Abstract
In patients with spontaneous intracerebral hemorrhage (sICH), hematoma expansion (HE) is associated with poor outcome. Spot sign and black hole sign are neuroimaging predictors for HE. This study was aimed to compare the predictive value of two signs for HE. Within 6 h after onset of sICH, patients were screened for the computed tomography angiography spot sign and the non-contrast computed tomography black hole sign. The sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) of two signs for HE prediction were calculated. The accuracy of two signs in predicting HE was analyzed by receiver-operator analysis. A total of 129 patients were included in this study. Spot sign was identified in 30 (23.3%) patients and black hole sign in 29 (22.5%) patients, respectively. Of 32 patients with HE, spot sign was observed in 19 (59.4%) and black hole sign was found in 14 (43.8%). The occurrence of black hole sign was significantly associated with spot sign (P < 0.001). The sensitivity, specificity, PPV, and NPV of spot sign for predicting HE were 59.38, 88.66, 63.33, and 86.87% respectively. In contrast, the sensitivity, specificity, PPV, and NPV of black hole sign for predicting HE were 43.75, 84.54, 48.28, and 82.00%, respectively. The area under the curve was 0.740 for spot sign and 0.641 for black hole sign. (P = 0.228) Both spot sign and black hole sign appeared to have good predictive value for HE, and spot sign seemed to be a better predictor.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Spontaneous intracerebral hemorrhage (sICH) is a severe subtype of stroke, which accounts for 10–15% of all cases of stroke and is related to high early mortality and poor outcome [1,2,3]. Hematoma expansion (HE) can be found in about 30% sICH patients and is associated with worse clinical outcome [4]. Some neuroimaging predictors have been found to be associated with HE, which may assist in the improvement of outcome in sICH patients [5,6,7]. The computed tomography angiography (CTA) spot sign, which was firstly defined by Wada et al. in 2007, was found to be significantly associated with HE [8]. The predictive value of spot sign for HE was confirmed by many other studies [9,10,11]. Meta-analysis also suggested spot sign on CTA was a reliable neuroimaging marker for prediction of HE [12]. In addition to predicting HE, CTA spot sign was also found to be related to clinical outcome and mortality in sICH patients [13, 14]. Although the spot sign seems to be a good indicator for predicting HE, its assessment is based on CTA, which is still unavailable in many medical institutions. Thus, the identification of potential predictors for HE on non-contrast computed tomography (NCCT) is still important. Recently, the black hole sign on NCCT, defined as the relatively hypoattenuated area within the hyperattenuated hematoma and with a clear border to nearby brain tissue, was suggested to be a novel predictor for HE [15]. The black hole sign seemed to be a simple and effective method to predict HE in sICH patients. However, the predictive roles of CTA spot sign and NCCT black hole sign have not been compared in the same cohort of sICH patients. Thus, this retrospective cohort study was performed to compare the predictive value of CTA spot sign and NCCT black hole sign for HE.
Methods
Study design
This retrospective study was performed based on the sICH database in the department of neurosurgery, West China Hospital, Sichuan University. This study was approved by the biomedical ethics committee of West China Hospital, Sichuan University. The inclusive criteria in this study were as follows: (1) adult sICH patients diagnosed by computed tomography (CT) or magnetic resonance imaging (MRI) scans; (2) CTA was performed within 6 h after ictus of sICH; (3) the follow-up NCCT was performed within 24 h after CTA. The exclusive criteria were as follows: (1) secondary intracerebral hemorrhage caused by aneurysm, arteriovenous malformation, moyamoya disease, or tumor; (2) insufficient imaging information including initial CTA or follow-up NCCT; (3) hematoma evacuation before follow-up NCCT. The management of patients followed the latest edition of American Heart Association/American Stroke Association (AHA/ASA) and European Stroke Organisation (ESO) guidelines [16, 17].
Clinical data
Baseline characteristics including age, sex, admission blood pressure, and medical history were collected. The included medical histories were hypertension, diabetes mellitus, previous stroke, and smoke and alcohol abuse. Results of coagulation tests at admission including platelet count, prothrombin time (PT), activated partial thromboplastin time (APTT), and international normalized ratio (INR) were also collected.
Imaging acquisition
In our hospital, CTA was a part of standard clinical examination when patients were admitted to the emergency room. CTA was performed on a dual-source 64-slice CT scanner (SOMATOM Definition Flash; Siemens Healthcare Sector, Forchheim, Germany), including NCCT scan (120 kV, 340 mA, contiguous 5-mm axial slices) and CTA scan. For CTA, 100 mL of ioversol (Iopamidol, Bracco Pharma Co, Shanghai, China; 370 mg I/mL) was intravenously injected at a rate of 4.8 mL/s via a power injector through an intravenous line using the following parameters: 80 kVp; 110 mA; slice thickness, 1 mm; and pitch, 1:1. Another NCCT was performed within 24 h after CTA for evaluation of hematoma size.
Detection of the black hole sign and the spot sign
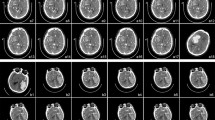
All the CT scans were reviewed by two neuroradiologists independently. The reviewers were blinded to the clinical condition of patients and evaluated the initial CTA and the follow-up NCCT. If disagreement about the black hole sign or the spot sign occurred, it would be judged by the discussion of two reviewers. In accordance with previous studies, the criteria for the spot sign in our study were as follows: (1) at least one focus of contrast pooling within the ICH: high Hounsfield unit (HU) value (>120); (2) discontinuous from normal or abnormal vasculature adjacent to the ICH; and (3) any size and morphology [18, 19]. The black hole sign on the NCCT was defined as follows: (1) relatively hypoattenuated area (black hole) encapsulated within the hyperattenuating hematoma; (2) the black hole could be round, oval, or rod-like but was not connected with the adjacent brain tissue; (3) the relatively hypoattenuated area should have an identifiable border; (4) the hematoma should have at least a 28 Hounsfield unit (HU) difference between the two density regions [15]. Illustrative black hole sign-positive NCCT images and spot sign-positive CTA images are shown in Fig. 1.
Measurement of hematoma volume
Volume of hematoma was calculated with the A × B × C/2 method from CT scans, in which A was the largest diameter on the largest hemorrhage slice, B was the maximal diameter perpendicular to A, and C was the vertical hematoma depth [20]. The HE was defined as an absolute increase >12.5 mL or a relative increase >33% in hematoma volume on follow-up NCCT [21].
Statistical analysis
All the statistical analyses were performed by SPSS 21.0 and Excel 2010. Baseline information including demographics, medical histories, blood pressure at admission, hematoma characteristics at admission, and results of coagulation tests were compared between patients with HE and those without HE. Statistical significance was assumed if a probability value is <0.05. Continuous values were expressed as mean and standard deviation (SD) and analyzed by the t test. Discontinuous variables were expressed as median and interquartile range (IQR) and analyzed by the Wilcoxon rank sum test. The categorical values were analyzed by chi-square analysis. Spearman’s correlation analysis was performed to investigate the association between the spot sign and the black hole sign. Multivariable logistic regression was also performed to adjust the odds ratio (OR) and 95% confidence interval (CI) of the black hole sign and the spot sign on HE. The roles of the black hole sign and the spot sign in predicting HE were analyzed by receiver-operator analysis. The area under the receiver-operating characteristic (ROC) curves of the black hole sign and the spot sign were compared with Z test. The interobserver reliability for the identification of the spot sign and the black hole sign was determined by calculation of κ values.
Results
From February 2015 to April 2016, a total of 129 consecutive patients meeting the inclusive criteria were enrolled in this study. The median time interval between onset of ICH to initial CTA was 4 h (3.5 h). The mean age was 59.0 ± 11.6 years with a range from 36 to 83 years. The mean baseline volume of hematoma was 26.15 ± 22.11 mL. Hematomas located in the lobe (18, 14.0%), basal ganglia (74, 57.4%), thalamus (16, 12.4%), cerebellum (15, 11.6%), and brain stem (6, 4.7%). HE was identified in 32 (24.8%) patients. The mean volume of HE was 24.68 ± 21.87 mL. The baseline characteristics of patients with and without HE are shown in Table 1. In this study, the spot sign was found in 30 patients and the black hole sign was found in 29 patients. Patients with HE had significantly higher prevalence of the spot sign and the black hole sign than those without HE. Interobserver reliability between two neuroradiologists to identify the spot sign and the black hole sign was satisfying with the κ value 0.918 and 0.932, respectively.
In 16 patients with both spot sign and black hole sign, 12 (75.00%) had HE. Seven (50.00%) of 14 patients with spot sign but no black hole sign had HE. Two (15.38%) out of 13 patients who had black hole sign but no spot sign had HE. A total of 86 patients had neither spot sign nor black hole sign and 11 (12.79%) of them had HE. Significant association between the spot sign and the black hole sign was found (Spearman correlation coefficient = 0.407, P < 0.001). Thus, multivariable analyses for the spot sign and the black hole sign were performed separately and both signs were associated with HE (Table 2 and Table 3).
The sensitivity, specificity, positive predictive value, and negative predictive value of the spot sign for predicting HE were 59.38, 88.66, 63.33, and 86.87%, respectively. The sensitivity, specificity, positive predictive value, and negative predictive value of the black hole sign for predicting HE were 43.75, 84.54, 48.28, and 82.00%, respectively. The ROC curves of the two signs for the prediction of HE in sICH patients are shown in Fig. 2. The area under the curve was 0.740 for the spot sign and 0.641 for the black hole sign. No significant difference was found between the area under ROC curves of the spot sign and the black hole sign (P = 0.228).
Discussion
In this study, we compared the predictive role of the CTA spot sign to that of the NCCT black hole sign in the same cohort of sICH patients. Occurrence of the black hole sign was found to be significantly associated with the spot sign. Both the spot sign and the black hole sign had good predictive values for HE. Although no significant difference was found in the predictive value for HE of the spot sign and the black hole sign, the spot sign tended to be a better neuroimaging predictor for HE.
Many previous studies showed the CTA spot sign was a good predictor for HE in sICH patients [8, 13, 14, 19, 22]. In our study, we also found the CTA spot sign had good predictive value for HE. The CTA spot sign was thought to reflect continuous bleeding from the ruptured cerebral vessels [18]. Although the previous studies suggested the predictive value of the spot sign, the results varied in different studies. In Wada et al.’s study, the sensitivity, specificity, positive predictive value, and negative predictive value of the spot sign were 91, 89, 77, and 96%, respectively [8]. However, Wang et al. reported the sensitivity, specificity, positive predictive value, and negative predictive value of the spot sign were 77.9, 93.2, 78.9, and 92.8% [22]. A meta-analysis including 18 studies suggested the pooled sensitivity and specificity of the spot sign were 53 and 88%, respectively. Several factors could influence the predictive value of the spot sign for HE. Firstly, the settings of CTA were various in different medical institutions. Higher tube current in CTA could provide better predictive accuracy of HE to the spot sign [23]. A 90-s delayed CTA was demonstrated to improve sensitivity of the spot sign for prediction of HE [11]. Longer scan timing of CTA could improve the predictive value of the spot sign for HE [24]. Secondly, the different sizes of hematoma may influence the prevalence of the spot sign. In patients with initial volume of hematoma >30 mL, the spot sign had the better predictive value for HE [22]. The lower volume of hematoma could be associated with the lower prevalence of the spot sign and lower possibility of HE [25]. Moreover, the interval between onset of sICH and CTA may also influence the accuracy of spot sign for HE prediction. The incidence of HE in patients with spot sign was found to decrease as onset-to-CTA time increased [26]. For sICH patients with spot sign, the shorter onset-to-CTA time and higher spot sign HU can improve the predictive value for HE [27]. Thus, although CTA spot sign has been shown to be a good predictor for HE, further studies are still needed to investigate the optimal method to predict HE with the spot sign.
Several previous studies discussed the association between heterogeneity of hematoma and HE. In Barras et al.’s study, density heterogeneity of hematoma predicted HE independently [28]. Blacquiere et al. also found density heterogeneity on NCCT was associated with HE at 24 h [5]. Boulouis et al. reported hypodensities within hematoma on NCCT could predict HE [29]. However, these studies did not provide a standardized and simple marker for HE prediction. The black hole sign was a simple neuroimaging marker based on the heterogeneity of hematoma on NCCT. It was firstly reported by Li et al. as a novel predictor for HE in sICH [15]. However, the association between the black hole sign and the spot sign was unclear. In our study, we found the occurrence of the black hole sign was significantly associated with the spot sign and patients with both spot sign and black hole sign were at high risk of HE. We also found the occurrence of HE was still relatively high in patients with spot sign but without black hole sign. However, the incidence of HE was low in patients with black hole sign but without spot sign. The black hole sign was considered to reflect different age of bleeding and the fresh bleeding could be hypoattenuating on NCCT [15], which seemed to share the similar underlying mechanism with the CTA spot sign. The sensitivity, specificity, positive predictive value, and negative predictive value of the black hole sign for predicting HE were reported to be 31.9, 94.1, 73.3, and 73.2%, respectively, which showed that the black hole sign could have good predictive value for HE [15]. In our study, the sensitivity and the negative predictive value of the black hole sign were higher and the specificity and the positive predictive value were lower. Many factors might influence the predictive value of the black hole sign. The time to baseline CT in this study (3.16 h in expanders and 4.00 h in non-expanders) was much longer than that in Li et al.’s study (2.3 h in expanders and 2.6 h in non-expanders). In addition, the initial volume of hematoma was different in our study (31.99 mL in expanders and 23.06 mL in non-expanders) from that in Li et al.’s study (33.1 mL in expanders and 14.1 mL in non-expanders). Further study is necessary to investigate the accurate factors influencing the predictive value of the black hole sign.
There are several limitations in this study. First, this was a single-center retrospective cohort study with the relatively small sample size. Second, only the CTA spot sign and the black hole sign were included in this study. Moreover, the onset-to-CTA time was relatively long in this study, which potentially affected the predictive value of both signs. Furthermore, the association between both signs and clinical outcome was not discussed in this study. Further multi-center prospective cohort studies with larger sample size, shorter onset-to-CTA time, and outcome assessment are needed.
Conclusion
This study compared the predictive value of the CTA spot sign and the NCCT black hole sign in the same cohort of sICH patients. The occurrence of the black hole sign was significantly associated with the spot sign. Both the spot sign and the black hole sign seemed to have good predictive value for HE and the spot sign appeared to be a better predictor for HE.
References
Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF (2001) Spontaneous intracerebral hemorrhage. N Engl J med 344(19):1450–1460. doi:10.1056/nejm200105103441907
Yang G, Shao GF (2016) Elevated serum IL-11, TNF alpha, and VEGF expressions contribute to the pathophysiology of hypertensive intracerebral hemorrhage (HICH). Neurological Sciences : Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 37(8):1253–1259. doi:10.1007/s10072-016-2576-z
Tan G, Hao Z, Lei C, Chen Y, Yuan R, Xu M, Liu M (2016) Subclinical change of liver function could also provide a clue on prognosis for patients with spontaneous intracerebral hemorrhage. Neurological Sciences : Official Journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology 37(10):1693–1700. doi:10.1007/s10072-016-2656-0
Guan J, Hawryluk GW (2016) Targeting secondary hematoma expansion in spontaneous intracerebral hemorrhage—state of the art. Front Neurol 7:187. doi:10.3389/fneur.2016.00187
Blacquiere D, Demchuk AM, Al-Hazzaa M, Deshpande A, Petrcich W, Aviv RI, Rodriguez-Luna D, Molina CA, Silva Blas Y, Dzialowski I, Czlonkowska A, Boulanger JM, Lum C, Gubitz G, Padma V, Roy J, Kase CS, Bhatia R, Hill MD, Dowlatshahi D (2015) Intracerebral hematoma morphologic appearance on noncontrast computed tomography predicts significant hematoma expansion. Stroke; a Journal of Cerebral Circulation 46(11):3111–3116. doi:10.1161/strokeaha.115.010566
Orito K, Hirohata M, Nakamura Y, Takeshige N, Aoki T, Hattori G, Sakata K, Abe T, Uchiyama Y, Sakamoto T, Morioka M (2016) Leakage sign for primary intracerebral hemorrhage: a novel predictor of hematoma growth. Stroke; a Journal of Cerebral Circulation 47(4):958–963. doi:10.1161/strokeaha.115.011578
Li Q, Zhang G, Huang YJ, Dong MX, Lv FJ, Wei X, Chen JJ, Zhang LJ, Qin XY, Xie P (2015) Blend sign on computed tomography: novel and reliable predictor for early hematoma growth in patients with intracerebral hemorrhage. Stroke; a Journal of Cerebral Circulation 46(8):2119–2123. doi:10.1161/strokeaha.115.009185
Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, Symons SP (2007) CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke; a Journal of Cerebral Circulation 38(4):1257–1262. doi:10.1161/01.STR.0000259633.59404.f3
Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, Romero JM, Viswanathan A, Greenberg SM, Rosand J, Goldstein JN (2012) CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care 17(3):421–428. doi:10.1007/s12028-012-9765-2
Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, Kobayashi A, Boulanger JM, Lum C, Gubitz G, Padma V, Roy J, Kase CS, Kosior J, Bhatia R, Tymchuk S, Subramaniam S, Gladstone DJ, Hill MD, Aviv RI (2012) Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. The Lancet Neurology 11(4):307–314. doi:10.1016/s1474-4422(12)70038-8
Ciura VA, Brouwers HB, Pizzolato R, Ortiz CJ, Rosand J, Goldstein JN, Greenberg SM, Pomerantz SR, Gonzalez RG, Romero JM (2014) Spot sign on 90-second delayed computed tomography angiography improves sensitivity for hematoma expansion and mortality: prospective study. Stroke; a Journal of Cerebral Circulation 45(11):3293–3297. doi:10.1161/strokeaha.114.005570
Du FZ, Jiang R, Gu M, He C, Guan J (2014) The accuracy of spot sign in predicting hematoma expansion after intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One 9(12):e115777. doi:10.1371/journal.pone.0115777
Han JH, Lee JM, Koh EJ, Choi HY (2014) The spot sign predicts hematoma expansion, outcome, and mortality in patients with primary intracerebral hemorrhage. Journal of Korean Neurosurgical Society 56(4):303–309. doi:10.3340/jkns.2014.56.4.303
Morotti A, Jessel MJ, Brouwers HB, Falcone GJ, Schwab K, Ayres AM, Vashkevich A, Anderson CD, Viswanathan A, Greenberg SM, Gurol ME, Romero JM, Rosand J, Goldstein JN (2016) CT angiography spot sign, hematoma expansion, and outcome in primary pontine intracerebral hemorrhage. Neurocrit Care 25(1):79–85. doi:10.1007/s12028-016-0241-2
Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, Wei X, Xie P (2016) Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke; a Journal of Cerebral Circulation 47(7):1777–1781. doi:10.1161/strokeaha.116.013186
Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, Thomas BM, Toni D, Unterberg A, Wagner M (2014) European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. International Journal of Stroke : Official Journal of the International Stroke Society 9(7):840–855. doi:10.1111/ijs.12309
Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke; a Journal of Cerebral Circulation 46(7):2032–2060. doi:10.1161/str.0000000000000069
Brouwers HB, Goldstein JN, Romero JM, Rosand J (2012) Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke; a Journal of Cerebral Circulation 43(12):3427–3432. doi:10.1161/strokeaha.112.664003
Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, Oleinik A, Lev MH, Gonzalez RG, Romero JM (2009) Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke; a Journal of Cerebral Circulation 40(9):2994–3000. doi:10.1161/strokeaha.109.554667
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M, Khoury J (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke; a Journal of Cerebral Circulation 27(8):1304–1305
Davis SM, Broderick J, Hennerici M, Brun NC, Diringer MN, Mayer SA, Begtrup K, Steiner T (2006) Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 66(8):1175–1181. doi:10.1212/01.wnl.0000208408.98482.99
Wang YH, Fan JY, Luo GD, Lin T, Xie DX, Ji FY, Zhang XT (2011) Hematoma volume affects the accuracy of computed tomographic angiography ‘spot sign’ in predicting hematoma expansion after acute intracerebral hemorrhage. Eur Neurol 65(3):150–155. doi:10.1159/000324153
Morotti A, Romero JM (2016) Effect of CTA tube current on spot sign detection and accuracy for prediction of intracerebral hemorrhage expansion
Tsukabe A, Watanabe Y, Tanaka H, Kunitomi Y, Nishizawa M, Arisawa A, Yoshiya K, Shimazu T, Tomiyama N (2014) Prevalence and diagnostic performance of computed tomography angiography spot sign for intracerebral hematoma expansion depend on scan timing. Neuroradiology 56(12):1039–1045. doi:10.1007/s00234-014-1430-3
Dowlatshahi D, Yogendrakumar V, Aviv RI, Rodriguez-Luna D, Molina CA, Silva Y, Dzialowski I, Czlonkowska A, Boulanger JM, Lum C, Gubitz G, Padma V, Roy J, Kase CS, Bhatia R, Hill MD, Demchuk AM (2016) Small intracerebral hemorrhages have a low spot sign prevalence and are less likely to expand. International Journal of Stroke : Official Journal of the International Stroke Society 11(2):191–197. doi:10.1177/1747493015616635
Dowlatshahi D, Brouwers HB, Demchuk AM, Hill MD, Aviv RI, Ufholz LA, Reaume M, Wintermark M, Hemphill JC 3rd, Murai Y, Wang Y, Zhao X, Wang Y, Li N, Sorimachi T, Matsumae M, Steiner T, Rizos T, Greenberg SM, Romero JM, Rosand J, Goldstein JN, Sharma M (2016) Predicting intracerebral hemorrhage growth with the spot sign: the effect of onset-to-scan time. Stroke; a Journal of Cerebral Circulation 47(3):695–700. doi:10.1161/strokeaha.115.012012
Kim SH, Jung HH, Whang K, Kim JY, Pyen JS, Oh JW (2014) Which emphasizing factors are most predictive of hematoma expansion in spot sign positive intracerebral hemorrhage? AJNR Am J Neuroradiol 56(2):86–90. doi:10.3340/jkns.2014.56.2.86
Barras CD, Tress BM, Christensen S, MacGregor L, Collins M, Desmond PM, Skolnick BE, Mayer SA, Broderick JP, Diringer MN, Steiner T, Davis SM (2009) Density and shape as CT predictors of intracerebral hemorrhage growth. Stroke; a Journal of Cerebral Circulation 40(4):1325–1331. doi:10.1161/strokeaha.108.536888
Boulouis G, Morotti A, Brouwers HB, Charidimou A, Jessel MJ, Auriel E, Pontes-Neto O, Ayres A, Vashkevich A, Schwab KM, Rosand J, Viswanathan A, Gurol ME, Greenberg SM, Goldstein JN (2016) Association between hypodensities detected by computed tomography and hematoma expansion in patients with intracerebral hemorrhage. JAMA Neurology 73(8):961–968. doi:10.1001/jamaneurol.2016.1218
Acknowledgements
Our great acknowledgement goes to the patients included in this study to make this study possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Yu, Z., Zheng, J., Ma, L. et al. The predictive accuracy of the black hole sign and the spot sign for hematoma expansion in patients with spontaneous intracerebral hemorrhage. Neurol Sci 38, 1591–1597 (2017). https://doi.org/10.1007/s10072-017-3006-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-017-3006-6