Abstract
The aim of the study was to assess the 90-day prognostic value of copeptin in a group of Chinese patients with acute intracerebral hemorrhage (ICH). In this study, all consecutive patients with first-ever ICH from 2010 to 2012 were recruited to participate in the study. On admission, plasma copeptin levels were measured by enzyme-linked immunosorbent assay. The Glasgow Coma Scale (GCS) and Hemphill ICH scores were assessed on admission blinded to plasma copeptin levels. For the assessment of functional outcome at 90 days, Modified Rankin Scale was used. During the study period, 271 patients were diagnosed as ICH and were included in the analysis. The median GCS score on admission was 11 points. Patients with an unfavorable outcomes and non-survivors had significantly increased plasma copeptin levels on admission (P < 0.001 for both). Copeptin was an independent prognostic marker of functional outcome and death [odds ratio 3.45 (95 % confidence intervals: 1.85–6.99) and 3.66 (2.42–8.28), respectively, P < 0.001 for both, adjusted for age, the hematoma volume and other predictors] in patients with ICH. In receiver operating characteristic curve analysis, copeptin could improve the Hemphill score in predicting 90-day functional outcome [area under the curve (AUC) of the combined model, 0.83; 95 % CI 0.74–0.90; P < 0.001] and mortality (AUC of the combined model, 0.88; 95 % CI 0.82–0.93; P < 0.001). In conclusion, our study suggests that copeptin levels are a useful tool to predict unfavorable functional outcome and mortality 90 days after ICH and have a potential to assist clinicians.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intracerebral hemorrhage (ICH) is more fatal and disabling than ischemic stroke and ranges from 10 to 20 cases per 100,000 population reflecting 10–15 % of all stroke patients [1]. ICH accounts for a high mortality and morbidity. Early prediction of outcome is crucial for optimized care and treatment decision. Dynamic factors such as hematoma volume expansion, edema formation or persistent high blood pressure are known to be associated with early neurologic deterioration and poor outcome [2]. Biomarkers are attracting increasing attention as potential predictors of outcome in ICH [3].
Copeptin is co-synthesized with vasopressin in the hypothalamus and is released into the portal circulation of the neurohypophysis. Copeptin is stable both in serum and plasma and can be easily measured in the circulation, in manual or fully automated chemiluminescence assays [4]. The role of copeptin is as yet unclear. Copeptin was recently suggested to play an important role in the correct structural formation of the arginine-vasopressin (AVP) precursor, as a prerequisite for its efficient proteolytic maturation [5].
Measurement of copeptin levels has been shown to be useful in a variety of clinical scenarios, particularly as a prognostic marker in patients with acute diseases such as lower respiratory tract infection, heart disease and stroke [4]. In critically ill patients, copeptin values increased significantly with the severity of the disease [6–8]. Copeptin levels have been found to be elevated in ischemic stroke, and high copeptin levels were highly predictive for poor function outcome and mortality [9, 10]. Furthermore, it is also found that copeptin was significantly associated with mortality and with a poor functional outcome in ICH [11, 12]. However, the small sample should be considered. The present study aimed to investigate the 90-day prognostic value of copeptin in a group of Chinese patients with ICH.
Subjects and methods
We conducted a prospective cohort study at the emergency department. From 2010 to 2012, all patients with first-ever acute ICH were included. All patients were admitted within 24 h of experiencing a new focal or global neurological event. Brain imaging (either CT or MRI) was performed routinely within 24 h after admission. Exclusion criteria were existing previous neurological disease, head trauma, use of antiplatelet or anticoagulant medication, autoimmune diseases with or without immunosuppressive therapy and presence of other prior systemic diseases including uremia, liver cirrhosis, malignancy, chronic heart or lung disease.
On arrival to the emergency department, the following demographical and clinical data were taken: gender, age and history of conventional vascular risk factors (hypertension, diabetes mellitus, atrial fibrillation, hyperlipoproteinemia, smoking habit and alcohol abuse) were obtained. Routine blood and biochemical tests, ECG, and a baseline brain CT or MRI scan were performed in all patients at admission. Hematoma volume was measured according to the previously reported formula ABC/2 [13] and was determined by one experienced neurologist unaware of the clinical and laboratory results. All patients received treatment according to current guidelines.
The study was approved by the ethics committee of Beijing Military Region General Hospital. The patients or their relatives gave written informed consent prior to entering the study. Two hundred age- and gender-matched healthy volunteers were assigned to the healthy control group. The median age of controls included in this study was 69 (interquartile ranges: IQR 58–80) years and 47 % were women.
Clinical status and severity of disease were assessed on admission. For clinical assessment, the Glasgow Coma Scale (GCS) and Hemphill ICH scores were used, and relevant co-morbidities were assessed with the Charlson co-morbidity index [14, 15]. For the assessment of functional outcome at 90 days, structured interviews were carried out by a trained medical student who was blinded to copeptin levels. Functional outcomes were measured with Modified Rankin Scale (mRS). A favorable outcome was defined as a score below 3 on the mRS [16].
Fasting venous blood was collected from all participants in vacutainer tubes and quickly centrifuged to avoid glycolysis. All blood samples were collected on the first day of admission [within 0–6 (n = 60), 6–12 (n = 98), 12–24 (n = 61), and 24–48 (n = 52) h from symptom onset], and copeptin was measured in accordance with standard detection methods in the hospital biochemistry department of this hospital. The concentration of copeptin in plasma was analyzed by enzyme-linked immunosorbent assay (ELISA) using commercial kits (Cusabio biotech co. ltd, Wuhan, Hubei Province, China) in accordance with the manufactures’ instructions. In our study, the lower detection limit was 0.5 pmol/L, intra-assay and inter-assay coefficients of variation were 3.8 % and 4.9 %. Median copeptin levels in 200 healthy individual was 4.1 pmol/L and the 97.5th percentile was 17.5 pmol/L. The median in healthy individuals was similar as published in other studies (4.2 pmol/L in Morgenthaler et al. [17] and 3.9 pmol/L in Zhang et al. [18]). The blood samples were run in duplicate. Researchers running ELISAs were blinded to all patient details.
Results are expressed as percentages for categorical variables and as medians (interquartile ranges, IQRs) for the continuous variables. Univariate data on demographic and clinical features were compared by Mann–Whitney U test or Chi-square test as appropriate. Correlations among continuous variables were assessed by the Spearman rank correlation coefficient. The influence of copeptin levels on functional outcome and death was assessed by logistic regression analysis, after adjusting for the main baseline variables in the univariate analyses. Results were expressed as adjusted odds ratios (OR) with the corresponding 95 % confidence interval (CI). Receiver operating characteristic (ROC) curves were utilized to evaluate the accuracy of copeptin to predict outcome and death. Thereby the area under the receiver operating characteristic curve (AUC) was a summary measure over criteria and cut-point choices. All statistical analyses were performed with SPSS for Windows, version 20.0 (SPSS Inc., Chicago, IL, USA). Statistical significance was defined as P < 0.05.
Results
From 365 screened patients, acute ICH was diagnosed in 271 patients, and all completed 3-month follow-up and were included in the analysis. The median age of patients included in this study was 69 (IQR 59–81) years and 46.9 % were women. The baseline characteristics of the 271 patients were described in Table 1. The median time from ICH onset to inclusion in the study was 5.9(IQR 3.8–13.2) h. The median GCS score on admission was 11 points (IQR 7–16). An unfavorable functional outcome was found in 82 patients (30.3 %) with a median mRS score of 4 (IQR 3–6). In our study, 34 patients died, thus the mortality rate was 12.5 %.
Plasma copeptin level on admission in patients was statistically significantly higher than that in healthy controls [16.2 (IQR 8.2–24.8) vs. 4.1 (IQR 3.4–9.2) pmol/L, respectively, P < 0.001]. Copeptin levels increased with increasing severity of ICH as defined by the Hemphill score. There was a modest correlation between levels of plasma copeptin and Hemphill score (r = 0.489, P < 0.001). In addition, the copeptin level correlated positively with hematoma volume (r = 0.462, P < 0.001; Fig. 1a) and negatively with GCS score (r = −0.346, P < 0.001; Fig. 1b). The results indicated that the plasma copeptin levels gradually increased with increasing age (r = 0.157, P = 0.004). There was no correlation between levels of plasma copeptin levels and sex (P = 0.563). Interestingly, no significant differences in copeptin levels were observed (analysis of variance (ANOVA): P = 0.213) depending on whether it was measured 0–3, 3–6, 6–12, 12–24, or 24–48 after symptom onset.
Plasma copeptin levels in patients with an unfavorable outcome were significantly greater than those in patients with a favorable outcome [29.1 (IQR 18.9–40.9) vs. 11.2 (IQR 6.5–19.4) pmol/L; P < 0.001; Fig. 2a]. In univariate logistic regression analysis, we calculated the ORs of log-transformed copeptin levels as compared with the Hemphill score, hematoma volume and other risk factors. With an unadjusted OR of 5.87 (95 % CI 3.11–11.16; P < 0.001), copeptin had a strong association with unfavorable functional outcome. After adjusting for all other significant outcome predictors, copeptin remained can be seen as an independent predictor of unfavorable outcome with an adjusted OR of 3.45 (95 % CI 1.85–6.99; P < 0.001) (see Table 2). With an AUC of 0.81 (95 % CI 0.73–0.88), copeptin showed a significantly greater discriminatory ability as compared with C-reactive protein (CRP) (AUC 0.61; 95 % CI 0.55–0.68; P < 0.001) and hematoma volume (AUC 0.76; 95 % CI 0.61–0.82; P < 0.001) and in the range of Hemphill score (AUC 0.79; 95 % CI 0.72–0.86; P < 0.001). Copeptin improved the Hemphill score (AUC of the combined model 0.87; 95 % CI 0.79–0.93; P < 0.001) (Fig. 3a).
Plasma copeptin levels in ICH patients with different groups. Mann–Whitney U test. All data are medians and interquartile ranges (IQR). Significantly higher in unfavorable outcome patients as compared to favorable (P < 0.001); significantly higher in non-survivors patients as compared to survivors patients (P < 0.001)
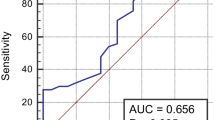
a Receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the functional outcome within 90 days based on the model incorporating 2 markers and the relative contribution of each biomarker alone (initial cohort); b receiver operator characteristic curve demonstrating sensitivity as a function of 1-specificity for predicting the mortality within 90 days based on the model incorporating 2 markers and the relative contribution of each biomarker alone (initial cohort)
Copeptin levels in 34 patients who died were more than 3 times greater as compared with patients who survived [41.4 (IQR 35.1–44.6) vs. 12.8 (IQR 7.4–21.9) pmol/L, respectively, P < 0.001; Fig. 2b). After adjusting for all other significant outcome predictors (such as age, Hemphill score and hematoma volume), copeptin level remained an independent predictor for mortality with an OR of 3.66 (95 % CI 2.42–8.28; P < 0.001). See the Table 2. Similarly, with an AUC of 0.84 (95 % CI 0.76–0.91), copeptin was in the range of Hemphill score (AUC 0.83; 95 % CI 0.74–0.89; P < 0.001). Copeptin also improved the Hemphill score (AUC of the combined model 0.90; 95 % CI 0.82–0.95; P < 0.001) (Fig. 3b).
Discussions
Copeptin is known to have prognostic value in a variety of diseases, as it reflects disease severity and thus the chance of recovery [19]. Katan et al. [20] found that copeptin was associated with lesion size and clinical severity on admission but was still an independent prognostic marker in patients with an acute ischemic stroke and Dong et al. [12] demonstrated for the first time that copeptin is an independent mortality marker in patients with ICH and its discriminative power was in the range of GCS score and hematoma volume which are known to be a strong individual outcome predictor. In our study, copeptin was correlated with hematoma volume, which in turn is associated with clinical severity and outcome. In multivariate logistic regression models of predictors of 90-day unfavorable functional outcome and death that included other confounding variables, the copeptin levels on admission were an independent predictor. The results of the present paper are consistent with reports of Zhang et al. [21], which indicated that copeptin is an independent predictor of the 90-day functional outcomes of ICH patients.
This large patient-based (n = 271) study shows an association of clinical severity and short-term prognosis with increased serum copeptin levels in Chinese subjects with ICH. This association was adjusted for the potentially confounding factors of age, sex, vascular risk factors, hematoma volume and clinical severity. In addition, two hundred age- and gender-matched healthy volunteers were assigned to the healthy control group. This design allowed us to examine the normal value of serum copeptin in our population. Furthermore, we demonstrated that plasma copeptin levels on admission in the ICH patients were significantly higher than those in healthy controls; and in patients who had unfavorable functional outcome or died, the copeptin levels on admission were significantly higher compared with levels in patients with favorable functional outcome or survivors. Our results indicated that the plasma copeptin levels gradually increased with increasing age, while there was no correlation between levels of plasma copeptin levels and sex. In contrast with this, Nickel et al. [4] found that copeptin levels were higher in the male volunteers compared with female.
In addition, in this study, median plasma copeptin level for ICH patients (16.2 pmol/L) was similar to that of intracerebral hemorrhage (18 pmol/L) [11], ischemic stroke (16.3 pmol/L) [22] and aneurysmal subarachnoid hemorrhage (23.8 pmol/L) [23] in previous reports. We also found that the copeptin level correlated positively with hematoma volume (r = 0.462, P < 0.001), which in turn is associated with clinical severity and outcome. In accordance with this hypothesis, Zweifel et al. [11] showed a correlation between the severity of hemorrhagic brain injury and copeptin level on admission. We could suggest that copeptin level at admission might reflect the initial hemorrhagic insult.
Copeptin predicted mortality and functional outcome in our ICH cohort and its discriminative power was in the range of Hemphill score. Other biomarkers have been shown to predict early neurologic deterioration and mortality in ICH patients, i.e. D-dimer [24], glutamate [25] and protein S100b [26]. Each of these biomarkers reflects a different pathophysiological process which also might have a specific therapeutic implication. In this context, copeptin might have an interesting potential as a new prognostic biomarker in combination with clinical features.
We assume that high level of copeptin may play a role in the process of ICH progression. Brain edema formation predicts an unfavorable outcome in ICH [27]. Therefore, copeptin levels might reflect developing or existing brain edema and might therefore be helpful in identifying patients at risk for brain edema formation who could profit from therapeutic interventions, such as the administration of a vasopressin antagonist [28]. Some authors have reported that AVP may play a role in the development of cerebral vasospasm [29] and ischemic brain edema [30]. Results obtained by Kozniewska et al. [31] indicated that brain cells adaptation to hyponatremia is impeded by vasopressin. Kozniewska et al. [32] suggested that vasopressin is one of the factors participating in vasogenic edema and cellular swelling after ICH. We speculate that copeptin and edema interact and cause a negative effect on ICH patients. Specific mechanisms need further investigation.
The following limitations of our study should be considered. A limitation of our study was that we could not monitor brain edema formation and link it with copeptin values. Secondly, without serial measurement of the circulating copeptin levels, this study yielded no data regarding when and how long copeptin is elevated in these patients. Another study by Dong et al. [12], however, found that after ICH, plasma copeptin level in patients increased during the 6-h period immediately peaked in 24 h. Thus, for the purposes of assessing the clinical utility of plasma copeptin as a predictor for unfavorable functional outcome and mortality, this is a reasonable approach because more demanding measurement protocols would not be practical in a clinical setting. In addition, it should be investigated whether serial copeptin testing further improves the risk stratification of ICH patients. Thirdly, data derived from a single-centre study always need to be replicated in larger multicentre studies.
Conclusions
Our study suggests that copeptin levels are a useful tool to predict unfavorable functional outcome and mortality 90 days after ICH and have a potential to assist clinicians.
References
Andersen KK, Olsen TS, Dehlendorff C, Kammersgaard LP (2009) Hemorrhagic and ischemic strokes compared: stroke severity, mortality, and risk factors. Stroke 40:2068–2072
Alvarez-Sabin J, Delgado P, Abilleira S, Molina CA, Arenillas J et al (2004) Temporal profile of matrix metalloproteinases and their inhibitors after spontaneous intracerebral hemorrhage: relationship to clinical and radiological outcome. Stroke 35:1316–1322
Delgado P, Alvarez Sabin J, Montaner J (2007) Biological markers in spontaneous intracerebral hemorrhage. Neurologia (Barcelona, Spain) 22:448–455
Nickel CH, Bingisser R, Morgenthaler NG (2012) The role of copeptin as a diagnostic and prognostic biomarker for risk stratification in the emergency department. BMC Med 10:7
Barat C, Simpson L, Breslow E (2004) Properties of human vasopressin precursor constructs: inefficient monomer folding in the absence of copeptin as a potential contributor to diabetes insipidus. Biochemistry 43:8191–8203
Morgenthaler NG, Müller B, Struck J, Bergmann A, Redl H, Christ-Crain M (2007) Copeptin, a stable peptide of the arginine vasopressin precursor, is elevated in hemorrhagic and septic shock. Shock 28:219–226
Stoiser B, Mörtl D, Hülsmann M, Berger R, Struck J, Morgenthaler NG et al (2006) Copeptin, a fragment of the vasopressin precursor, as a novel predictor of outcome in heart failure. Eur J Clin Invest 36:771–778
Voors AA, von Haehling S, Anker SD, Hillege HL, Struck J, Hartmann O et al (2009) C-terminal provasopressin (copeptin) is a strong prognostic marker in patients with heart failure after an acute myocardial infarction: results from the OPTIMAAL study. Eur Heart J 30:1187–1194
De Marchis GM, Katan M, Weck A et al (2013) Copeptin adds prognostic information after ischemic stroke results from the CoRisk study. Neurology 80:1278–1286
Dong X, Tao DB, Wang YX et al (2013) Plasma copeptin levels in Chinese patients with acute ischemic stroke: a preliminary study. Neurol Sci 34:1591–1595
Zweifel C, Katan M, Schuetz P, Siegemund M, Morgenthaler NG, Merlo A et al (2010) Copeptin is associated with mortality and outcome in patients with acute intracerebral hemorrhage. BMC Neurol 10:34
Dong XQ, Huang M, Yu WH et al (2011) Change in plasma copeptin level after acute spontaneous basal ganglia hemorrhage. Peptides 32:253–257
Kothari RU, Brott T, Broderick JP, Barsan WG, Sauerbeck LR, Zuccarello M et al (1996) The ABCs of measuring intracerebral hemorrhage volumes. Stroke 27:1304–1305
Goldstein LB, Samsa GP, Matchar DB, Horner RD (2004) Charlson index comorbidity adjustment for ischemic stroke outcome studies. Stroke 35:1941–1945
Gill MR, Reiley DG, Green SM (2004) Interrater reliability of Glasgow Coma Scale scores in the emergency department. Ann Emerg Med 43:215–223
Bonita RBR (1988) Modification of rankin scale: recovery of motor function after stroke. Stroke 19:1497–1500
Morgenthaler NG, Struck J, Alonso C, Bergmann A (2006) Assay for the measurement of copeptin, a stable peptide derived from the precursor of vasopressin. Clin Chem 52:112–119
Zhang JL, Yin CH, Zhang Y et al (2013) Plasma copeptin and long-term outcomes in acute ischemic stroke. Acta Neurol Scand 128:372–380
Fenske W, Stork S, Blechschmidt A, Maier SG, Morgenthaler NG, Allolio B (2009) Copeptin in the differential diagnosis of hyponatremia. J Clin Endocrinol Metab 94:123–129
Katan M, Fluri F, Morgenthaler NG, Schuetz P, Zweifel C, Bingisser R, Muller K, Meckel S, Gass A, Kappos L et al (2009) Copeptin: a novel, independent prognostic marker in patients with ischemic stroke. Ann Neurol 66:799–808
Zhang A, Li J, Li X et al (2013) The prognostic value of copeptin for acute intracerebral hemorrhage patients. Exp Ther Med 5:467–470
Urwyler SA, Schuetz P, Fluri F, Morgenthaler NG, Zweifel C, Bergmann A, Bingisser R, Kappos LB, Steck AB, Engelter SB, Müller BF, Christ-Crain M, Katan M (2010) Prognostic value of copeptin: one-year outcome in patients with acute stroke. Stroke 41:1564–1567
Zhu XD, Chen JS, Zhou F et al (2011) Detection of copeptin in peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Crit Care 15:R288
Delgado P, Alvarez-Sabin J, Abilleira S, Santamarina E, Purroy F, Arenillas JF, Molina CA, Fernandez-Cadenas I, Rosell A, Montaner J (2006) Plasma d-dimer predicts poor outcome after acute intracerebral hemorrhage. Neurology 67:94–98
Castillo J, Davalos A, Alvarez-Sabin J, Pumar JM, Leira R, Silva Y, Montaner J, Kase CS (2002) Molecular signatures of brain injury after intracerebral hemorrhage. Neurology 58:624–629
Delgado P, Alvarez Sabin J, Santamarina E, Molina CA, Quintana M, Rosell A, Montaner J (2006) Plasma S100B level after acute spontaneous intracerebral hemorrhage. Stroke 37:2837–2839
Gebel JM Jr, Jauch EC, Brott TG, Khoury J, Sauerbeck L, Salisbury S, Spilker J, Tomsick TA, Duldner J, Broderick JP (2002) Relative edema volume is a predictor of outcome in patients with hyperacute spontaneous intracerebral hemorrhage. Stroke 33:2636–2641
Molnar AH, Varga C, Berko A, Rojik I, Parducz A, Laszlo F, Laszlo FA (2008) Inhibitory effect of vasopressin receptor antagonist OPC-31260 on experimental brain oedema induced by global cerebral ischaemia. Acta Neurochir (Wien) 150:265–271
Delgado TJ, Arbab MAR, Warberg J, Svendgaard NA (1988) The role of vasopressin in acute cerebral vasospasm. J Neurosurg 68:266–273
Shuaib A, Wang CX, Yang T, Noor R (2002) Effects of nonpeptide V1 vasopressin receptor antagonist SR-49059 on infarction volume and recovery of function in a focal embolic stroke model. Stroke 33:3033–3037
Kozniewska E, Gadamski R, Klapczynska K, Wojda R, Rafalowska J (2008) Morphological changes in the brain during experimental hyponatremia. Do vasopressin and gender matter? Folia Neuropath 46:165–170
Kozniewska E, Romaniuk K (2008) Vasopressin in vascular regulation and water homeostasis in the brain. J Physiol Pharmacol 59(Suppl 8):109–116
Acknowledgments
All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, ZJ., Ou, YQ., Li, X. et al. The 90-day prognostic value of copeptin in acute intracerebral hemorrhage. Neurol Sci 35, 1673–1679 (2014). https://doi.org/10.1007/s10072-014-1809-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-014-1809-2