Abstract
This study aims to investigate the mechanism of electroacupuncture (EA) in promoting behavioral recovery after focal cerebral ischemia/reperfusion. The SD rats received filament occlusion of the right middle cerebral artery for 2 h followed by reperfusion for 1, 3, 7, 14, and 21 days, respectively. Rats were randomly divided into sham group, model group and EA group. After 2 h of the reperfusion, EA was given at bilateral “Hegu” point (LI 4) in the EA group. Neurobehavioral evaluation, the expression of stem cell factor (SCF), its receptor c-kit and matrix metalloproteinase-9 (MMP-9) protein and mRNA in the cortical ischemic region were measured. EA treatment can improve behavioral recovery after ischemia/reperfusion. Compared with the sham group, the positive cells and mRNA expression of SCF, c-kit, MMP-9, the protein expression of SCF were increased significantly in the model and EA groups (P < 0.001). Compared with the model group, the positive cells, protein and mRNA expression of SCF were increased significantly in EA groups (P < 0.01). The positive cells and mRNA expression of c-kit were increased in EA groups beginning at 3 day and remained significantly high thereafter. The expression of MMP-9 positive cells and mRNA were deceased significantly in the 1 day subgroup in EA (P < 0.01), but increased significantly in the 3, 7 days subgroups (P < 0.01). We conclude that EA treatment up-regulates the positive cells and mRNA expression of SCF, c-kit and MMP-9 after cerebral ischemia/reperfusion. EA may promote neurobehavioral recovery by increasing the protein and mRNA expression of SCF, c-kit and MMP-9 after cerebral ischemia/reperfusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cerebral ischemia has been one of the common diseases leading to both high deformity and mortality. Researches focused on the cure and prevention of cerebral ischemia has never been stopped. Electroacupuncture, as a tradition non-medicine therapy, plays a definite role in improving cerebral ischemia recovery and is widely used in clinic. However, the exact mechanism is unclear [1]. Recent research has indicated that endothelial progenitor cells (EPCs) participate in not only vasculogenesis of embryo stage but also vascularization through self-mobilization, migration and differentiation after birth [2]. EPCs can also promote neuranagenesis and angiogenesis after cerebral ischemia [3, 4].
Traditional Chinese medicine (TCM) states that EA can make brain clear, dredge channel-qi (life energy) and opsonize meridians. EA can improve physical movement in patients with stroke, increase the microvascular number around infracts area and decrease cerebral edema and inflammation induced brain damage. Our previous study demonstrated that EA can not only up-regulate the angiogenesis factors, such as VEGF, Ang-1, FDGF and its receptor Fit-1, but also down-regulate the inhibiting factors: Endostatin et al., which both facilitated neovascularization [5, 6]. In recent years, some reports have demonstrated that EA can promote EPCs mobilization in bone marrow (BM) and migration in peripheral blood (PB) [7, 8], which is beneficial to vasculogenesis after ischemia/reperfusion. However, how EA may affect EPCs and promote vasculogenesis has not been well studied.
Stem cell factor (SCF) is a multifunctional cytokines, which has found in recent years. Activated by matrix metalloproteinase-9 (MMP-9), SCF leads to mobilization, migration and homing of EPCs through binding to its receptor c-kit. Many reports have held that SCF/c-kit pathway could promote neuranagenesis after cerebral ischemia [9, 10], however, whether EA improves recovery after ischemia through this pathway remains unclear. In our research, we will make a rat model of focal cerebral ischemia/reperfusion, use point acupuncture as a way of treatment, observe the protein and mRNA expression of SCF and c-kit, probe the function of the SCF/c-kit after cerebral ischemia/reperfusion and discuss the underlying mechanism of EA.
Materials and methods
Animals and experimental groups
All the experimental procedures were approved by the Ethics Committee of Chongqing Medical University and all procedures were in accordance with the National Institutes of Health Guidelines for Animal Research (Guide for the Care and Use of Laboratory Animals). Eighty (250–300 g) Sprague–Dawley rats were randomly assigned to three groups: normal, the model group and EA group. The model group and EA group were further randomly divided into five subgroups receiving reperfusion 1, 3, 7, 14 and 21 days after 2 h ischemia.
Focal cerebral ischemia
Transient focal cerebral ischemia was induced by MCAO as previously described by Longa and Luo et al. [11, 12]. Briefly, rats were fasted for 12 h but were allowed free access to water before surgery. Rats were anesthetized by intraperitoneal injection of 3.5 % chloral hydrate (1 ml/100 g). Rectal temperature was monitored and maintained at 36.5–37.5 °C by surface heating and cooling. The right common carotid artery, internal carotid artery and external carotid artery were surgically exposed. The external carotid artery was isolated and coagulated. A 4–0 nylon suture (Ethicon Nylon Suture; Ethicon Inc, Osaka, Japan) with its tip rounded (diameter: 0.25–0.27 mm; length: 18–22 mm) by heating near a flame was inserted into the internal carotid artery through the external carotid artery stump and gently advanced to occlude the MCA until it met a slight resistance. The average depth that the nylon filament reached was 1.8–2.0 cm. The section was sutured. Reperfusion was accomplished by withdrawing the suture after 2 h of ischemia. After recovery from anesthesia, the rats were returned to their cages with free access to water and food.
Electroacupuncture stimulation
In the EA group, the rats were anesthetized by intraperitoneal injection of 3.5 % chloral hydrate (1 ml/100 g). According to the Experimental Animals Meridians Mapping and described Hua et al. [13], the “Hegu” acupoint (LI4), which was located at the bilateral fore between 1, 2 metacarpal, was stimulated with the intensity of 1 mA (the rats limbs slight tremor) and frequency of 40/60 Hz for 15 min by using the G6805-2 EA Instrument (Beijing Xinsheng Ltd). The depth of inserting needle was 0.4–0.6 cm, the needle gauge was 0.4 cm, and first stimulation was finished at 1 h after reperfusion, once a day and the longest time did not exceed 7 day.
Neurobehavioral evaluation
Neurobehavioral evaluation was carried out in MCAO and EA groups (n = 5 for each). After cerebral ischemia/perfusion was made at different time points, rats were neurologically assessed by an observer who was unaware of animal grouping. The neurological function was scored on a five-point scale reported by Longa et al.: 0, no deficit; 1, failure to extend left forepaw fully; 2, circling to the left; 3, falling to the left; 4, no spontaneous walking with a depressed level of consciousness.
Immunohistochemistry analysis
Immunohistochemistry was performed as previously described by Zhang et al. [14]. Briefly, brains were fixed in 4 % phosphate-buffered paraformaldehyde (PFA) by transcardial perfusion. For paraffin sections, brains were embedded in para-plast and cut into coronal sections 10 μm in thickness on a rotary microtome. Paraffin sections were dewaxed and antigens retrieved by immersing slides in 0.01 mol/L citrate buffer, pH 6.0, heating in a microwave oven (high for 3 min, low for 5 min), cooling to room temperature, and then washing with PBS for 5 min. Endogenous peroxides was blocked in 3 % H2O2 for 15 min, and then incubated for 30 min with 10 % normal goat serum to block non-specific binding. Sections were incubated with anti-SCF antibody (1:100, Santa Cruz), anti-c-kit antibody (1:50, Bioss) and anti-MMP-9 antibody (1:100, Santa Cruz) at 4 °C overnight. Then, the slides were rewarmed for 60 min at room temperature. After washing, slides were incubated with appropriate biotinylated secondary antibody (Zhongshan Biotechnology Company, Beijing) for 30 min at room temperature. And then, slides were incubated in horseradish peroxides (Zhongshan Biotechnology Company, Beijing) for 30 min at room temperature. After washing with PBS, 3,3-diaminobenzidine (Zhongshan Biotechnology Company, Beijing) was added for color development, and sections counterstained with haematoxylin. Brown granules in cells under microscope were defined as positive signals. Negative control was performed by omission of the primary antibodies.
RT-PCR analysis
The rats from each subgroup at each time point were anesthetized with 3.5 % chloral hydrate (1 ml/100 g); their brains were removed immediately, and 50–100 mg tissue around ischemic area was cut down and put into liquid nitrogen. The whole process should avoid pollution by outer mRNA. Total RNA was extract from tissues with TRIzol reagent according to the manufacturer’s instructions (TaKaRa Biotechnology Ltd., Japan). To generate cDNA, reverse transcribe was strictly carried out with reverse transcriptase (TaKaRa Biotechnology Ltd., Japan). Each cDNA sample was then amplified by PCR using the oligonucleotide primers shown in Table 1. For amplification, the following thermocycler program was used: 94 °C for 5 min, 94 °C for 30 s; annealing (for temperature, see Table 1) for 30 s, 72 °C for 30 s, 72 °C for 5 min, repeated for 35 cycles from step 2 to step 4. The amplified products were resolved on ethidium bromide-stained 1.5 % agarose gels. The densities results were analyzed by Quantity One software (Bio-Rad).
Western blot analysis
100 g new Brain tissue was extracted in 0.5 ml ice-cold protein lysis buffer containing 20-mM Tris pH 7.5, 150-mM NaCl, 1 % TritonX-100, 2.5-mM sodium pyrophosphate, 1 mMEDTA, 1 % Na3VO4, 0.5 μg/mL leupeptin, 1 mM PMSF (Beyotime Technologies, Wuhan, China). The protein concentration of the brain tissue was measured by the Bradford method (Beyotime, Technologies, Wuhan, China). Equal amounts (50 μg) of total protein extracts were prepared and mixed with 4× sample buffer. Protein sample (50 μg) were separated on 10 % SDS-PAGE and transferred to a polyvinylidene fluoride membrane. All blots were blocked for 90 min at room temperature with 10 % non-fat dry milk in Tris-buffered saline containing 0.1 % Tween 20 (TBS-T) pH 7.4. After that, the filters were incubated with the primary antibodies (anti-rat IgG made in rabbit for SCF, 1:100, Santa Cruz; anti-rat β-actin made in rabbit, 1:2500, Beijing 4A Biotech Co, Ltd) diluted in blocking buffer at 4 °C overnight. Then, the filters were rewarmed for 30 min at room temperature. After washing with TBS-T, the membrane was incubated with horseradish peroxidase-conjugated secondary antibody (anti-rabbit IgG, 1:4000, Santa Cruz Biotechnology) at room temperature for 90 min. Finally, immunoreactions were visualized by electrochemiluminescence (Beyotime Technologies, Wuhan, China). The densities results were analyzed by Quantity One software (Bio-Rad).
Statistical analysis
Data were analyzed using SPSS 16.0 for Windows statistical analyses software. Normal distribution data were expressed as mean ± SD (standard deviation). Multiple group comparisons were performed by a one-way ANOVA, followed by LSD’s test. Neurobehavioral evaluation was compared by Kruskal–Wallis test followed by the Mann–Whitney U test with Bonferroni correction. Differences were considered statistically significant at P < 0.05.
Results
Neurobehavioral evaluation
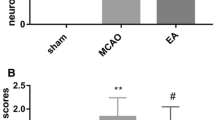
After ischemia, the rats showed neurological deficit behavior at every time point. The neurological deficit score of model group and EA group gradually decreased, but no significant difference was found at 1 day (P = 0.695) and 3 day (P = 0.434) after reperfusion. With time went on, the rats’ neurological function had slightly recovered. The neurological deficit score of EA group was lower compared with model group. Moreover, the difference had a statistic value at 7 day (P = 0.023) and 14 day (P = 0.023) after reperfusion (Fig. 1a), suggesting that EA treatment improves behavioral recovery after ischemia/reperfusion.
At 1, 3 days after reperfusion, the neurological deficit score of model group and EA group gradually decreased, but no significant difference was found (## P > 0.05). At 7, 14 days after reperfusion, the neurological deficit score of EA group was lower compared with model group (**P < 0.05). ## P > 0.05 versus control group at 1 and 3 days after reperfusion; **P < 0.05 versus control group at 7 and 14 days after reperfusion
Expression of SCF protein and mRNA
Semi-quantification of SCF expression was examined by immunohistochemical analysis. The SCF-positive cells were shown in neurons glial cells and ECs, which all located in pre-infarct. SCF immunoreactivity in both model and EA groups was higher than that in sham group (P < 0.001). SCF-positive cells were up-regulated from 1 to 7 days, arrived at the peak at 7 day and remained significantly high for up to 21 day. The SCF-positive cells expression in EA group were higher than that in model group at the same time point and the difference between EA and model groups had a statistic value at 1 day (P = 0.0080), 3 day (P < 0.0001), 7 day (P = 0.0010), 14 day (P = 0.0060) and 21 day (P < 0.0001) (Fig. 2a, b).
Immunohistochemical staining for SCF-positive cell expression in the penumbral cortex at 1, 3, 7, 14 and 21 days after reperfusion. The photomicrographs showed SCF expression in the penumbral cortex of rats that received sham, MCAO and MCAO+EA. Graph (a) shows the representative results of SCF expression in the sham group, MCAO at 7 day and MCAO+EA group at 7 day, respectively. Graph (b) shows the quantification of SCF expression in the penumbral cortex in all groups. Scale bar = 25 µm. # P < 0.01 versus sham group; *P < 0.01 versus model group
The Western blot analysis was carried out to examine the SCF protein. In model group, SCF expression began to increase at 3 day, reached the peak at 7 day but decreased at 14 day after reperfusion. In EA group the SCF protein expression was higher than that in model group at the same time point, the difference between EA and model groups had a statistic value at 3 day (P < 0.001), 7 day (P < 0.001) and 14 day ((P = 0.0080) (Fig. 3a, b). And the RT-PCR analysis was conformed to Western blot, the SCF mRNA expression was higher than that in model group at the same time point, and the difference between EA and model groups had a statistic value at 3 day (P < 0.0001), 7 day (P < 0.0001) and 14 day (P < 0.0001) (Fig. 4a, b).
Cerebral tissue homogenates were obtained from the ischemic hemisphere at 3, 7 and 14 days after reperfusion. The expression of SCF proteins in different groups was measured by Western blot analysis. Graph (a) shows the representative results of Western blot analysis that received sham, MCAO and MCAO+EA at 3, 7 and 14 days after reperfusion. Graph (b) shows the quantification of SCF protein expression in all groups. Values in graph (b) are expressed as relative density of SCF and presented as mean ± SD (n = 5). # P < 0.01 versus sham group; *P < 0.01 versus model group
Cerebral tissue homogenates were obtained from the ischemic hemisphere at 3, 7 and 14 days after reperfusion. The expression of SCF mRNA in different groups was measured by RT-PCR analysis. Graph (a) shows the representative results of RT-PCR analysis that received sham, MCAO and MCAO+EA at 3, 7 and 14 days after reperfusion. Graph (b) shows the quantification of SCF mRNA expression in all groups. Values in the graph (b) are expressed as relative density of SCF and presented as mean ± SD (n = 5). # P < 0.01 versus sham group; *P < 0.01 versus model group.
Expression of c-kit protein and mRNA
The immunohistochemical analysis was performed to identify the expression of c-kit. The c-kit immunoreactivity was examined in cortex, lateral ventricle, ECs neurons and glial cells, which located in pre-infarct. The expression of c-kit in model and EA groups was up-regulated compared with that in sham group (P < 0.0001). The c-kit expression in model group increased from 1 to 7 days, and reached the peak at 7 day then began to decrease. In EA group the c-kit expression was higher than that in model group at the same time point, and the difference between EA and model groups had a statistic value at 3 day (P = 0.010), 7 day (P = 0.014), at 14 day (P = 0.018) and 21 day (P = 0.037) except 1 day after reperfusion (P = 0.313) (Fig. 5a, b).
Immunohistochemical staining for c-kit-positive cell expression in the penumbral cortex at 1, 3, 7, 14 and 21 days after reperfusion. The photomicrographs show c-kit expression in the penumbral cortex of rats that received sham, MCAO and MCAO+EA. Graph (a) shows the representative results of c-kit expression in the sham group, MCAO at 7 days and MCAO+EA group at 7 days, respectively. Graph (b) shows the quantification of c-kit expression in the penumbral cortex in all groups. Scale bar = 25 µm. # P < 0.01 versus sham group; *P < 0.01 versus model group
The RT-PCR analysis was performed to examine the expression of c-kit mRNA at 3, 7, 14 days after reperfusion. The expression of c-kit mRNA was conformed to immunohistochemical; the peak was still at 7 day. In EA group, the c-kit mRNA expression was higher than that in model group at the same time point, the difference between EA and model groups had a statistic value at 3 day (P = 0.0010), 7 day (P = 0.012), at 14 day (P = 0.0020) (Fig. 6a, b).
Cerebral tissue homogenates were obtained from the ischemic hemisphere at 3, 7 and 14 days after reperfusion. The expression of c-kit mRNA in different groups was measured by RT-PCR analysis. Graph (a) shows the representative results of RT-PCR analysis that received sham, MCAO and MCAO+EA at 3, 7 and 14 days after reperfusion. Graph (b) shows the quantification of c-kit mRNA expression in all groups. Values in graph (b) are expressed as relative density of c-kit and presented as mean ± SD (n = 5). # P < 0.01 versus sham group; *P < 0.01 versus model group
Expression of MMP-9 protein and mRNA
Immunohistochemical technique was applied to investigate the expression of MMP-9-immunoreactive cells in the peri-infarct area. The expression of MMP-9-positive cells kept on a low level in sham group. After cerebral ischemia, MMP-9-positive cells were not only found in neurons and ECs but also in microglia and glial cells around ischemia cortex. In model group, MMP-9 expression increased at 1 day and reached the peak at 3 day but decreased at 7 day after reperfusion. In EA group the MMP-9 expression was lower than that in model group at 1 day (P < 0.0001), while higher at 3 day (P < 0.0001) and 7 day (P = 0.010) after reperfusion (Fig. 7a, b).
Immunohistochemical staining for MMP-9-positive cell expression in the penumbral cortex at 1, 3 and 7 days after reperfusion. The photomicrographs show MMP-9 expression in the penumbral cortex of rats that received sham, MCAO and MCAO+EA. Graph (a) shows the representative results of MMP-9 expression in sham group, MCAO at 3 days and MCAO+EA group at 3 days, respectively. Graph (b) shows the quantification of MMP-9 expression in the penumbral cortex in all groups. Scale bar = 25 µm. # P < 0.01 versus sham group; *P < 0.01 versus model group
In order to verify these results at the mRNA level, the RT-PCR analysis was performed. As a result, the changes of MMP-9 mRNA kept pace with immunohistochemical. In EA group the MMP-9 mRNA expression was lower than that in model group at 1 day (P < 0.0001), while higher at 3 day (P < 0.0001) and 7 day (P = 0.0010) after reperfusion (Fig. 8a, b).
Cerebral tissue homogenates were obtained from the ischemic hemisphere at 1, 3 and 7 days after reperfusion. The expression of MMP-9 mRNA in different groups was measured by RT-PCR analysis. Graph (a) representative results of RT-PCR analysis that received sham, MCAO and MCAO+EA at 1, 3 and 7 days after reperfusion. Graph (b) shows the quantification of MMP-9 mRNA expression in all groups. Values in graph (b) are expressed as relative density of c-kit and presented as mean ± SD (n = 5). # P < 0.01 versus sham group; *P < 0.01 versus model group
Discussion
Stem cell factor (SCF) is a hypoxia-inducible cytokine that has been reported to be strongly up-regulated at sites of stroke, myocardial infarction, and artery ligation [9, 15, 16]. Recently, it has been shown that SCF/c-kit binding protects cortical neurons from apoptosis and excitotoxicity in vitro, and that the neuroprotective effect is medicated by MEK/ERK and PI3 K/Akt signal transduction pathways [17]. Jin et al. [9] reported that SCF/c-kit can promote the neurogenesis after cerebral ischemia. So SCF/c-kit is believed to play a significant role after cerebral ischemia.
In this study, SCF expression was up-regulated from 1 to 7 days after reperfusion, arrived at the peak at 7 day and remained significantly high for up to 21 day. This was consistent with previous report, in which SCF expression reached the peak at 7 day after reperfusion, and SCF could promote neuronal stem cells (NSCs) proliferation, migration and neuranagenesis [9, 10]. Similarly, it is known that SCF involves in EPCs mobilization, migration and proliferation in diabetes mellitus [18] and myocardial ischemia [19]. Thus, it is possible that after brain ischemia/reperfusion, SCF may function as a protective mechanism that contributes to injury repair by facilitating EPCs/NSCs proliferation. In addition, by biding to its receptor c-kit presented on the surface of EPCs [20], SCF can stimulate hematopoietic stem cells (HSCs) proliferation and differentiation, motivate the HSCs/EPCs to PB, and promote vasculogenesis [21]. It was demonstrated in our study that c-kit expression began to increase at 3 day after reperfusion, reached the peak at 7 day after reperfusion, which further supports the role of EPCs in development and self-repair after ischemia/reperfusion.
EA treatment improved behavioral recovery and SCF/c-kit expression after cerebral ischemia/reperfusion, demonstrating that EA is effective in ischemia/reperfusion repair. Similar finding was reported in our lab previously. In a rat model of ischemia/reperfusion model, EA stimulation of “hegu” point resulted in the presence of AC133 in ischemia penumbra microvascular and pre-infarction cells in brain tissue [22]. AC133 is one of the surface markers of stem cells. It is not presented in mature vascular endothelial cells, but selectively presented in stem cells and EPCs [23]. Thus EA treatment may facilitate behavioral recovery by facilitating EPC migration.
Our study found that MMP-9 prominently increased after reperfusion. Interestingly, EA treatment decreased MMP-9 expression at 1 day but significantly increased its expression at 3 and 7 days after reperfusion. MMP-9 plays dual role in cerebral ischemia. On one hand it can degrade extracellular matrix (ECMs), destroy the vascular basement membrane and aggravate the inflammation and edema [24]; on the other hand it can promote the EPCs mobilization [25]. SCF has two existing forms: sKitL (soluble kit ligand) and mKitL (membrane bound kit ligand). In the process of EPCs mobilization, the sKitL serves as a key role [26]. With the help of mKitL, EPCs binds to bone marrow stromal Cells. In the presence of MMP-9, mKitL turns into sKitL, which facilitates dissociation of EPCs and stromal. Consequently, more EPCs mobilize to PB from BM [23]. It was found that the mobilization of stem cells was inhibited in MMP-9−/− mice, and MMP-9 inhibitors can decrease the EPCs mobilization. Although the mechanisms underlying the differential regulation of MMP-9 in acute and chronic phases are not known, EA treatment may play protective role in this regard. EA ameliorates brain edema by inhibiting MMP-9 in acute stage, while increases MMP-9 expression in chronic phases which results in facilitated EPCs migration. In supporting of this, Dong et al. [27] reported that EA preconditioning could decrease MMP-9 expression and activity, attenuate brain edema and BBB disruption caused by subsequent cerebral ischemia. After EA stimulation of the “quchi”, “zusanli” points, EPCs in rats blood increased after acute focal cerebral ischemia/reperfusion, this change was concerned with MMP-9 [28].
SCF can facilitate HSCs/EPCs survival, adherence, mobilization and migration [29]. It has found that up-regulation of EPCs can induce neovascularization in ischemia tissue. Matsumoto et al. [30] demonstrated that the inhibitor of Link protein (the inhibitor protein of SCF/c-kit) can promote the SCF/c-kit pathway than induce HSCs/EPCs mobilization, and promote the vasculogenesis and fracture healing. In inflammation, likewise, SCF/c-kit promotes pathological vasculogenesis through EPCs mobilization from BM to PB [31]. Kim et al. [32] demonstrated that SCF/c-Kit signaling directly enhanced the neovascularization activity of EPCs in vivo and in vivo; therefore suggesting that highly up-regulated SCF in ischemic tissues promotes vascular regeneration by directly facilitating EPC-mediated neovascularization as well as providing abundant EPCs at injured tissues. In our study, EA may increase expression of MMP-9 and the binding of SCF to its receptor c-kit, results in the promotion of the EPCs mobilization, migration and homing, which facilitate the vasculogenesis and recovery after cerebral ischemia. At present, our study cannot give enough evidence to demonstrate “whether the IR injury caused the c-kit and MMP-9 or the c-kit and MMP-9 was responsible for the IR injury”. So in our future study, we will make further research to find reliable evidence to illustrate this issue.
Abbreviations
- EPCs:
-
Endothelial progenitor cells
- SCF:
-
Stem cell factor
- MMP-9:
-
Matrix metalloproteinase-9
- EA:
-
Electroacupuncture
- PBS:
-
Phosphate buffer saline
- NSCs:
-
Neuronal stem cells
- HSCs:
-
Hematopoietic stem cells
- SKitL:
-
Soluble kit ligand
- MKitL:
-
Membrane bound kit ligand
- PB:
-
Peripheral blood
- BM:
-
Bone marrow
References
Wang CX, Li ZR, Chen BY (2006) Protective effect of electroacupuncture on cerebral function via ameliorating oxidative stress in MCAO rats. Neurosci Bull 21:153–157
Murayama T, Tepper OM, Silver M et al (2002) Determination of bone marrow-derived endothelial progenitor cell significance in angiogenic growth factor-induced neovascularization in vivo. Exp Hematol 30:967–972
Ohta T, Kikuta K, Imamura H et al (2006) Administration of ex vivo-expanded bone marrow-derived endothelial progenitor cells attenuates focal cerebral ischemia-reperfusion injury in rats. Neurosurgery 59:679–686
Fan Y, Shen F, Frenzel T et al (2010) Endothelial progenitor cell transplantation improves long-term outcome in mice. Ann Neurol 67:488–497
Ma JX, Luo Y (2007) Effect of electroacupuncture on expression of angiogenic growth factors and anti-angiogenic growth factors in the brain tissue of the rat after focal cerebral ischemia reperfusion. Chin Acupunct Moxibustion 27:129–133
Wu L, Luo Y, Zhang SS (2010) Effects of electroacupuncture on mRNA and protein expression of placental growth factor and Flt-1 and promotes revascularization in the brain after focal cerebral ischemia/reperfusion in SD rats. Chin J Arterioscler 18:589–593
Cai SX, Yu WJ, Zhang L et al (2009) Effect of electroacupuncture on plasma endogenous endothelial progenitor cell counts in cerebral ischemia/reperfusion rats. Acupunct Res 34:114–119
Zhang T, Lin T, Wang XZ et al (2009) The influence of electroacupuncture pretreatment to endothelial progenitor cells and vessel endothelial growth factor in marrow and peripheral blood of cerebral ischemia/reperfusion rats. Chin J Rehabil Med 24:428–432
Jin KL, Mao XO, Sun XJ et al (2002) Stem cell factor stimulates neurogenesis in vitro and in vivo. J Clin Invest 110:311–319
Sun LX, Lee J, Fine HA (2004) Neuronally expressed stem cell factor induces neural stem cell migration to areas of brain injury. J Clin Invest 113:1364–1374
Longa EZ, Weinstein PR, Carlson S et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91
Luo Y, Dong WW (2002) An experimentaI study on focaI cerebraI ischemia/reperfusion model in Wistar rats with suture method. J Chongqing Med Univ 27:1–4
Hua XB, Li CR, Zhou HL et al (1991) Development of the atlas of acupoints for rat. Exp Anim and Anim 1:1–5
Zhang HL, Gu ZL, Savitz SI et al (2008) Neuroprotective effects of prostaglandin A1 in rat models of permanent focal cerebral ischemia are associated with nuclear factor-kappaB inhibition and peroxisome proliferators- activated receptor gamma up-regulation. J Neurosci Res 86:1132–1141
Han ZB, Ren H, Zhao H et al (2008) Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF). Carcinogenesis 29:1853–1861
Fazel S, Cimini M, Chen L et al (2006) Cardioprotective ckit + cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest 116:1865–1877
Dhandapani KM, Wade FM, Wakade C et al (2005) Neuroprotection by stem cell factor in rat cortical neurons involves AKT and NFkappaB. J Neurochem 95:9–19
Abu El-Asrar AM, Struyf S, Opdenakker G et al (2010) Expression of stem cell factor/c-kit signaling pathway components in diabetic fibrovascular epiretinal membranes. Mol Vis 16:1098–1107
Kuang D, Zhao X, Xiao GX et al (2008) Stem cell factor/c-kit signaling mediated cardiac stem cell migration via activation of p38 MAPK. Basic Res Cardiol 103:265–273
Asahara T, Murohara T, Sullivan A et al (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 5320:964–967
Takahashi T, Kalka C, Masuda H et al (1999) Ischemia-and cytokine induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5:434–438
Zhang SS, Luo Y, Wu L (2010) Role of phosphoinositide 3-kinase/AKT signaling pathway in promoting angiogenesis in rats with focal cerebral ischemia/reperfusion using electrical acupuncture. Acta Academiae Medicinae Militaris Tertiae 32:2488–2491
Wang YN, Xu JM (2007) Progress on marker of hematopoietic stem cell-ACl33. Chin J New Drugs Clin Rem 26:708–714
Romanic AM, White RF, Arleth AJ et al (1998) Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke 29:1020–1030
Heissig B, Hattori K, Dias S et al (2002) Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell 109:625–637
Huang EJ, Nocka KH, Buck J et al (1992) Differential expression and processing of two cell associated forms of the kit ligand KL-1 and KL-2. Mol Biol Cell 3:349–362
Dong H, Fan YH, Zhang W et al (2009) Repeated electroacupuncture preconditioning attenuates matrix metalloproteinase-9 expression and activity after focal cerebral ischemia in rats. Neurol Res 31:853–858
Zhao Y, Chen SJ, Yu WJ et al (2010) The effect of electroacupuncture on endogenous EPCs and serum cytokines in cerebral ischemia-reperfusion rat. J Biomed Eng 27:1322–1326
Smith MA, Court EL, Smith JG (2001) Stem cell factor: laboratory and clinical aspects. Blood Rev 15:191–197
Matsumoto T, Ii M, Nishimura H et al (2010) Lnk-dependent axis of SCF-cKit signal for osteogenesis in bone fracture healing. J Exp Med 207:2207–2223
Dentelli P, Rosso A, Balsamo A et al (2007) C-kit, by interacting with the membrane-bound ligand, recruits endothelial progenitor cells to inflamed endothelium. Blood 109:4264–4271
Kim KL, Meng Y, Kim JY et al (2011) Direct and differential effects of stem cell factor on the neovascularization activity of endothelial progenitor cells. Cardiovascular Res 92:132–140
Acknowledgments
The research was supported by the Special Fund for Scientific Research of Doctorial Subjects in College and Universities of Ministry of Education (NO:20095503110001) and the Program of the Traditional Chinese Medicine Research of Chongqing Municipal Health Bureau (2005-B-24).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, T., Luo, Y., Sun, H. et al. Electroacupuncture improves behavioral recovery and increases SCF/c-kit expression in a rat model of focal cerebral ischemia/reperfusion. Neurol Sci 34, 487–495 (2013). https://doi.org/10.1007/s10072-012-1081-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-012-1081-2