Abstract
Early developmental environment can have profound effects on individual physiology, behaviour, and learning. In birds and mammals, social isolation during development is known to negatively affect learning ability; yet in other taxa, like reptiles, the effect of social isolation during development on learning ability is unknown. We investigated how social environment affects learning ability in the family-living tree skink (Egernia striolata). We hypothesized that early social environment shapes cognitive development in skinks and predicted that skinks raised in social isolation would have reduced learning ability compared to skinks raised socially. Offspring were separated at birth into two rearing treatments: (1) raised alone or (2) in a pair. After 1 year, we quantified spatial learning ability of skinks in these rearing treatments (N = 14 solitary, 14 social). We found no effect of rearing treatment on learning ability. The number of skinks to successfully learn the task, the number of trials taken to learn the task, the latency to perform the task, and the number of errors in each trial did not differ between isolated and socially reared skinks. Our results were unexpected, yet the facultative nature of this species’ social system may result in a reduced effect of social isolation on behaviour when compared to species with obligate sociality. Overall, our findings do not provide evidence that social environment affects development of spatial learning ability in this family-living lizard.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animals learn by acquiring, processing, storing, and then acting on information collected from their environment (Dukas 2009; Shettleworth 2010; Buchanan et al. 2013). An individual’s ability to learn can be adaptive by influencing behaviours with ecological significance, like foraging, competition, mating, anti-predatory behaviour, and dispersal (Dukas 2009; Buchanan et al. 2013). For example, American bird grasshoppers (Schistocerca americana) that readily learnt a foraging task exhibited a 20% higher growth rate than non-learners (Dukas and Bernays 2000); great tit (Parus major) parents that learnt a novel task had higher offspring survival and more offspring (Cauchard et al. 2013); and male satin bowerbird (Ptilonorhynchus violaceus) problem-solving performance relates positively to their mating success (Keagy et al. 2009; but see Isden et al. 2013 for contrasting results with spotted bowerbirds, Chlamydera maculata). These studies provide evidence of a link between animal learning and fitness (but see Thorton et al. 2014 for methodological concerns). Although learning is a crucial trait for the survival and reproduction of some species, there are many factors that affect learning ability. Environmental severity (Shettleworth 2010, pp 371–394; Roth et al. 2010), rapid environmental change as experienced during urbanization (Sih et al. 2011; Sol et al. 2013), experimental methods (Noble et al. 2012), and sociality (Zuberbühler and Byrne 2006; Burkart and van Schaik 2009) are known to affect learning ability. In addition, individual-specific traits such as sex (Carazo et al. 2014), personality (Sih and Del Giudice 2012; Carazo et al. 2014), age (Noble et al. 2014), as well as early developmental environment (Stamps and Groothuis 2010; Clark et al. 2013) are linked to learning ability.
The social environment during early development can influence an individual’s learning ability throughout their lifetime (Cacioppo and Hawkley 2009). This relationship between social environment and learning ability was first demonstrated in the 1960s through Harlow’s research on rhesus macaques (Macaca mulatta). Rhesus macaques live in large, mixed-sex groups (~10 individuals; Melnick et al. 1984), and females care for their young from birth until the birth of their next offspring (Fooden 2000). Harlow’s research isolated juvenile rhesus macaques from any social interaction; development in social isolation debilitated these individuals in many ways, including significantly impairing learning ability (Harlow et al. 1965). Subsequently, numerous studies have also demonstrated a negative relationship between social isolation and learning in rats (Rattus norvegicus; Greenough et al. 1972; Morgan et al. 1975; Einon 1980; Juraska et al. 1984; Holson 1986), although a few studies examining rats and chickens (Gallus gallus domesticus) have found variable and/or positive effects of isolation on learning (Wongwitdecha and Marsden 1996; Frisone et al. 2002; Goerlich et al. 2012). Overall, it is well established that social environment, or lack thereof, can affect learning ability in mammals and birds. So far, studies have been taxonomically biased towards endotherms (e.g. birds and mammals) with obligate social systems. There has been little research on how social isolation affects learning in ectotherms (e.g. fish and reptiles).
There is increasing evidence that reptiles exhibit diverse social systems that can be kin-based (Doody et al. 2012; Gardner et al. 2015). For example, Australian skinks in the Egernia group exist in stable social aggregations, some with kin, some exhibiting long-term monogamy, and even parental care of offspring (Chapple 2003; Gardner et al. 2015; While et al. 2015). Egernia striolata (the Australian tree skink) is known to aggregate in social groups consisting of mating adult pairs, parents with offspring, and juveniles (Bonnett 1999; Duckett et al. 2012). Yet, interestingly, the social structure of E. striolata is highly variable both within and between populations. Within populations, skinks can be either found alone or in groups of variable size (2–10 skinks; Bustard 1970; Bonnett 1999). Across the tree skink’s range, different social systems have been described between populations. In arboreal populations, tree skinks have been found in small groups (maximum of three individuals) and most often found alone (Bustard 1970; Cunningham et al. 2007). Yet, in other arboreal and in saxicolous populations, tree skinks were most often in larger social groups (<10 lizards) of closely related individuals (Swanson 1976; Ehmann 1992, p. 242; Bonnett 1999; Michael and Cunningham 2010; Duckett et al. 2012). In the wild, groups consisting of parents and offspring are the most common, yet groups of only juveniles do exist (Bonnett 1999; Duckett et al. 2012, Riley unpubl. data). These juvenile-only groups vary in size, ranging from pairs to four individuals; often juveniles are also observed on their own (Bonnett 1999; Michael and Cunningham 2010; Duckett et al. 2012). This social nature of E. striolata makes it a good model for studying the influence of social environment on learning ability. We examined the effect of development in social isolation versus within a social group, and hypothesized that development in social isolation would affect the learning ability of E. striolata. As the Egernia group of skinks exhibit similar social behaviours to birds and mammals, we expected that social environment would similarly affect development of reptile behaviour. Thus, we predicted that (1) fewer skinks raised in social isolation would learn a spatial maze task, and (2) it would take longer for skinks raised in isolation to learn the task compared to skinks raised socially.
Methods
Study species, collection, and husbandry
Tree skinks are a viviparous skink found across south-eastern Australia. They inhabit hollow limbs of, and cracks under the bark of, standing trees or within fallen timber, as well as crevices on rock outcrops (Cogger 2014, p. 549).
We collected 15 gravid female E. striolata from near Albury, New South Wales (−35.98′S, 146.97′E), and held them at Macquarie University until parturition. Parturition occurred from 10 February to 12 March 2014. Offspring were separated from females and randomly allocated into two treatments, social and isolated, on 14 April 2014 (after baseline behavioural trait assays occurred; Riley unpublished data). The social treatment consisted of two unrelated juveniles housed together (N = 14 lizards within seven pairs; four males and ten females); in the isolated treatment, lizards were housed alone (N = 14 lizards; eight males and six females). Juvenile social groupings of similar sizes have been reported for wild populations of E. striolata (Chapple 2003), although social groups most often consist of parent(s) and offspring (Chapple 2003). Including parents in our social treatment was logistically not feasible because adult Egernia, particularly females, are known to be highly aggressive towards juveniles (O’Connor and Shine 2004; Sinn et al. 2008). In fact, infanticide is common in multiple Egernia group spp. (Lanham and Bull 2000; Post 2000; O’Connor and Shine 2004), and there are even instances wherein females eat their own offspring (E. stokesii, Lanham and Bull 2000; E. striolata, Riley pers. obs. 2015). We housed juveniles within their rearing treatments for approximately 1 year before we conducted our learning assay (17 May to 4 June 2015).
Learning assay
We quantified the learning ability of juvenile E. striolata (N = 28) with a spatial learning task. During the assay, we housed juveniles in a paper-lined rectangular arena (base dimensions 390 mm W × 580 mm L × 455 mm H) containing a water dish and a refuge (120 mm W × 175 mm L × 38 mm H). A 100-W heat lamp directed at the refuge, which allowed lizards to thermoregulate, lighted each arena. We did not feed lizards during the assay; the only food they received was the food reward (1.25 ml of puréed fruit; Heinz® apple and mango, apple, and pear) offered twice daily, and eaten only if the trial was completed successfully. Prior to trials commencing, we gave lizards 24 h to acclimate to their novel housing area.
We tested spatial learning ability using a vertical maze. This is a biologically relevant task, because in the wild E. striolata forage within their rock and tree habitats by vertically climbing from one crevice to another (Riley pers. obs. 2015). In our spatial learning task, the lizards had to navigate a set of five ladders and three ledges to access a food reward (see Supplementary Video 1). In stage one of the task, lizards had to choose between one of three mesh ladders running from the ground to one of two wooden ledges (Fig. 1). If done correctly, in stage two, lizards then had a choice between one of two ladders running from these wooden ledges to a third ledge that held the food reward (Fig. 1). Incorrect ladders at all stages were partially covered with clear tape, so the lizard could not completely climb them but they looked identical to the correct ladder. The slippery, clear tape covered the mesh ladders starting at 50 mm above the ground (50 mm is approximately half the body length of our skinks; Fig. 1). So, unless the lizard attempted the climb the ladder, it could not feel or see a difference between the ladders at ground level. We randomized the position of the correct first ladder to control for lateralization bias (Fig. 1). In other words, either the first left-most ladder or the second right ladder was climbable, or vice versa. We randomly assigned an equal number of lizards to each set-up. This task was attached to a laminated plywood board (390 × 305 mm), and during trials, it was placed along the side of the trial tub opposite to the refuge (Fig. 1). The task had both intra-maze spatial cues (e.g. black circle on right and diagonal stripes on left) and extra-maze spatial cues (e.g. the location of items outside the trial bin) that the lizards could have used to navigate the task (Fig. 1).
Schematic diagram of our spatial learning assay arena as set-up at the beginning of our trials. The clear tape covering the incorrect mesh ‘ladders’ was not visible, but is included in the diagram for clarity. The task, the vertical spatial learning maze, was insertable and was only within the arena during the trial
At the beginning of each trial, we first removed the water dish and placed the lizard within its refuge at the opposite end of the arena to the task (Fig. 1). We would then place the task-board within the housing bin, and then, marking the start of the trial, remove the refuge. The trial was remotely video-recorded using CCTV cameras (model H.264, CCTV security systems, Melbourne, VIC) for 1 h. We conducted two trials per day, in the morning (09:00–10:00 h) and the afternoon (12:00–14:00 h) with a minimum of 2 h between trials. All lizards were given a maximum of 30 trials to attempt the task; nevertheless, due to variability in lizard behaviour, the total number of trials completed varied between individuals. Most skinks attempted the first stage of the task for 30 trials, but one skink only interacted with the first stage of the task for 25 trials. Similarly, most lizards attempted the full task for 30 trials (N = 24), but one skink interacted with the task for 25 trials, one skink interacted with the task for 26 trials, and another two skinks interacted with the task for 28 trials.
From the videos, we scored: (1) successful completion of task, (2) latency to perform the task successfully, and (3) number of errors made during each trial. Successful completion of the task was considered in two stages (Fig. 1). First, the lizard had to climb the correct first ladder and reach the ledge. If the lizard attempted to climb (had a minimum of both forelimbs on a ladder) any of the incorrect ladders, the task was marked as unsuccessful. Second, once on the first ledge, the lizards had to move across the gap between the two ledges, climb the second correct ladder, gain access to the final ledge, and access the food reward (see Supplementary Video 1). When lizards were situated on the first ledge, we observed that they preferred to grip onto the exposed portion of the incorrect ladder’s mesh with one, or more, limbs to allow stability, while they were attempted to move across the ledges. So, we marked the second stage of the task as successful if the lizard (1) moved horizontally, or diagonally across the first ledge and did not encounter the tape-covered portion of the incorrect ladder, and then (2) climbed the correct second ladder. If, instead, the lizard moved vertically up the incorrect ladder and encountered the tape-covered portion, it was marked as unsuccessful. We separately assessed if each lizard correctly performed the first stage of the task (e.g. climbed the correct first ladder; Fig. 1), and the full task (e.g. climbed both the correct first and second ladders). We then classified each lizard as a ‘learner’ or a ‘non-learner’ by examining the tally of correct/incorrect choices (Tables S1 and S2). Following Noble et al. (2014), we considered a lizard to be a ‘learner’ if it successfully performed the task a minimum of 5/6 consecutive times. We scored latency to perform the task by recording the time (s) from the start of the trial (as marked by lifting the refuge from the arena) until the lizard placed its head in the food dish. We scored latency for the full task only, and for each trial regardless of whether the task was initially completed successfully. For example, if a lizard initially climbed an incorrect ladder but then completed the task, it would have been unsuccessful at the task, but we would still measure latency until it accessed the food reward. For the full task only, we also tallied how many times a lizard climbed incorrect ladders before it performed the full task correctly or the trial ended. For all behaviours (task success for the first stage and full task, latency, number of errors), there were high levels of congruence in our scoring (see Supplementary Materials).
Assessment of learning criteria
We assessed robustness of our learning criteria by tallying the number of correct/incorrect choices from the last trial in the learning criterion to the lizard’s last trial (e.g. if a lizard performed 5/6 trials correctly, we started the tally at the 6th trial; Tables S1 and S2). We only tested the learning criteria for a subset of lizards that had six or more trials after the trial in which they reached the criterion. We tested whether this tally of correct/incorrect choices was significant according to an exact binomial choice test. For the first stage of the task, 21/23 (91%) of lizards performed the task correctly significantly more than expected by chance. For the full learning task, 16/17 (94%) of the skinks performed the task correctly significantly more than expected by chance. These results suggest our learning criterion was sufficient in categorizing lizards that learnt from those that did not.
Statistical analyses
We analysed our data using generalized linear mixed effects models (GLMM) with a Bayesian Markov chain Monte Carlo (MCMC) sampling approach. We used mixed effect models (GLMMs) to incorporate the dependency among observations of lizards from the same litter, as well as repeated observations of the same individual into our analyses (Dobson and Barnett 2008). MCMC is a simulation technique that we used to obtain the distribution of each parameter in our GLMMs, and this technique requires specification of a probability distribution (prior) for the analysis (Masson 2011; Zurr et al. 2013; Gelman et al. 2014, pp. 3–27; Kruschke 2014, pp. 7–59). We preliminarily ran our GLMMs with multiple priors, but there was negligible difference between model results with varying priors. So, we used default diffuse uniform priors for our fixed effects, and for the random effect variance–covariance matrix our prior specification was V = \(\left[ { \begin{array}{*{20}c} 1 & 0 \\ 0 & 1 \\ \end{array} } \right]\) and nu = 0.002 (Hadfield 2010). In brief, diffuse priors assign equal probabilities to all possibilities and typically yield parameter estimates that are not too different from frequentist statistical analyses (Zurr et al. 2013, pp. 66–72; Kruschke 2014, pp. 7–59). Analyses were performed in R v 3.0.3 using the MCMCglmm package (Hadfield 2010; R Core Team 2016).
In each model, we estimated model parameters 2,000,000 times (iterations), discarded the first 10,000 estimations (burn-in), and only sampled the parameter every 1,000th estimate (thinning interval). We repeated this procedure three separate times (chains) to reduce the autocorrelation of successive samples from one chain (Zurr et al. 2013, pp. 66–72). We verified convergence of chains using the Gelman–Rubin test in the R package coda (Plummer et al. 2015). We also visually inspected all plots of our chains to ensure they were well mixed (i.e. were sampling randomly). Autocorrelation of the chains for both fixed and random effects was assessed to ensure levels were low (lag <0.1) using the autocorr function in R, and we also performed Geweke and Heidelberg autocorrelation diagnostics (all from the R package coda; Plummer et al. 2015).
Data from the first stage of the task and the full task were analysed separately, but the variables included in each of the models (1–3) were the same (see Table 1 for details):
-
1.
This binomial MCMC-GLMM examined whether the probability of learning a task (learner = 1, non-learner = 0) was influenced by rearing treatment (isolated or social). We also controlled for sex (fixed effect) and mother identity (random effect).
-
2.
This Poisson MCMC-GLMM examined whether the number of trials taken to learn the task was influenced by rearing treatment, while controlling for lizard sex and mother identity.
-
3.
This binomial MCMC-GLMM examined whether the probability of task success during each trial was influenced by rearing treatment. The model also included the fixed effects of sex, trial number, and an interaction between treatment and trial number. It also included lizard and mother identity as random effects.
-
4.
This Gaussian MCMC-GLMM examined whether latency to successfully complete the task (transformed with a square-root transformation to ensure normality of residuals) was influenced by rearing treatment. The model also included the fixed effects of sex, trial number, and an interaction between treatment and trial number, as well as the random effects of lizard and mother identity.
-
5.
This Poisson MCMC-GLMM examined whether the number of errors made during each trial was affected by rearing treatment. The model also included the fixed effects of sex, trial number, and an interaction between treatment and trial number, as well as the random effects of lizard and mother identity.
We report the mode of the MCMC sample and 95% credible intervals for our parameter estimates. Parameter estimates were considered significant when the credible intervals did not include 0, and the pMCMC values calculated by MCMCglmm were <0.05 (Hadfield 2010). When we predicted fitted lines from the models for visualization of differences in response variables between rearing treatments, we set sex, our secondary fixed factor, to the intercept-level value. Data for this study are available from https://dx.doi.org/10.6084/m9.figshare.3984111.v1.
Results
First stage of learning task (three-ladder choice)
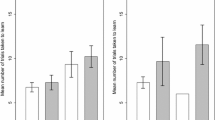
Twenty-five of 28 (89%) of the lizards met our learning criterion for choosing the correct first stage ladder (out of three possibilities). Whether a lizard learnt or did not learn the first stage of the task did not depend on rearing treatment (Table 1): 12/14 (86%) socially reared and 13/14 (93%) isolated lizards were categorized as learners. Rearing treatment also did not affect the number of trials taken to learn stage one of the task. Socially reared skinks took on average 15 trials (95% CI 10–19) to learn stage one of the task, and isolated skinks took on average 14 trials (95% CI 11–17). Males were less likely to learn the first stage of the task than females, but there was no sex-effect on the number of trials taken to learn the task and this observed sex-effect was not consistent when we examined the full task (Table 1).
Rearing treatment did not affect probability of task success during each trial (Table 1; Fig. 2a). There also was no sex-effect on the probability of task success during each trial (Table 1). Yet, probability of task success during each trial increased over time (as trial number increased), which indicates that, regardless of rearing treatment, tree skinks were learning stage one of the task (Table 1; Fig. 2a).
Predicted probabilities of task success during each trial did not differ between developmental treatments (social: light grey shading and dashed line; isolated: dark grey shading and solid line) for either a stage one or b the full spatial learning task. The darkest shade of grey is where the 95% predicted credible intervals, which are represented by shaded polygons around predicted probabilities, overlap
Full learning task (three-ladder choice and then a two-ladder choice)
When we considered the learning task in its entirety (three-ladder choice followed by a two-ladder choice), 19/28 (68%) of skinks met our learning criterion. Whether a lizard learnt the full task or not did not depend on rearing treatment (Table 1): 9/14 (64%) socially reared and 10/14 (71%) isolated lizards were categorized as learners. Rearing treatment did not affect number of trials taken to learn the full task (Table 1): socially reared skinks took an average of 16 trials (95% CI 11–21) to learn the task, and isolated skinks took an average of 17 trials (95% CI 14–19). The probability of learning the full task and the number of trials taken to learn the task were not significantly affected by sex (Table 1).
Similarly, rearing treatment did not affect probability of task success, latency, or number of errors made during each trial (Table 1; Fig. 2b). Socially reared skinks took an average of 1269 s to complete the task (95% CI 1261–1278), and made on average 0.90 incorrect choices during a trial (95% CI 0.83–0.97). Isolated skinks took on average 1321 s to complete the task (95% CI 1313–1328) and made on average 1.26 incorrect choices during a trial (95% CI 1.20–1.32). There were no sex-effects on probability of task success during each trial, latency, or number of errors made during each trial (Table 1). Probability of task success during each trial increased over time (as trial number increased; Fig. 2b), and latency to complete the task (Fig. S1) and number of errors (Fig. S2) during a trial both decreased over time (Table 1). These results are evidence that tree skinks were learning the full task.
Discussion
Our prediction that social isolation during development would negatively affect learning ability in E. striolata was not supported. An almost equal number of skinks in our two treatments (social vs. isolated rearing environment) were categorized as ‘learners’ in our spatial learning task. Moreover, the number of trials it took skinks to learn the task did not differ between rearing treatments. We found no effect of rearing treatment on probability of task success during each trial, latency until task success, and number of errors made during the trial. All our findings, across analyses for both the first stage of the task and the full task, consistently demonstrate no evidence for an effect of social isolation on learning ability of a social skink.
The key to why we found this unexpected result may lie in the tree skink’s variable social system. As noted above, the social structure of E. striolata is quite variable; within one population, individuals can vary from being solitary to highly aggregative with kin (Bustard 1970; Bonnett 1999; Duckett et al. 2012). This natural flexibility in group size and variation in individual sociability may mean that development in isolation is simply a normal option in the wild, as such social isolation is possibly less stressful for this species. Thus, there are limited negative consequences to this social state. For example, in domestic chickens, stress (or lack of it) has been suggested as a mechanism that regulates learning ability (Goerlich et al. 2012). In this study, isolated chicks actually made more correct choices in an associative learning task. These chicks had a reduced stress response, which likely resulted in a higher coping ability and an enhanced learning ability. It would be beneficial to follow up our study on E. striolata by measuring stress levels in both our isolated and socially reared treatments to examine whether stress may be the mechanism that explains our unexpected findings. All in all, the plastic social nature of E. striolata may buffer these lizards from the extreme negative effects of social isolation previously observed in studies on mammals and birds. These previous studies often examined the effects of social isolation on species with more complex, more rigid, and obligate social structure.
An alternative hypothesis could be that the presence or absence of a parent during development may affect tree skink behaviour. As neither of our rearing treatments included parents due to logistical constraints (see Methods section), any potential effects of removing a parent were not quantified. In the wild, the most common tree skink social group does consist of parents and offspring (Bonnett 1999; Duckett et al. 2012). Although both juveniles and adults can be found alone, social groups can also consist of adults only, juveniles only, or parents and offspring (Bonnett 1999; Duckett et al. 2012, Riley pers obs 2016). In fact, in multiple Egernia group sp., offspring benefit from the presence of parents and gain added protection, closer to optimal thermoregulation, and increased access to prey (O’Connor and Shine 2004; Langkilde et al. 2007; Sinn et al. 2008). Thus, as offspring benefit from the presence of parents in Egernia group sp., one might expect there could be parental effects on offspring behaviour. It is still unknown whether juveniles benefit from the presence of parents in E. striolata, yet it is an aspect to consider in the early development of behaviour of this species.
Although our study did not find any evidence that social isolation negatively affects spatial learning in tree skinks, there are other lizard behaviours that could be affected by social isolation. Personality traits and an individual’s ability to interact with conspecifics are known to be altered by social environment during development in mammals and birds (Harlow et al. 1965; Naguib et al. 2011). Hatchling veiled chameleons (Chameleo calyptratus) raised in isolation were more submissive when interacting with conspecifics and took longer to attack prey in a foraging task (Ballen et al. 2014). However, adult C. calyptratus are largely intolerant of conspecifics (De Vosjoli and Ferguson 1995, pp. 81–89), so our understanding of social environment on lizard behaviour would benefit from further research on a known social species. Social isolation may also hinder the ability an individual has to process and interpret social cues and information. Thus, isolation may affect social learning ability because lack of social cues during development may obstruct information transfer between conspecifics. While we found no effect of social isolation on individual learning ability, the same may not be true of social learning and warrants further investigation.
As the sociality of reptiles is becoming increasingly recognized (Doody et al. 2012; Gardner et al. 2015), it is crucial to also study the consequences and impact that being social has on reptilian behaviour, ecology, and evolution. Understanding the consequences of sociality for reptiles is practically important for captive research, breeding programs, and conservation. Management, conservation, and research programs may need to implement group housing of social species to reduce potential negative impacts of isolation on these animals’ development. Our study did not find any evidence that social isolation negatively affects spatial learning ability in the social tree skink. However, more research is required to better understand the negative effects of social isolation on other behavioural and learning traits of this species. Because lizards have relatively rudimentary parental care and species vary from mainly solitary to highly social, they may represent a unique opportunity to easily manipulate early social environment and examine how behavioural development can be shaped by sociality.
References
Ballen C, Shine R, Olsson M (2014) Effects of early social isolation on the behaviour and performance of juvenile lizards, Chamaeleo calyptratus. Anim Behav 88:1–6. doi:10.1016/j.anbehav.2013.11.010
Bonnett MP (1999) The ecology, behaviour and genetic relationships of a population of Egernia striolata. Unpublished Honours thesis, Flinders University of South Australia, Adelaide, Australia
Buchanan KL, Grindstaff JL, Pravosudov VV (2013) Condition dependence, developmental plasticity, and cognition: implications for ecology and evolution. Trends Ecol Evol 28:290–296. doi:10.1016/j.tree.2013.02.004
Burkart JM, van Schaik CP (2009) Cognitive consequences of cooperative breeding in primates? Anim Cogn 13:1–19. doi:10.1007/s10071-009-0263-7
Bustard HR (1970) A population study of the scincid lizard Egernia striolata in northern New South Wales. Proc K Ned Akad Wet C 73:202
Cacioppo JT, Hawkley LC (2009) Perceived social isolation and cognition. Trends Cogn Sci 13:447–454. doi:10.1016/j.tics.2009.06.005
Carazo P, Noble DWA, Chandrasoma D, Whiting MJ (2014) Sex and boldness explain individual differences in spatial learning in a lizard. Proc Biol Sci 281:20133275. doi:10.1098/rspb.2013.3275
Cauchard L, Boogert NJ, Lefebvre L, Dubois F, Doligez B (2013) Problem-solving performance is correlated with reproductive success in a wild bird population. Anim Behav 85:19–26. doi:10.1016/j.anbehav.2012.10.005
Chapple DG (2003) Ecology, life-history, and behavior in the Australian scincid genus Egernia, with comments on the evolution of complex sociality in lizards. Herpetol Monogr 17:145–180. doi:10.1655/0733-1347(2003)017[0145:elabit]2.0.co;2
Clark BF, Amiel JJ, Shine R, Noble DWA, Whiting MJ (2013) Colour discrimination and associative learning in hatchling lizards incubated at ‘hot’ and ‘cold’ temperatures. Behav Ecol Sociobiol 68:239–247. doi:10.1007/s00265-013-1639-x
Cogger HG (2014) Reptiles and amphibians of Australia, 7th edn. CSIRO Publishing, Clayton
Cunningham RB, Lindenmayer DB, Crane M, Michael D, MacGregor C (2007) Reptile and arboreal marsupial response to replanted vegetation in agricultural landscapes. Ecol Appl 17:609–619. doi:10.1890/05-1892
De Vosjoli P, Ferguson G (1995) Care and breeding of Panther, Jackson’s, Veiled, and Parson’s chameleons. i5 Publishing, Los Angeles
Dobson AJ, Barnett A (2008) An introduction to generalized linear models. CRC Press, Boca Raton, pp 45–66, 229–256
Doody JS, Burghardt GM, Dinets V (2012) Breaking the social-non-social dichotomy: a role for reptiles in vertebrate social behavior research? Ethology 119:95–103. doi:10.1111/eth.12047
Duckett PE, Morgan MH, Stow AJ (2012) Tree-dwelling populations of the skink Egernia striolata aggregate in groups of close kin. Copeia 2012:130–134. doi:10.1643/ce-10-183
Dukas R (2009) Learning: mechanisms, ecology and evolution. In: Dukas R, Ratcliffe JM (eds) Cognitive ecology II. The University of Chicago Press, Chicago, pp 7–26
Dukas R, Bernays EA (2000) Learning improves growth rate in grasshoppers. Proc Natl Acad Sci USA 97:2637–2640. doi:10.1073/pnas.050461497
Ehmann H (1992) Encyclopedia of Australian animals: reptiles. Angus and Robertson, Sydney
Einon D (1980) Spatial memory and response strategies in rats: age, sex and rearing differences in performance. Q J Exp Psychol 32:473–489. doi:10.1080/14640748008401840
Fooden J (2000) Systematic review of the rhesus macaque, Macaca mulatta (Zimmermann, 1780). Field Zool 96:1–180. doi:10.5962/bhl.title.7192
Frisone DF, Frye CA, Zimmerberg B (2002) Social isolation stress during the third week of life has age-dependent effects on spatial learning in rats. Behav Brain Res 128:153–160. doi:10.1016/S0166-4328(01)00315-1
Gardner MG, Pearson SK, Johnston GR, Schwarz MP (2015) Group living in squamate reptiles: a review of evidence for stable aggregations. Biol Rev Camb Philos Soc. doi:10.1111/brv.12201
Gelman A, Carlin JB, Stern HS, Rubin DB (2014) Bayesian data analysis, vol 3. Chapman and Hall/CRC Press, Boca Raton
Goerlich VC, Nätt D, Elfwing M, Macdonald B, Jensen P (2012) Transgenerational effects of early experience on behavioral, hormonal and gene expression responses to acute stress in the precocial chicken. Horm Behav 61:711–718. doi:10.1016/j.yhbeh.2012.03.006
Greenough WT, Madden TC, Fleischmann TB (1972) Effects of isolation, daily handling, and enriched rearing on maze learning. Psychon Sci 27:279–280. doi:10.3758/bf03328961
Hadfield JD (2010) MCMC methods for multi-response generalized linear mixed models: the MCMCglmm R package. J Stat Softw 33:1–22. doi:10.18637/jss.v033.i02
Harlow HF, Dodsworth RO, Harlow MK (1965) Total social isolation in monkeys. Proc Natl Acad Sci USA 54:90–97. doi:10.1073/pnas.54.1.90
Holson RR (1986) Feeding neophobia: a possible explanation for the differential maze performance of rats reared in enriched or isolated environments. Physiol Behav 38:191–201. doi:10.1016/0031-9384(86)90154-X
Isden J, Panavi C, Dingle C, Madden J (2013) Performance in cognitive and problem-solving tasks in male spotted bowerbirds does not correlate with mating success. Anim Behav 86:829–838. doi:10.1016/j.anbehav.2013.07.024
Juraska JM, Henderson C, Müller J (1984) Differential rearing experience, gender, and radial maze performance. Dev Psychobiol 17:209–215. doi:10.1002/dev.420170302
Keagy J, Savard JF, Borgia G (2009) Male satin bowerbird problem-solving ability predicts mating success. Anim Behav 78:809–817. doi:10.1016/j.anbehav.2009.07.011
Kruschke J (2014) Doing Bayesian data analysis: a tutorial with R, JAGS and Stan. Academic Press, Cambridge
Langkilde T, O’Connor D, Shine R (2007) Benefits of parental care: do juvenile lizards obtain better-quality habitat by remaining with their parents? Austral Ecol 32:950–954. doi:10.1111/j.1442-9993.2007.01783.x
Lanham EJ, Bull CM (2000) Maternal care and infanticide in the Australian skink, Egernia stokesii. Herpetol Rev 31:151–152. doi:10.1016/j.anbehav.2004.02.014
Masson ME (2011) A tutorial on a practical Bayesian alternative to null-hypothesis significance testing. Behav Res Methods 43:679–690. doi:10.3758/s13428-010-0049-5
Melnick DJ, Pearl MC, Richard AF (1984) Male migration and inbreeding avoidance in wild rhesus monkeys. Am J Primatol 7:229–243. doi:10.1002/ajp.1350070303
Michael DR, Cunningham RB (2010) The social elite: habitat heterogeneity, complexity and quality in granite inselbergs influence patterns of aggregation in Egernia striolata (Lygosominae: Scincidae). Austral Ecol 35:862–870. doi:10.1111/j.1442-9993.2009.02092.x
Morgan MJ, Einon DF, Nicholas D (1975) The effects of isolation rearing on behavioural inhibition in the rat. Q J Exp Psychol 27:615–634. doi:10.1080/14640747508400524
Naguib M, Flörcke C, van Oers K (2011) Effects of social conditions during early development on stress response and personality traits in great tits (Parus major). Dev Psychobiol 53:592–600. doi:10.1002/dev.20533
Noble DWA, Carazo P, Whiting MJ (2012) Learning outdoors: male lizards show flexible spatial learning under semi-natural conditions. Biol Lett 8:946–948. doi:10.1098/rsbl.2012.0813
Noble DWA, Byrne RW, Whiting MJ (2014) Age-dependent social learning in a lizard. Biol Lett 10:20140430. doi:10.1098/rsbl.2014.0430
O’Connor DE, Shine R (2004) Parental care protects against infanticide in the lizard Egernia saxatilis (Scincidae). Anim Behav 68:1361–1369. doi:10.1016/j.anbehav.2004.02.014
Plummer M, Best N, Cowles K, Vines K (2015) Coda: output analysis and diagnostics for MCMC. R package version 0.13-3. http://CRAN.R-project.org/package=coda
Post MJ (2000) The captive husbandry and reproduction of the Hosmer’s Skink Egernia hosmeri. Herpetofauna 30:2–6
Roth TC, LaDage LD, Pravosudov VV (2010) Learning capabilities enhanced in harsh environments: a common garden approach. Proc R Soc B Biol 277:3187–3193. doi:10.1098/rspb.2010.0630
R Core Team (2016) A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/
Shettleworth SJ (2010) Cognition, evolution, and behavior, 2nd edn. Oxford University Press, New York
Sih A, Del Giudice M (2012) Linking behavioural syndromes and cognition: a behavioural ecology perspective. Philos Trans R Soc Lond B 367:2762–2772. doi:10.1098/rstb.2012.0216
Sih A, Ferrari MCO, Harris DJ (2011) Evolution and behavioural responses to human-induced rapid environmental change. Evol Appl 4:367–387. doi:10.1111/j.1752-4571.2010.00166.x
Sinn DL, While GM, Wapstra E (2008) Maternal care in a social lizard: links between female aggression and offspring fitness. Anim Behav 76:1249–1257. doi:10.1016/j.anbehav.2008.06.009
Sol D, Lapiedra O, González-Lagos C (2013) Behavioural adjustments for a life in the city. Anim Behav 85:1101–1112. doi:10.1016/j.anbehav.2013.01.023
Stamps JA, Groothuis TGG (2010) Developmental perspectives on personality: implications for ecological and evolutionary studies of individual differences. Philos Trans R Soc Lond B 365:4029–4041. doi:10.1098/rstb.2010.0218
Swanson S (1976) Lizards of Australia. Angus and Robertson, Sydney, p 43
Thorton A, Isden J, Madden JR (2014) Toward wild psychometrics: linking individual cognitive differences to fitness. Behav Ecol 25:1299–1301. doi:10.1093/beheco/aru095
While GM, Chapple DG, Gardner MG et al (2015) Egernia lizards. Curr Biol 25:R593–R595. doi:10.1016/j.cub.2015.02.070
Wongwitdecha N, Marsden CA (1996) Effects of social isolation rearing on learning in the Morris water maze. Brain Res 715:119–124. doi:10.1016/0006-8993(95)01578-7
Zuberbühler K, Byrne RW (2006) Social cognition. Curr Biol 16:R786–R790. doi:10.1016/j.cub.2006.08.046
Zurr AF, Hilbe JM, Ieno EN (2013) A beginner’s guide to GLM and GLMM with R: a frequentist and Bayesian perspective for ecologists. Highland Statistics Ltd, Newburgh
Acknowledgements
We thank G. While and M. Favre for their assistance in the field and laboratory, as well as J. Baxter-Gilbert and F. Kar for their artistic and statistical advice.
Funding
Financial support for this research was provided by the Australian Research Council (DP130102998, awarded to MJW and RWB), Natural Sciences and Engineering Research Council of Canada (scholarship to JLR), the Australasian Society for the Study of Animal Behaviour, the Australian Museum, and Macquarie University. DWAN was supported by an ARC Discovery Early Career Research Award (DE150101774) and UNSW Vice Chancellors Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All the authors declare they have no conflict of interest.
Ethical approval
We followed guidelines for the care and use of animals as laid out by the Association for the Study of Animal Behaviour. Experimental protocols were approved by the Macquarie University Animal Ethics Committee (ARA # 2013/039). Collection of skinks was approved by the New South Wales National Parks and Wildlife Service, Office of Environment and Heritage (License # SL101264). Female skinks were captured either by hand, noosing or Eliot trap and were placed in cloth bags until they could be transported by vehicle to Macquarie University from Albury, New South Wales, in an insulated box. We observed no injuries resulting from our cognition experiment.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MOV 73617 kb)
Rights and permissions
About this article
Cite this article
Riley, J.L., Noble, D.W.A., Byrne, R.W. et al. Does social environment influence learning ability in a family-living lizard?. Anim Cogn 20, 449–458 (2017). https://doi.org/10.1007/s10071-016-1068-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-1068-0