Abstract
Background
Intracranial hemorrhages is one of the major causes of mortality and morbidity worldwide, and there is still no effective biomarker to predict prognosis.
Aim
We aimed to determine the effectiveness of high sensitive troponin I (hs-cTn-I) levels to predict the prognosis of spontaneous intracerebral hemorrhage (sICH) by comparing Glasgow Coma Score (GCS) and hematoma volume with hs-cTn-I levels.
Methods
This study was planned as a retrospective observational study. Patients with available data, over 18 years old and sICH were included in the study. Cerebral computed tomography images were evaluated by a senior radiologist. Hematoma volume was calculated using the ABC/2 formula.
Results
The study comprised 206 individuals in total 78 (37.86%) women and 128 (62.13%) men. Forty-four (21.35%) of patients died. The sensitivity of GCS, hs-cTn-I, and hematoma volume values were 86.36%, 66.67%, and 59.46%, respectively, with corresponding specificities of 78.75%, 93.02%, and 87.58%. Patients with hs-cTn-I values over 26, GCS values of ≤ 9, and hematoma volume values above 44.16 were found to have higher risk of mortality (p = 0.011; p < 0.001; p < 0.001, respectively). The mortality rates were found to be increased 2.586 (IQR: 1.224–5.463) times in patients with hs-cTn-I values above 26, 0.045 times (IQR: 0.018–0.115) in patients with GCS values ≤ 9, and 7.526 times (IQR: 3.518–16.100) in patients with hematoma volume values above 44.16.
Conclusions
Our findings suggest that hs-cTn-I values exceeding 26 units may serve as effective biochemical markers for predicting the prognosis of patients with sICH.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acute cerebrovascular accident is the second leading cause of death worldwide, with intracranial hemorrhages (ICH) comprising 10% to 15% of all stroke cases [1,2,3]. Mortality rates reaching as high as 30–40% from ICH and the severe morbidity observed in recovered individuals make this condition a significant public health concern. Therefore, prompt diagnosis, timely initiation of necessary treatments, and correct assessment of these patients prognosis are crucial.
Tests for high sensitive troponins (hs-cTn) have become increasingly common over the past ten years. These kits are primarily used to diagnose acute myocardial infarction (AMI), as they can measure troponin concentrations that are 10 to 100 times lower than regular conventional, prior methods [4]. However, it's important to note that elevated hs-cTn levels may also occur in heart muscle disorders like atrial fibrillation with rapid ventricular response, congestive heart failure, and cardiomyopathy, as well as in non-cardiac conditions such as pulmonary embolism, severe sepsis, chronic renal failure, and acute ischemic stroke [5,6,7]. Many theories have been proposed regarding this rise in troponin levels in stroke victims, although the exact cause still remains unclear. A well recognized theory suggests that acute cerebral lesions increase intracranial pressure, which in turn stimulates the sympathoadrenal system. It is hypothesized that the subsequent myocyte degeneration (myocytolysis) triggers a release of catecholamines, thereby elevating serum troponin levels [8, 9].
Prognosis of hemorrhagic stroke patients remains a challenge to estimate using biochemical screening, and a viable biomarker has yet to be discovered. In this study, we compared high sensitive troponin-I (hs-cTn-I) levels with two established prognostic indicators Glasgow Coma Score (GCS) and hematoma volume in spontaneous intracerebral hemorrhage (sICH) patients, to better understand the role that hs-cTn-I plays in the prediction of the prognosis of this condition.
Materials and methods
Study design
After the ethics committee approval (Health Science University, Adana City Research and Training Hospital, Ethics Committee, Meeting Number:80, Decision Number:1398, Date: 06/May/2021). We included patients diagnosed with sICH in the emergency department between 1 Jan 2020 and 31 Dec 2021 in this retrospective observational study. Patient data was obtained from hospital automation system.
Cerebral computed tomography images were evaluated by a senior radiologist. Hematoma volume was calculated using the ABC/2 formula. In this formula, A is the greatest hemorrhage diameter by CT, B is the diameter 90 degrees to A, and C is the approximate number of CT slices with hemorrhage multiplied by the slice thickness. Systolic blood pressure (SBP), diastolic blood pressure (DBP), demographic data, Glasgow Coma Scale, prothrombin time (PT) international normalized ratio (INR), activated partial thromboplastin time (APTT), hs-cTn-I levels were noted.
Over 18 years old and patients with avaible data were included in the study. Patients with ishcemic stroke, traumatic intracranial hemorrhages, mass, and lesions apart from ICH were excluded. We also excluded patients under 18 years old, patients with missed data, patients diagnosed with acute coronary syndrome, patients who arrested in or outside the hospital without diagnosis, patients with oncologic and rheumatologic diagnosis and patients who underwent chemotherapy/radiotherapy.
Laboratory analyses
PTZ, INR, APTT, and hs-cTn-I levels were noted. Blood coagulation parameters were measured Sysmex Corporation (1–5-1 Wakinohama -Kaigandori, Chuo-ku, Kobe 651–0073, Japan) device. Hs-cTn-I levels were measured Beckman Coulter Dxl 800 (Beckman Coulter, Inc. 250 S KraemerBlvd. Brea, CA 92821, USA) device.
Statistical analysis
The statistical analysis of the collected data was conducted using the SPSS (Statistical Package for the Social Sciences) 25.0 package software. Categorical measurements were summarized using numbers and percentages, while continuous data were summarized using mean and standard deviation, with minimum–maximum and median values provided when necessary categorical terms were compared using the chi-square test. Shapiro–Wilk test was employed to determine whether the study's parameters had a normal distribution. Mann Whitney-U test was used for parameters that did not follow a normal distribution. Sensitivity and specificity values of hs-cTn-I, GCS and hematoma volume were calculated based on the patients' mortality factor, and the cut-off value was determined through analysis of the area under the ROC curve. The statistical significance level was set as 0.05 for all tests.
Results
The study comprised 206 individuals in total 78 (37.86%) women and 128 (62.13%) men. Forty-four (21.35%) of patients died. Table 1 presents the patient characteristics categorized according to mortality status.
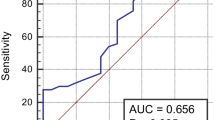
Table 2 shows the results of the diagnostic tests conducted for hematoma volume, hs-cTn-I, and GCS levels that were evaluated in relation to the study's mortality variable. With a sensitivity of 86.36%, the GCS score was shown to have the best diagnostic test performance. This was followed by a hs-cTn-I with the value of 0.852. In addition, the sensitivity of the hematoma volume value was 59.46%; the sensitivity of the hs-cTn-I value was 66.67%; and the sensitivity of the GCS value was found to be 86.36% (Fig. 1).
Table 3 shows the relevant parameters according to the cut-off values found in Table 2 for hs-cTn-I, GCS, and hematoma volume. Of all the patients, 42 (20.4%) had hs-cTn-I values greater than 26, 74 (35.9%) had a GCS values of 9 or lower, and 41 (19.9%) had hematoma volume values greater than 44.16.
Patients with hs-cTn-I values over 26, GCS values of 9 and lower, and Hematoma volume values above 44.16 were found to have higher risk of mortality (p = 0.011; p < 0.001; p < 0.001, respectively). Patients with hs-cTn-I levels above 26 had 2.586 times increased risk of mortality (IQR: 1.224–5.463), those with GCS scores of 9 or lower had 0.045 times increased risk of mortality (IQR: 0.018–0.115), and individuals with hematoma volumes above 44.16 had a 7.526 times increased risk of mortality (IQR: 3.518 -16.100) (Table 4).
There was no significant difference between the patients' GCS values (mild, moderate, severe) and hs-cTn-I levels (p = 0.051), however a significant difference was found between hematoma volume values and GCS groups (p < 0.001). The mean hematoma volume values of patients with mild GCS values were found to be higher than those of patients with moderate and severe GCS levels (p = 0.023; p < 0.001, respectively), as determined by the Post Hoc Bonferroni test used to explore the differences between the groups (Table 5).
Discussion
While the prognosis for sICH can be predicted by a number of etiological factors and imaging findings, including the patient's age, gender, history of hypertension, use of anticoagulants, GCS, hematoma volume, and early hematoma growth, a biochemical marker that can fully estimate the prognosis has not yet been discovered. The purpose of this study was to evaluate the prognostic role of hs-cTn-I in sICH by comparing hs-cTn-I levels with other prognostic variables that have been proved effective in numerous studies, such as GCS and hematoma volume, alongside a number of etiological factors that are useful for prognosis estimation. In our study, the sensitivity of GCS, hs-cTn-I, and hematoma volume values were 86.36%, 66.67%, and 59.46%, respectively, with corresponding specificities of 78.75%, 93.02%, and 87.58%. We also observed that the rate of hematoma volume values exceeding 44.16 and the hs-cTn-I values exceeding 26 were significantly high in patients with mortality, as were the GCS values of 9 and below. The mortality rates were found to be increased 2.586 (IQR: 1.224–5.463) times in patients with hs-cTn-I values above 26, 0.045 times (IQR: 0.018–0.115) in patients with GCS values 9 or less, and 7.526 times (IQR: 3.518–16,100) in patients with hematoma volume values above 44.16. Based on these findings, we believe that hs-cTn-I values can be considered as a valuable biochemical diagnostic marker to determine the prognosis of sICH.
Age is a significant clinical predictor of early mortality in ICH, as indicated in previous studies [10, 11]. Anticoagulant use and a history of hypertension also contribute to higher rates of morbidity and mortality [12]. Additionally, male gender has been identified as another risk factor [13, 14]. In-hospital mortality for individuals between the ages of 18 and 65 was found to be relatively low (14.9%) in the study carried out by Bernardo et al. [15]. Celikbilek et al. observed a higher mortality rate among patients older than 65, supported by a 2021 study which proposed that the average age in the mortality group was considerably higher [14, 16]. Biffi et al.'s study demonstrated that poor outcomes in warfarin-related bleeding were associated with the dose-dependent response of the INR levels [17]. Two separate studies found that individuals using warfarin were at a higher risk of developing ICH, while Zubkov et al. found no such association [18,19,20]. Ariesen et al. and Celikbilek et al. found male gender to be a risk factor, while Sturgeon et al. and Efstathiou et al. observed no relation between ICH and patients' gender [13, 14, 21, 22]. The study by Muresan et al. found no statistically significant difference in the age, gender, and history of hypertension between patients who died and those who survived, contrary to results of the study of Al-Khaled et al. where the mortality rates were higher in the patients with older age, male gender, history of hypertension, and patients using oral anticoagulants [23, 24]. According to results of our research, patients who died had significantly higher INR values; age, gender, and blood pressure readings were not statistically different in the mortality group, and the overall mortality rate was 21.35%.
Hematoma volume emerges as an important factor for predicting mortality and morbidity of patients with ICH. In Hedge et al.'s study a total of 61 (33.33%) patients had hematoma volumes greater than 30, and 57% of these patients died; these patients with hematoma volumes greater than 30 had considerably higher mortality rates compared to patients with hematoma volumes less than 30 [25]. A number of other studies revealed similar findings [16, 26,27,28]. Meanwhile, there was no statistically meaningful difference in the mortality rates within the patient group with hematoma volume more than 30 ml in the research of Qin et al. [29]. In our study, we observed that patients with a hematoma volume greater than 44.16 had a significantly higher mortality rate, with an average increase of 7.526 times.
Several previous studies have found that low GCS results at admission are independent predictors for mortality [27, 30,31,32]. Bhatia et al. and Ironside et al. found that poor GCS values, high hemorrhage volume, and intraventricular bleeding are all independent predictors of increased mortality risk [27, 28]. Patients with a GCS value of less than 8 and a bleeding volume greater than 70 cm3 had an 88% mortality risk, according to the Dolgun et al. [26]. Again, Hedge et al. stated 73% of patients with a GCS < 8 died within three months in their study [25] Similar to previous research, individuals with a GCS of less than 9 had significantly higher mortality rates in our study.
One biomarker that is crucial for the diagnosis of myocardial infarction is troponin. In addition to being used in the diagnosis of MI, hs-Troponins have recently gained significance in predicting the outcome of various other diseases. Regarding the ICH research, Garrett and He noted that elevated cardiac troponin levels are predictive of death in ICH patients, however Qin et al.'s more recent studies did not find a similar, substantial correlation between troponin and hematoma volume, death, or GCS [28, 32, 33]. Still, the same study suggested that hematoma volumes greater than 30 ml are one of the biggest risk factors for myocardial enzyme abnormalities following ICH, with poor GCS also being one of these risk factors [29, 33, 34]. Again, Troponin was linked to increased mortality rates and a poor prognosis in both ischemic stroke and sICH, according to a study by Alkhachroum et al. that evaluated 1655 stroke patients [35]. Our study shows meaningful correlations between hematoma volume, GCS ratios, mortality rates and hs-cTn-I levels surpassing the 26-point cutoff.
In conclusion, our findings suggest that troponin values exceeding 26 units may serve as effective biochemical markers for predicting the prognosis of patients with sICH.
Data availability
Data and materials are reachable from hospital automation information systems.
References
WHO The top 10 causes of death. 9 December 2020. https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death
Van Asch CJ, Luitse MJ, Rinkel GJ et al (2010) Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9:167–176. https://doi.org/10.1016/S1474-4422(09)70340-0
Tsao CW, Aday AW, Almarzooq ZI and others (2022) Heart disease and stroke statistics–2022 update: a report From the American Heart Association. Circulation 145:e153–e639. https://doi.org/10.1161/CIR.0000000000001052
Garg P, Morris P, Fazlanie AL and others (2017) Cardiac biomarkers of acute coronary syndrome: from history to high-sensitivity cardiac troponin. Intern Emerg Med 12(2):147–155. https://doi.org/10.1007/s11739-017-1612-1
Latini R, Masson S, Anand IS and others (2007) Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 116(11):1242–1249. https://doi.org/10.1161/CIRCULATIONAHA.106.655076
Rosjo H, Varpula M, Hagve TA and others (2011) Circulating high sensitivity troponin T in severe sepsis and septic shock: distribution, associated factors, and relation to outcome. Intensive Care Med 37(1):77–85. https://doi.org/10.1007/s00134-010-2051-x
Aksu A, Avci A, Yolcu S and others (2023) The relationship between infarct volume and high sensitivity troponin I level in patients diagnosed with ischemic stroke. Ir J Med Sci 192(2):901–906. https://doi.org/10.1007/s11845-022-03048-0
Palma JA, Benarroch EE (2014) Neural control of the heart: recent concepts and clinical correlations. Neurology 83:261–271. https://doi.org/10.1212/WNL.0000000000000605
Beissner F, Meissner K, Bar KJ, Napadow V (2013) The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. J Neurosci 33:10503–10511. https://doi.org/10.1523/JNEUROSCI.1103-13.2013
Pinho J, Costa AS, Araujo JM et al (2019) Intracerebral hemorrhage outcome: A comprehensive update. J Neurol Sci 398:54–66. https://doi.org/10.1016/j.jns.2019.01.013
Trifan G, Testai FD (2020) Systemic Immune-Inflammation (SII) index predicts poor outcome after spontaneous supratentorial intracerebral hemorrhage. J Stroke Cerebrovasc Dis 29(9):105057. https://doi.org/10.1016/j.jstrokecerebrovasdis.2020.105057
Greenberg SM, Ziai WC, Cordonnier C and others (2022) Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 53:00–00. https://doi.org/10.1161/STR.0000000000000407
Ariesen MJ, Claus SP, Rinkel GJE, Algra A (2003) Risk factors for intracerebral hemorrhage in the general population: A systematic review. Stroke 34(8):2060–2065. https://doi.org/10.1161/01.STR.0000080678.09344.8D
Celikbilek A, Goksel BK, Zararsiz G, Benli S (2013) Spontaneous intra-cerebral hemorrhage: A retrospective study of risk factors and outcome in a Turkish population. J Neurosci Rural Pract 40(03):271–277. https://doi.org/10.4103/0976-3147.118770
Bernardo F, Rebordao L, Machado S et al (2019) In-hospital and long-term prognosis after spontaneous intracerebral hemorrhage among young adults aged 18–65 years. J Stroke Cerebrovasc Dis: Off J Nat Stroke Assoc 28(11):104350. https://doi.org/10.1016/j.jstrokecerebrovasdis.2019.104350
Aydın IE, Yıldırım Ç, Savrun ST et al (2021) The Effect of Hemorrhage Volume on Mortality in Spontaneous Intracerebral Hemorrhages. Van Tıp Derg 28(3):389–392. https://doi.org/10.5505/vtd.2021.81084
Biffi A, Battey TW, Ayres AM and others (2011) Warfarin-related intraventricular hemorrhage: imaging and outcome. Neurology 77:1840–1846. https://doi.org/10.1212/WNL.0b013e3182377e12
Lee SH, Ryu WS, Roh JK (2009) Cerebral microbleeds are a risk factor for warfarin-related intracerebral hemorrhage. Neurology 72:171–176. https://doi.org/10.1212/01.wnl.0000339060.11702.dd
Lovelock CE, Cordonnier C, Naka H and others (2010) Edinburgh Stroke Study Group. Antithrombotic drug use, cerebral microbleeds, and intracerebral hemorrhage: a systematic review of published and unpublished studies. Stroke 41:1222–1228. https://doi.org/10.1161/STROKEAHA.109.572594
Zubkov AY, Mandrekar JN, Claassen DO et al (2008) Predictors of outcome in warfarin-related intracerebral hemorrhage. Arch Neurol 65:1320–1325. https://doi.org/10.1001/archneur.65.10.1320
Delcourt C, Sato S, Zhang S and others (2017) Intracerebral hemorrhage location and outcome among INTERACT2 participants. Neurology 88(15):1408–1414. https://doi.org/10.1212/WNL.0000000000003771
Takeuchi S, Wada K, Nagatani K et al (2013) Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurg Focus 34(5):E5. https://doi.org/10.3171/2013.2.FOCUS12424
Al-Khaled M, Awwad S, Bruning T (2020) Nontraumatic spontaneous intracerebral hemorrhage: Baseline characteristics and early outcomes. Brain Behav 10(1):e01512. https://doi.org/10.1002/brb3.1512
Muresan EM, Golea A, Vesa SC et al (2022) Admission emergency department point-of-care biomarkers for prediction of early mortality in spontaneous ıntracerebral hemorrhage. In Vivo 36:1534–1543. https://doi.org/10.21873/invivo.12864
Hegde A, Menon G (2018) Modifying the intracerebral hemorrhage score to suit the needs of the developing world. Ann Indian Acad Neurol 21(4):270–274. https://doi.org/10.4103/aian.AIAN_419_17
Dolgun H, Hanalioglu S, Gurses L et al (2020) Surgical treatment of spontaneous ıntracerebral hematomas. ACU Sağlık Bil Derg 11(3):439–446. https://doi.org/10.31067/0.2020.293
Ironside N, Chen CJ, Dreyer V et al (2020) Location-specific differences in hematoma volume predict outcomes in patients with spontaneous intracerebral hemorrhage. Int J Stroke 15(1):90–102. https://doi.org/10.1177/1747493019830589
Bhatia R, Singh H, Singh S and others (2013) A prospective study of in-hospital mortality and discharge outcome in spontaneous intracerebral hemorrhage. Neurol India 61(3):244–248. https://doi.org/10.4103/0028-3886.115062
Qin G, Dai C, Feng S, Wu G (2022) Changes of electrocardiogram and myocardial enzymes in patients with ıntracerebral hemorrhage. Hindawi Dis Markers 9309444. https://doi.org/10.1155/2022/9309444
Cheung RTF, Zou LY (2003) Use of the original, modified, or new intracerebral hemorrhage score to predict mortality and morbidity after intracerebral hemorrhage. Stroke 34(7):1717–1722. https://doi.org/10.1161/01.STR.0000078657.22835.B9
Hemphill JC 3rd, Bonovich DC, Besmertis L et al (2001) The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 32:891–897. https://doi.org/10.1161/01.str.32.4.891
Broderick JP, Brott TG, Duldner JE et al (1993) Volume of intracerebral hemorrhage: A powerful and easy-to-use predictor of 30-day mortality. Stroke 24(7):987–993. https://doi.org/10.1161/01.str.24.7.987
Garrett MC, Komotar RJ, Starke RM et al (2010) Elevated troponin levels are predictive of mortality in surgical intracerebral hemorrhage patients. Neurocrit Care 12:199–203. https://doi.org/10.1007/s12028-009-9245-5
He Y, Liu Q, Wang J et al (2020) Prognostic value of elevated cardiac troponin I in patients with intracerebral hemorrhage. Clin Cardiol 43:338–345. https://doi.org/10.1002/clc.23320
Alkhachroum AM, Miller B, Chami T et al (2019) A troponin study on patients with ischemic stroke, intracerebral hemorrhage and subarachnoid hemorrhage: Type II myocardial infarction is significantly associated with stroke severity, discharge disposition and mortality. J Clin Neurosci 64:83–88. https://doi.org/10.1016/j.jocn.2019.04.005
Acknowledgements
There is no person, instution or company to acknowledgement.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Dr. Ulger Huseyin, Dr. Icme Ferhat, Dr. Avci Akkan, Dr. Avci Begum Seyda and Dr Parlatan Cenk: conceptualization, methodology, investigation, and writing – original draft. Dr. Icme Ferhat, Dr. Avci Akkan and Dr. Aksay Erdem: resources, formal analysis, and writing – review and editing. Dr. Icme Ferhat, Dr. Ulger Huseyin: conceptualization, methodology, and writing – review and editing. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethical approval
The ethics committee of the Adana City Training and Research Hospital approved the study.
The study was performed according to the recommendations set by the The Declaration of Helsinki on Medical Research involving Human Subjects.
Informed consent
Written informed consent was not necessary because no patient data has been included in the manuscript.
Conflict of interest
The authors have no conflicts of interests to declare.
Human rights
This manuscript was carried out in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Any part of this paper is not under consideration for publishing or published in anywhere else.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ulger, H., Icme, F., Parlatan, C. et al. Prognostic relationship between high sensitivity troponin I level, hematoma volume and glasgow coma score in patients diagnosed with spontaneous intracerebral hemorrhage. Ir J Med Sci (2024). https://doi.org/10.1007/s11845-024-03737-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11845-024-03737-y