AIS patients and controls in exploring stage
In the exploring stage, 10 AIS patients and 10 controls were included to explore the expressions of 28 pro-angiogenic and anti-angiogenic miRNAs, and no difference of baseline characteristics was observed between groups (Table 1). The mean age was 59.8 ± 8.9 years in the AIS patients and 61.1 ± 9.4 in the controls. Additionally, the numbers of females in the AIS patients and controls were both 5 (50%). The mean value of BMI was 21.5 ± 2.5 kg/m2 in the AIS patients and 21.2 ± 2.1 kg/m2 in the controls. Hypertension was found in 8 (80%) AIS patients and 7 (70%) controls. And the numbers of cases with diabetes mellitus in the AIS patients and controls were both 2 (20%). In addition, hyperlipidemia was found in 6 (60%) AIS patients and 7 (70%) controls. The numbers of patients with hyperuricemia in the AIS patients and controls were 2 (20%) and 3 (30%), respectively. In addition, 3 (30%) AIS patients and 2 (20%) controls were diagnosed with atrial fibrillation. Smoking history was found in 3 (30%) AIS patients and 3 (30%) controls.
DEMs in exploring stage
As shown in Table 2, the plasma levels of miR-126 (p = 0.034), miR-19a (p = 0.034), miR-130a (p = 0.023), miR-378 (p = 0.034), miR-296 (p = 0.023), and miR-101 (p = 0.041) decreased in the AIS patients compared with the controls, while the expressions of miR-221 (p = 0.008), miR-222 (p = 0.034), miR-218 (p = 0.008), miR-206 (p = 0.016), and miR-185 (p = 0.001) in plasma were found elevated in the AIS patients compared to the controls (Table 2). These 11 DEMs were then included in the validating stage at a large sample size.
Characteristics of AIS patients and controls in validating stage
In the validating stage, 11 DEMs were further evaluated in 106 AIS patients and 110 controls. There was no difference of baseline characteristics in the AIS group and control group (Table 3). The mean age was 60.8 ± 9.7 years in the AIS group and 58.6 ± 15.2 years in the control group. And there were 58 (55%) females in the AIS group and 51 (46%) females in the control group. The BMI values of the AIS patients and controls were 20.7 ± 2.3 and 21.1 ± 2.7, respectively. Hypertension was found in 81 (76%) AIS patients and 85 (77%) controls. Thirty-four (32%) AIS patients and 26 (24%) controls have diabetes mellitus. And patients with hyperlipidemia were 52 (49%) in the AIS group and 61 (55%) in the control group. Thirty-three (31%) AIS patients and 28 (25%) controls were diagnosed with hyperuricemia, while 29 (27%) AIS patients and 21 (19%) controls had atrial fibrillation. The numbers of patients that had a smoking history were 26 (25%) and 38 (35%) in the AIS patients and controls.
Determination of DEMs in validating stage
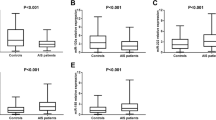
As displayed in Fig. 2, the expression of miR-126 (p < 0.001), miR-130a (p < 0.001), and miR-378 (p = 0.033) in plasma was found to be decreased in the AIS patients compared to the controls. In addition, miR-222 (p = 0.032), miR-218 (p = 0.002), and miR-185 (p = 0.011) plasma expressions were found to be elevated in the AIS patients compared to the controls. However, no difference of the expression of miR-19a (p = 0.497), miR-296 (p = 0.857), miR-101 (p = 0.702), and miR-206 (p = 0.145) in plasma was found between the AIS patients and controls.
Diagnostic value of candidate miRNAs for AIS
To assess the plasma level of 11 DEMs for risk of AIS, univariate and multivariate logistic regression analysis was performed (Table 4). The data of univariate logistic regression analysis illuminated that miR-126 (p < 0.001), miR-130a (p < 0.001), miR-378 (p = 0.017), miR-222 (p = 0.017), miR-218 (p = 0.001), and miR-185 (p = 0.009) were predicting factors for risk of AIS. All factors with p value <0.1 were analyzed by multivariate logistic regression analysis afterwards, and the analysis showed that miR-126 (p = 0.000) and miR-130a (p = 0.001) were independent protective factors for AIS while miR-222 (p = 0.036), miR-218 (p = 0.007), and miR-185 (p = 0.043) were independent risk factors for AIS.
As shown in Fig. 3, ROC curve analysis was performed to assess the diagnostic value of the five DEMs that could predict the risk of AIS independently in the validating stage. And the area under curve (AUC) values of miR-126, miR-130a, miR-222, miR-218, and miR-185 plasma levels for predicting AIS were 0.654 (95% CI 0.580–0.728), 0.642 (95% CI 0.568–0.175), 0.584 (95% CI 0.508–0.661), 0.624 (95% CI 0.549–0.699), and 0.601 (95% CI 0.525–0.676), respectively. When combining the five DEMs, AUC increased to 0.767 (95% CI 0.705–0.829) with sensitivity of 87.7% and specificity of 54.5% at best cutoff point, which exhibited a good diagnostic value for AIS.
Correlation of DEMs with NIHSS score
The associations of 11 DEMs with NIHSS score were determined by the Spearman two-way test, and results presented that miR-126 (p < 0.001), miR-378 (p < 0.001), and miR-101 (p = 0.418) were negatively correlated with NIHSS score, while miR-222 (p < 0.001), miR-218 (p = 0.313), and miR-206 (p = 0.482) were positively associated with NIHSS score (Fig. 4).
Correlations of 11 DEM plasma levels with NIHSS scores. a MiR-126. b MiR-19a. c MiR-130a. d MiR-378. e MiR-296. f MiR-101. g MiR-221. h MiR-222. i MiR-218. j MiR-206. k MiR-185. The Spearman two-way test was used to assess the correlations of Gensini scores with six differentially expressed miRNA levels. p < 0.05 was considered significant
Discussion
AIS counts for approximately 80% of all strokes, among all patients with AIS, those with diabetes, hypertension, smoking history, drinking history, and hypercholesterolemia, and patients in old age, are at high risk [26]. Clinical outcomes of AIS, which consist of long-term disabilities and neurological deficits, are usually not satisfactory [3]. Interventions for improving clinical outcomes include intravenous thrombolysis, recombinant tissue plasminogen activator (IV rtPA) therapy, and mechanical thrombectomy, while most of these therapies only improve function when they are applied on patients in the early onset of stroke [2]. Thus, diagnosis and intervention of stroke in the early onset are crucial for AIS management.
The aberrant expressions of miRNAs in patients with AIS and the roles of miRNAs in AIS pathophysiology as well as their clinical associations with AIS have been reported in several studies [27]. For instance, miR-223 might play a role in AIS pathogenesis by upregulating insulin-like growth factor-1 [27], which disclosed that miRNAs might took part in the regulation of endothelial cells and angiogenesis in AIS.
Previous studies illuminated that several miRNAs could be potential biomarkers for diagnosis of AIS, such as serum exosome miR-9 and miR-124 [28]. Furthermore, a study characterized that the combination of miR-15a, miR-16, and miR-17-5p had a better diagnostic value for AIS compared with individual diagnostic values of miR-15a, miR-16, and miR-17-5p [29]. In this study, univariate and multivariate logistic regression analysis displayed that miR-126, miR-130a, miR-222, miR-218, and miR-185 were independent predicting factors for AIS. Moreover, our data presented an elevation of AUC when combining miR-126, miR-130a, miR-222, miR-218, and miR-185 compared to the AUCs for the five separate DEMs. Those results may be attributed to miR-126 and miR-130a, which were demonstrated to be pro-angiogenic miRNAs in multiple studies, while miR-222, miR-218, and miR-185 play negative roles in endothelial cell functions [18, 23, 30,31,32,33,34,35,36,37,38,39]. To our knowledge, of all the five DEMs in the validating stage of our study, only miR-126 has been reported in another study to be aberrantly expressed in the AIS patients. In the study of Long G et al., the plasma expression of miR-126 was discovered to be decreased in ischemic stroke patients until 24 weeks post stroke, which is in accordance with our study [11]. However, the dysregulations of miR-130a, miR-378, miR-222, miR-281, and miR-185 in plasma of patients with AIS were firstly reported by our study.
miR-126, located in chromosome 9q34.3, was found to be specifically and increasingly expressed in human endothelial cells [30]. Overexpression of miR-126 suppresses high-glucose migration and tube construction of endothelial cells of rhesus macaque through downregulating vascular endothelial growth factor A (VEGFA) and PIK3R2 [33]. In a line of research conducted on intracerebral hemorrhage (ICH) mice, miR-126 exhibits a protective role and was discovered to take part in the angiogenesis of ICH [32]. MiR-130a regulates the homeobox A5 (HOXA5) homeobox gene GAS, which is a growth arrest-specific homeobox, thereby mediates the angiogenic phenotype of vascular endothelial cells [34]. Additionally, Lu C et al. discovered that miR-130a plays a crucial role in cardiac dysfunction by inhibiting phosphatase and tensin homolog (PTEN) expression through stimulating PI3K/Akt signaling [35]. miR-222 locates near on Xp11.3 chromosome and was proved to target stem cell factor (SCF) and c-Kit and thus obstructs endothelial cell migration, proliferation, and function as anti-angiogenic miRNA in vitro [36, 37]. Dentelli P et al. identified signal transducer and activator of transcription 5A (STAT5A) as a target of miR-222, and miR-222 modulates neovascularization through modulating STAT5A [38]. MiR-218 was illustrated to be a tumor-suppressive miRNA in multiple studies, and in the research of Guan B et al., they found in prostate cancer that miR-218 obstructed tumor angiogenesis through targeting the mTOR component RICTOR [18]. In addition, an experiment on oxygen-induced retinopathy mice revealed an inhabitation effect on neovascularization of miR-218 via downregulating the level of Roundabout 1 [39]. miR-185 was elucidated to differently express in endothelial cells under hypoxia; miR-185 negatively regulates angiogenesis in human microvascular endothelial cells by directly interacting with stromal interaction molecule 1 [23]. Moreover, miR-101 and miR-206 demonstrated inhabitation effects of angiogenesis in several types of carcinomas [17, 40], while for miR-378, it was elucidated to enhance cell survival, tumor growth, and angiogenesis via mediating suppressor of fused gene (SuFu) and Fus-1 levels [19]. In the present study, we identified 11 DEMs in the exploring stage and observed that miR-126 and miR-130a markedly declined in the AIS patients while miR-222, miR-218, and miR-185 obviously increased in the AIS patients in the validating stage. Moreover, the Mann-Whitney analysis showed that miR-126, miR-378, and miR-101 were negatively associated with NIHSS score, while miR-222, miR-218, and miR-206 were positively correlated with NIHSS score. Former studies have demonstrated the pro-angiogenic roles of miR-126 and miR-130a as well as the anti-angiogenic roles of miR-222, miR-218, and miR-185 in various diseases including several cardiovascular or neurological deficits [18, 23, 30,31,32,33,34,35,36,37,38,39], which partially supported our results.
There were some limitations to this study: (1) blood samples were took from patients only once post the onset of symptoms and the difference of miRNA expression in the early and late onset of AIS was not compared in our study, (2) the roles of the selected miRNAs in relapse and prognosis of AIS were not evaluated in our study, and (3) the detailed mechanisms of DEMs in the pathogenesis of AIS were not evaluated in our study. Therefore, further studies contain more blood samples collected from various time spots after the onset of AIS symptoms; investigation of detailed mechanisms and evaluation of prognosis are needed in the future.
In conclusion, our study manifested that circulating miR-126, miR-130a, miR-222, miR-218, and miR-185 could be served as promising and independent biomarkers for risk of AIS and miR-126, miR-378, miR-222, miR-101, miR-218, and miR-206 could be used for disease severity management of AIS.
References
Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Moran AE, Sacco RL, Anderson L, Truelsen T, O'Donnell M, Venketasubramanian N, Barker-Collo S, Lawes CM, Wang W, Shinohara Y, Witt E, Ezzati M, Naghavi M, Murray C, Global Burden of Diseases I, Risk Factors S, the GBDSEG (2014) Global and regional burden of stroke during 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet 383(9913):245–254
Prabhakaran S, Ruff I, Bernstein RA (2015) Acute stroke intervention: a systematic review. JAMA 313(14):1451–1462. doi:10.1001/jama.2015.3058
Hankey GJ (2016) Stroke. Lancet. doi:10.1016/S0140-6736(16)30962-X
Yates LA, Norbury CJ, Gilbert RJ (2013) The long and short of microRNA. Cell 153(3):516–519. doi:10.1016/j.cell.2013.04.003
Rink C, Khanna S (2011) MicroRNA in ischemic stroke etiology and pathology. Physiol Genomics 43(10):521–528. doi:10.1152/physiolgenomics.00158.2010
Tan JR, Koo YX, Kaur P, Liu F, Armugam A, Wong PT, Jeyaseelan K (2011) microRNAs in stroke pathogenesis. Curr Mol Med 11(2):76–92
Khoshnam SE, Winlow W, Farbood Y, Moghaddam HF, Farzaneh M (2017) Emerging roles of microRNAs in ischemic stroke: as possible therapeutic agents. J Stroke 19(2):166–187. doi:10.5853/jos.2016.01368
Zhan X, Jickling GC, Tian Y, Stamova B, Xu H, Ander BP, Turner RJ, Mesias M, Verro P, Bushnell C, Johnston SC, Sharp FR (2011) Transient ischemic attacks characterized by RNA profiles in blood. Neurology 77(19):1718–1724. doi:10.1212/WNL.0b013e318236eee6
Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke 39(3):959–966. doi:10.1161/STROKEAHA.107.500736
Jickling GC, Ander BP, Zhan X, Noblett D, Stamova B, Liu D (2014) microRNA expression in peripheral blood cells following acute ischemic stroke and their predicted gene targets. PLoS One 9(6):e99283. doi:10.1371/journal.pone.0099283
Long G, Wang F, Li H, Yin Z, Sandip C, Lou Y, Wang Y, Chen C, Wang DW (2013) Circulating miR-30a, miR-126 and let-7b as biomarker for ischemic stroke in humans. BMC Neurol 13:178. doi:10.1186/1471-2377-13-178
Yang ZB, Zhang Z, Li TB, Lou Z, Li SY, Yang H, Yang J, Luo XJ, Peng J (2014) Up-regulation of brain-enriched miR-107 promotes excitatory neurotoxicity through down-regulation of glutamate transporter-1 expression following ischaemic stroke. Clin Sci (Lond) 127(12):679–689. doi:10.1042/CS20140084
Liu P, Zhao H, Wang R, Wang P, Tao Z, Gao L, Yan F, Liu X, Yu S, Ji X, Luo Y (2015) MicroRNA-424 protects against focal cerebral ischemia and reperfusion injury in mice by suppressing oxidative stress. Stroke 46(2):513–519. doi:10.1161/STROKEAHA.114.007482
Zhao H, Wang J, Gao L, Wang R, Liu X, Gao Z, Tao Z, Xu C, Song J, Ji X, Luo Y (2013) MiRNA-424 protects against permanent focal cerebral ischemia injury in mice involving suppressing microglia activation. Stroke 44(6):1706–1713. doi:10.1161/STROKEAHA.111.000504
Fan Y, Shi C, Li T, Kuang T (2017) microRNA-454 shows anti-angiogenic and anti-metastatic activity in pancreatic ductal adenocarcinoma by targeting LRP6. Am J Cancer Res 7(1):139–147
Du C, Weng X, Hu W, Lv Z, Xiao H, Ding C, Gyabaah OA, Xie H, Zhou L, Wu J, Zheng S (2015) Hypoxia-inducible MiR-182 promotes angiogenesis by targeting RASA1 in hepatocellular carcinoma. J Exp Clin Cancer Res 34:67. doi:10.1186/s13046-015-0182-1
Tang XR, Wen X, He QM, Li YQ, Ren XY, Yang XJ, Zhang J, Wang YQ, Ma J, Liu N (2017) MicroRNA-101 inhibits invasion and angiogenesis through targeting ITGA3 and its systemic delivery inhibits lung metastasis in nasopharyngeal carcinoma. Cell Death Dis 8(1):e2566. doi:10.1038/cddis.2016.486
Guan B, Wu K, Zeng J, Xu S, Mu L, Gao Y, Wang K, Ma Z, Tian J, Shi Q, Guo P, Wang X, He D, Du Y (2016) Tumor-suppressive microRNA-218 inhibits tumor angiogenesis via targeting the mTOR component RICTOR in prostate cancer. Oncotarget. doi:10.18632/oncotarget.14131
Xue D, Yang Y, Liu Y, Wang P, Dai Y, Liu Q, Chen L, Shen J, Ju H, Li Y, Tan Z (2016) MicroRNA-206 attenuates the growth and angiogenesis in non-small cell lung cancer cells by blocking the 14-3-3zeta/STAT3/HIF-1alpha/VEGF signaling. Oncotarget 7(48):79805–79813. doi:10.18632/oncotarget.12972
Zhang T, Liu W, Zeng XC, Jiang N, Fu BS, Guo Y, Yi HM, Li H, Zhang Q, Chen WJ, Chen GH (2016) Down-regulation of microRNA-338-3p promoted angiogenesis in hepatocellular carcinoma. Biomed Pharmacother 84:583–591. doi:10.1016/j.biopha.2016.09.056
Hirakawa T, Nasu K, Abe W, Aoyagi Y, Okamoto M, Kai K, Takebayashi K, Narahara H (2016) miR-503, a microRNA epigenetically repressed in endometriosis, induces apoptosis and cell-cycle arrest and inhibits cell proliferation, angiogenesis, and contractility of human ovarian endometriotic stromal cells. Hum Reprod 31(11):2587–2597. doi:10.1093/humrep/dew217
Gao F, Sun M, Gong Y, Wang H, Wang Y, Hou H (2016) MicroRNA-195a-3p inhibits angiogenesis by targeting Mmp2 in murine mesenchymal stem cells. Mol Reprod Dev 83(5):413–423. doi:10.1002/mrd.22638
Hou J, Liu L, Zhu Q, Wu Y, Tian B, Cui L, Liu Y, Li X (2016) MicroRNA-185 inhibits angiogenesis in human microvascular endothelial cells through targeting stromal interaction molecule 1. Cell Biol Int 40(3):318–328. doi:10.1002/cbin.10572
Wu F, Yang Z, Li G (2009) Role of specific microRNAs for endothelial function and angiogenesis. Biochem Biophys Res Commun 386(4):549–553. doi:10.1016/j.bbrc.2009.06.075
Liu HT, Xing AY, Chen X, Ma RR, Wang YW, Shi DB, Zhang H, Li P, Chen HF, Li YH, Gao P (2015) MicroRNA-27b, microRNA-101 and microRNA-128 inhibit angiogenesis by down-regulating vascular endothelial growth factor C expression in gastric cancers. Oncotarget 6(35):37458–37470. doi:10.18632/oncotarget.6059
Prugger C, Luc G, Haas B, Morange PE, Ferrieres J, Amouyel P, Kee F, Ducimetiere P, Empana JP, Group PS (2013) Multiple biomarkers for the prediction of ischemic stroke: the PRIME study. Arterioscler Thromb Vasc Biol 33(3):659–666. doi:10.1161/ATVBAHA.112.300109
Wang Y, Zhang Y, Huang J, Chen X, Gu X, Wang Y, Zeng L, Yang GY (2014) Increase of circulating miR-223 and insulin-like growth factor-1 is associated with the pathogenesis of acute ischemic stroke in patients. BMC Neurol 14:77. doi:10.1186/1471-2377-14-77
Ji Q, Ji Y, Peng J, Zhou X, Chen X, Zhao H, Xu T, Chen L, Xu Y (2016) Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One 11(9):e0163645. doi:10.1371/journal.pone.0163645
Wu J, Du K, Lu X (2015) Elevated expressions of serum miR-15a, miR-16, and miR-17-5p are associated with acute ischemic stroke. Int J Clin Exp Med 8(11):21071–21079
van Solingen C, Seghers L, Bijkerk R, Duijs JM, Roeten MK, van Oeveren-Rietdijk AM, Baelde HJ, Monge M, Vos JB, de Boer HC, Quax PH, Rabelink TJ, van Zonneveld AJ (2009) Antagomir-mediated silencing of endothelial cell specific microRNA-126 impairs ischemia-induced angiogenesis. J Cell Mol Med 13(8A):1577–1585. doi:10.1111/j.1582-4934.2008.00613.x
Parker LH, Schmidt M, Jin SW, Gray AM, Beis D, Pham T, Frantz G, Palmieri S, Hillan K, Stainier DY, De Sauvage FJ, Ye W (2004) The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature 428(6984):754–758. doi:10.1038/nature02416
Kong F, Zhou J, Zhou W, Guo Y, Li G, Yang L (2017) Protective role of microRNA-126 in intracerebral hemorrhage. Mol Med Rep. doi:10.3892/mmr.2017.6134
Yang WZ, Yang J, Xue LP, Xiao LB, Li Y (2016) MiR-126 overexpression inhibits high glucose-induced migration and tube formation of rhesus macaque choroid-retinal endothelial cells by obstructing VEGFA and PIK3R2. J Diabetes Complicat. doi:10.1016/j.jdiacomp.2016.12.004
Chen Y, Gorski DH (2008) Regulation of angiogenesis through a microRNA (miR-130a) that down-regulates antiangiogenic homeobox genes GAX and HOXA5. Blood 111(3):1217–1226. doi:10.1182/blood-2007-07-104133
Lu C, Wang X, Ha T, Hu Y, Liu L, Zhang X, Yu H, Miao J, Kao R, Kalbfleisch J, Williams D, Li C (2015) Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J Mol Cell Cardiol 89(Pt A):87–97. doi:10.1016/j.yjmcc.2015.10.011
Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G (2006) MicroRNAs modulate the angiogenic properties of HUVECs. Blood 108(9):3068–3071. doi:10.1182/blood-2006-01-012369
Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ (2009) MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem Biophys Res Commun 381(1):81–83. doi:10.1016/j.bbrc.2009.02.013
Dentelli P, Rosso A, Orso F, Olgasi C, Taverna D, Brizzi MF (2010) microRNA-222 controls neovascularization by regulating signal transducer and activator of transcription 5A expression. Arterioscler Thromb Vasc Biol 30(8):1562–1568. doi:10.1161/ATVBAHA.110.206201
Han S, Kong YC, Sun B, Han QH, Chen Y, Wang YC (2016) microRNA-218 inhibits oxygen-induced retinal neovascularization via reducing the expression of roundabout 1. Chin Med J 129(6):709–715. doi:10.4103/0366-6999.178013
Lee DY, Deng Z, Wang CH, Yang BB (2007) MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting SuFu and Fus-1 expression. Proc Natl Acad Sci U S A 104(51):20350–20355. doi:10.1073/pnas.0706901104
Acknowledgements
This work was not supported by any special fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The Ethics Committee of Cangzhou Central Hospital approved this study. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Jin, F., Xing, J. Circulating pro-angiogenic and anti-angiogenic microRNA expressions in patients with acute ischemic stroke and their association with disease severity. Neurol Sci 38, 2015–2023 (2017). https://doi.org/10.1007/s10072-017-3071-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3071-x