Abstract
Based on behavioral evidence, the domestic dog has emerged as a promising comparative model of human self-control. However, while research on human inhibition has probed heterogeneity and neuropathology through an integration of neural and behavioral evidence, there are no parallel data exploring the brain mechanisms involved in canine inhibition. Here, using a combination of cognitive testing and awake neuroimaging in domestic dogs, we provide evidence precisely localizing frontal brain regions underpinning response inhibition in this species and demonstrate the dynamic relationship between these regions and behavioral measures of control. Thirteen dogs took part in an in-scanner go/no-go task and an out-of-scanner A-not-B test. A frontal brain region was identified showing elevated neural activity for all subjects during successful inhibition in the scanner, and dogs showing greater mean brain activation in this region produced fewer false alarms. Better performance in the go/no-go task was also correlated with fewer errors in the out-of-scanner A-not-B test, suggesting that dogs show consistent neurobehavioral individual differences in cognitive control, as is seen in humans. These findings help establish parity between human and canine mechanisms of self-control and pave the way for future comparative studies examining their function and dysfunction.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Comparative models of inhibition hold promise for better understanding human control processes and their relationship with both general cognitive faculty and disease states (Casey et al. 1997; Logan et al. 1997; Sagvolden et al. 2005). To date, inhibition has been predominately explored in primates and rodents (Schoenbaum et al. 2002), but recent behavioral data suggest the domestic dog as a productive new model (Tapp et al. 2003; Wobber et al. 2009; Topál et al. 2009; Miller et al. 2010, 2012; Bray et al. 2014, 2015). Dogs are readily available for study, easily trained, and are ideal subjects for noninvasive research. In addition, because dogs share a social ecology with humans that requires frequent exercise of control mechanisms, understanding inhibition in the dog, including how it may be enhanced and why it sometimes fails, should be of broad interest to pet owners.
In humans, variability in control processes, up to and including the pathological, has been linked to variability in structure and function in frontal brain regions serving control (Forstmann et al. 2008). Dogs also show high inter-individual variability in a range of behavioral and cognitive domains, including inhibition (Adams et al. 2000; Jones and Gosling 2005; Bray et al. 2014, 2015; Riemer et al. 2014). However, the corresponding neural data necessary to ground the dog’s control processes in a neural substrate and situate them in relation to those of humans and other species have been lacking.
An older literature relying predominantly on lesion methods has shown that self-control and inhibitory processes in the domestic dog rely, at least partly, on frontal brain regions (Brutrowski and Mempel 1961; Brutkowski and Dabrowska 1963, 1966; Stępień et al. 1963). The best evidence from these studies suggested that inhibition of a positively conditioned response was most likely to be disrupted by lateral frontal lesions (Dabrowska and Szafranska-Kosmal 1972). These lesion studies provided important preliminary evidence of frontal brain function in dogs, but were confounded by a number of factors typical to lesion studies. First, as acknowledged in each of these papers, technical limitations interfered with standardization of lesion location across subjects. Second, without the clear anatomical and functional boundaries found in subcortical regions, selection of experimental lesion sites in the cortex is problematic. Cortical landmarks are variable in the human brain (Thompson et al. 1996), and this problem may be amplified by high morphological heterogeneity in the dog cranium and brain (Wayne 1986). Third, most brain functions are not strictly modular, occurring instead across networks (Farah 1994). Regional ablation may remove cortex important for a certain type of processing, but also sever connections between other regions not lesioned. Fourth, because these brain lesions were irreversible, they did not allow for exploration of dynamic function and change in brain regions over time. Finally, and most importantly, lesions studies are all-or-none within a particular region. If there are individual or task differences in recruitment of a particular region, as is the case with cognitive control and inhibition in humans and other primates (Forstmann et al. 2008), lesion studies cannot illuminate this. Noninvasive fMRI with dogs addresses each of these shortcomings to varying extents and has the benefit of being much more humane.
Here we used a combination of noninvasive brain imaging (Berns et al. 2012, 2014; Cook et al. 2014; for review see Cook et al. 2015) and behavioral measures to explore the mechanisms behind one widely studied component of control: response inhibition (cf., Eagle et al. 2008).
Prior fMRI experiments with dogs from both our laboratory and others (e.g., Andics et al. 2014; Jia et al. 2014) have relied on passive exposure protocols. To study control mechanisms, we trained 13 awake, unrestrained dogs to perform an active go/no-go task while undergoing fMRI. Dogs were trained to touch a paddle with their noses when hearing a conditioned whistle stimulus (the “go” signal) and to inhibit this response when the whistle sounded after onset and during continued presentation of a specific (“no-go”) visual stimulus. Dogs were rewarded with food for successful paddle touches on go trials, and for successful inhibition on no-go trials. In a third trial type, dogs received a highly familiar raised hand signal indicating incipient reward. All subjects had months of prior experience with this conditioned reward cue. The whistle was never paired with the raised hand reward cue, which was used as a neutral condition in this experiment. To identify brain regions involved in inhibition, we compared fMRI activity on successful no-go versus neutral trials. Although dogs were required to hold still during both no-go and neutral trials, our hypothesis was that holding still during the pre-potent whistle go cue presented on the no-go trials would require greater neural inhibitory activation than holding still for the familiar neutral condition. Importantly, both trial types resulted in food reward, meaning that contrasts in BOLD activation could not be explained by reference to anticipation of reward.
In a second out-of-scanner experiment, the same dogs took part in a variant of the widely used A-not-B task for assessing cognitive control (Piaget 1954; Andrews Espy et al. 1999; Amici et al. 2008; MacLean et al. 2014). While this test has been used comparatively to make species-level distinctions, our interest was in individual variability. Therefore, in our procedure, after preliminary baiting to a specific reward location (A), dogs were tested on the number of trials required to switch to a second reward location (B).
We hypothesized that: (1) successful inhibition on the no-go trials would differentially recruit frontal brain regions, (2) subjects producing fewer false alarms on the go/no-go task would show greater frontal activation and (3) false alarm rate on the in-scanner go/no-go task would predict performance on the out-of-scanner A-not-B task.
Experiment 1
Methods
Subjects
From our previous imaging work with dogs (Berns et al. 2012, 2014; Cook et al. 2014; Dilks et al. 2015), sample sizes of 10 or more have been enough to show effects at the group level. For the current study, we scanned all dogs in our project who were able to complete the necessary training in a timely manner. Subjects were dogs (N = 13, Table 1) in the Atlanta community. All were pets and/or released service dogs whose owners volunteered their time for fMRI training and experiments. All dogs used in the current study had participated in prior fMRI research and had previously completed at least one fMRI session in which two hand signals were presented by their primary trainer, one indicating forthcoming food reward and the other indicating no reward (Cook et al. 2014). Accordingly, all dogs had demonstrated an ability to remain still during training and scanning for periods of 30 s or greater. However, despite success in previous experiments, in this more complex experiment, two dogs, Callie and Ohana, exhibited excessive motion in the scanner and were subsequently excluded from fMRI analysis because of insufficient observations after motion censoring (see below).
Training
For participation in previous experiments (details in: Berns et al. 2012, 2014; Cook et al. 2014), dogs took part in a training program using behavior shaping, desensitization, habituation and behavior chaining to prepare them to be comfortable within the physical confines of the MRI bore and with the loud noise produced by scanning.
In the current experiment, dogs received a highly familiar signal (raised left hand, signifying incipient receipt of food reward) used in previous experiments, which required no new training. The experiment also involved two new stimuli: a short auditory whistle blow from their handler (7 kHz dog training whistle) and a crossed, raised arms “X” signal from their handler (Fig. 1; a typical training session is shown in Video S1 in the Supplemental Material available online). The whistle constituted the “go” signal for this experiment, and in response the dogs were trained to perform a nose-touch to a small plastic target fixed ~2 cm from the front of their nose on their stationing apparatus. The “X” constituted the “no-go” signal, during which dogs were trained to hold still, inhibiting the otherwise rewarded response to the whistle signal. Successful target touches on go trials were rewarded with food, as were successful inhibitions on no-go trials. Experimental comparisons of brain activity were between neutral and successful no-go trials as discussed below.
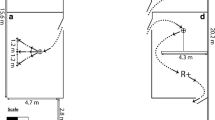
Previously trained neutral stimulus (raised left palm) is displayed on the left, the no-go signal (crossed arms) on the right. The whistle was never blown during the neutral stimulus and was always blown during the no-go stimulus. In both trial types, remaining still in the stationing apparatus for ~10 s was rewarded
Training for the go/no-go task involved instruction once every 2 weeks at our training facility overseen by core project staff and regular training at home overseen by each dog’s primary handler. During training sessions, each dog cooperatively stationed him- or herself in a custom-made foam chinrest placed inside of a mock MRI coil. Presentation of signals was contingent on the dogs being able to station calmly and with minimal motion.
We first trained the nose-touch in response to the whistle. Initially, each dog was prompted to touch the target upon commencement of handler communication consisting of points and verbal cues in conjunction with the onset of the whistle stimulus. Next, the visual and verbal cues were faded out until the dog would touch the target with his or her nose upon solely hearing the whistle stimulus. Correct target touches were rewarded with food. When each dog’s targeting behavior was under conditioned control (defined as target response to 90 % of whistle signals within a single session), the neutral hand signal (familiar from prior experiments) was introduced to training sessions. On approximately one-third of training trials, instead of blowing the whistle the handler would present the neutral hand signal for 3–10 s, and if the dog refrained from targeting during presentation would reward the dog with food. The whistle was never blown during presentation of the neutral hand signal. Importantly, likely because all dogs had extensive experience with the neutral hand signal from prior experiments, there were few false alarms during the neutral hand signal, even during training. When these did occur, the handler would remove the hand signal, wait for a short period (approximately 5 s) and repeat the trial.
When dogs showed a targeting response to the whistle on 90 % or more of presentations and refrained from targeting during the neutral hand signal on 90 % or more of presentations, the no-go (“X”) hand signal was introduced. This signal was interspersed with go and neutral trials. Within 1–3 s of presenting the “X,” the owner would blow the whistle at very low volume. The X-hand signal was always presented prior to the whistle blowing on no-go trials. If the dog refrained from targeting for at least 1 s, they received a food reward. Over successive trials and sessions, the amplitude of the whistle on no-go trials was increased until comparable to that used on go trials. Then, the duration the dog was required to hold still after X-plus-whistle was extended by successive approximations, until the dog could reliably remain stationary for a period of 10 s after the whistle. When a dog was able to respond appropriately to both the go and no-go signals on 80 % or more of trials within a single session during practice, they were judged ready for the live scanning task. Training time to reach these criteria ranged from 2 to 4 months.
MRI scanning
In simplest terms, a typical fMRI analysis compares relative blood oxygen level dependent (BOLD) signal (associated with neural activation; Logothetis et al. 2001) across different brain regions during different conditions. Regions showing the greatest change in activation levels can be identified statistically and theoretically are those most relevant to task performance. To identify brain regions supporting inhibition, we compared the activation during successful inhibition trials with the activation during neutral trials. Of note, we used neutral trials for comparison with inhibition trials because successful “go” trials could not be analyzed (the head motion produced by touching the target resulted in these images being discarded). Although holding still during the neutral condition may require some measure of inhibition, dogs never received a whistle signal during the neutral condition. Thus, we hypothesized that inhibiting response during no-go trials, which always included a whistle signal, would produce greater activation in inhibition-relevant brain regions than during the neutral condition alone.
All scanning for the current experiment was conducted with a Siemens 3 T Trio whole-body scanner. General scanning procedure was identical to that in Berns et al. (2014), where detailed scanning parameters and data processing procedures can be found.
For each subject a T2-weighted structural image was previously acquired during one of our earlier experiments using a turbo spin-echo sequence optimized for rapid acquisition (25–30 2 mm slices, TR = 3940 ms, TE = 8.9 ms, flip angle = 131°, 26 echo trains, 128 × 128 matrix, FOV = 192 mm). These structural scans were used here as targets to register (i.e., spatially transform) the lower-resolution functional scans, as is typically done in fMRI (cf., Smith et al. 2004).
Functional scans used a single-shot echo-planar imaging (EPI) sequence to acquire volumes of 24 sequential 2.5 mm slices with a 20 % gap (TE = 25 ms, TR = 1200 ms, flip angle = 70°, 64 × 64 matrix, 3 mm in-plane voxel size, FOV = 192 mm). These parameters allow us to “take a picture” of brain activity across the whole brain once every 1.2 s. Each picture/time point is referred to as a “volume.” Slices were oriented dorsally to the dog’s brain (coronal to the magnet, as in the sphinx position the dogs’ heads were positioned 90° from the prone human orientation) with the phase-encoding direction right to left. Sequential slices were used to minimize between-plane offsets from participant movement, while the 20 % slice gap minimized the “crosstalk” that can occur with sequential scan sequences. Between one and four runs of up to 500 functional volumes were acquired for each subject, each run lasting approximately 6–12 min.
Experimental design
An event-based design (cf., Dale 1999) was used, with neutral, no-go and go trials presented at semi-random intervals. Experimental runs included between 20 and 26 trials each. Each run included 10 go trials (on which the whistle was blown with no accompanying hand signal), 4 neutral trials (on which the raised left hand signal was presented with no whistle for 10 s), and between 6 and 12 no-go trials (on which the X-hand signal was displayed, the whistle was blown within 1–3 s of signal presentation and the hand signal was maintained for 10 s following the whistle blow). Trials were interspersed, and each dog received the same schedule of signals during testing, except that some no-go trials were repeated once if a dog was making a high proportion of false alarms (touching the target on no-go trials). Our aim was to acquire 20+ successful inhibition no-go trials for each subject.
Dogs were stationed in the magnet bore as shown in Fig. 2, using their custom chin rests with the plastic target affixed as during training. All signals were presented by the dog’s primary handler, who was standing directly in front of the dog at the opening of the magnet bore. The handlers dispensed rewards (hot dog pieces) by hand following successful trials (touching the target on go trials and refraining from touching the target for the full duration of no-go and neutral trials).
An observer stood next to the handler, out of view of the dog. In accordance with a previously established experimental schedule, the observer instructed the handler regarding which trial type (go, no-go, neutral) to employ. Using a mirror placed inside the magnet bore, the observer was able to determine whether the dog touched the target on any particular trial, and instructed the handler to provide or withhold food accordingly. Following each run, the dog was taken out of the scanner and allowed to walk around, drink water and rest.
Event recording and functional data preprocessing
Trial events (i.e., timing of the onset of go trials, and onset and offset of no-go and neutral trials) were recorded by the aforementioned observer via a four-button MRI-compatible button box.
Data preprocessing (cf., Churchill et al. 2012) was conducted using AFNI (NIH; Cox 1996) and its associated functions, and most steps were identical to those listed in Berns et al. (2014). In brief, two-pass, six-parameter affine motion correction was used with a hand-selected reference volume for each dog. Because dogs moved between trials, aggressive censoring [i.e., removing bad volumes from the fMRI time sequence (cf., Power et al. 2015)] was carried out. Any movement during the 1.2 s window in which a volume is acquired compromises the BOLD data for that time point. The majority of censored volumes followed the consumption of food. On average, 42 % of total EPI volumes were retained for each subject (from 16.5 to 59 %). This was somewhat lower than in previous experiments using reward and no-reward signals (where we retained ~50 % of volumes on average; Berns et al. 2014; Cook et al. 2014), likely due to the head motion required to touch the target. Two dogs were excluded on the basis of excessive motion throughout the exercise. In these two subjects, Callie and Ohana, even after motion correction, we retained only 27 and 16.5 % of volumes, which led to unusable motion artifacts in many of the remaining images and left insufficient condition repetitions for analysis. This reduced sample size for fMRI results to 11 dogs, although behavioral measures were still valid for comparison across all 13 dogs.
For each subject, a general linear model (GLM; cf., Friston et al. 1995) was estimated for each voxel in the brain using the fMRI analysis program 3dDeconvolve (part of the AFNI suite). The task-related regressors in this model were: (1) go [whistle onset], (2) inhibit [duration of X-hand trials on which the dog did not touch the target], (3) false alarm [duration of X-hand trials on which the dog touched the target) and (4) neutral [duration of raised left hand]. Put simply, this type of standard fMRI analysis computes a parallel univariate regression at each point in the brain (voxel), attempting to fit the time course of activity in each voxel in the brain with the timing of the conditions included as regressors, after accounting for the intrinsic lag of the blood oxygenation (BOLD) response. This produces a beta weight for each voxel for each regressor that can then be directly compared between conditions to determine their relative contribution to brain activity in that voxel. Some dogs did not produce false alarms during scanning, so their models did not include the third task-related regressor.
Motion time courses generated by the motion correction were also included in the model as nuisance regressors to further control for motion effects. A constant and linear drift term was included for each run to account for baseline shifts in brain activity between runs as well as slow drifts unrelated to the experiment.
Whole-brain group analyses
For group-level statistics, a high-resolution MRI canine brain atlas (Datta et al. 2012) was used as the template space into which all individual results were spatially transformed for group comparisons. The appropriate individual-level contrast ([inhibit − neutral] obtained from the GLM as described above) was extracted and normalized to template space using the Advanced Normalization Tools (ANTs) software (Avants et al. 2011). Once the individual results were in template space, they were averaged together, producing mean brain activation values at each voxel for the [inhibit − neutral] contrast across all subjects. Then AFNI’s 3dttest++ was used to compute a voxelwise t test across all dogs with the null hypothesis that each voxel had a mean value of zero. This type of group test identifies regions where, on average, subjects show a differential response in brain activation between contrasted conditions. To allow for subject heterogeneity and to better localize the brain regions associated with the contrast of [inhibit − neutral], we also included regressors for each dog’s hit rate (HR) and false alarm rate (FA).
Because this process results in the computation of a massive number of statistical tests across the brain, correction for multiple comparisons is necessary. Following standard practice in the human neuroimaging field, we then calculated the average smoothness of the residuals using the AFNI functions 3dFWHMx and 3dClustsim to estimate the significance of different cluster sizes across an anatomical frontal brain mask (cf., Hagler et al. 2006). This mask was anatomically consistent with definitions of frontal regions from older lesion work examining dog frontal cortex (Dabrowska and Szafranska-Kosmal 1972) and included all voxels rostral to the rostralmost portion of the corpus callosum minus the olfactory bulb, olfactory cortex and the basal ganglia. This procedure corrected for statistics across multiple voxels in the frontal region to specify the likelihood of falsely identifying a region as active, called the familywise error, or FWE (cf., Nichols and Hayasaka 2003). In general, the FWE depends both on the uncorrected P value at each voxel (P unc), in this case generated by the group mean t test specific to the [no-go − neutral] contrast, and the spatial extent of what is considered an area of activation. The smoothness of the residuals gives the latter. Thus, for a given P unc, an area of activation would need to surpass a threshold size of contiguous voxels, all less than P unc, to be considered significant with a FWE < 0.05.
Within any such cluster, we then extracted the average value for each dog, representing the percent increase in BOLD activation within the region on successful inhibit versus neutral trials for each subject. We then tested for correlation of activity within this region with the subject’s false alarm rate (percentage of no-go trials on which the subject made an incorrect go response), used as a direct measure of go/no-go task performance and inhibitory capability. Due to motion, brain activity could not be measured during false alarms or go trials, so false alarm rate represented an overall in-scanner behavioral performance measure that was independent from the inhibit-neutral contrast and resultant brain activation values. Because false alarm rates are fundamentally a proportion, and bounded at 0 and 1, linear regression is inappropriate. We therefore used a logit model (with each dog weighted by her number of no-go trials), which is a validated approach for modeling proportional dependent variables (Long 1997; Baum 2008).
Motion parameters were assessed for each subject for each experimental condition, as in Cook et al. (2014), to determine whether artifacts produced by condition-specific patterns of motion might explain any apparent condition-specific changes in BOLD.
To assess experimental validity, a paired t test was used to compare number of false alarms on no-go trials (with whistle) and on neutral trials (no whistle). If false alarms were produced in equal measure in both conditions, this would indicate that the subjects were not discriminating these two contexts, and the presentation of the whistle signal on no-go trials did not require increased inhibitory control.
Results
Whole-brain group analysis of [inhibit − neutral] conditions yielded a significant activation in left ventral frontal cortex (P FWE < 0.05, Fig. 3). The cluster was centered over the cortex lateral to the ventral pre-sylvian sulcus, and extended rostrally and ventrally into proreal and orbital gyri. Mean scan-to-scan movement did not differ significantly between the no-go and neutral conditions (successful no-go = 0.63 mm, neutral = 0.52 mm, pairwise t(10) = −0.68, P = 0.51, two-tailed), suggesting that the pattern of differential activation could not be accounted for by condition-specific motion. In addition, out of the 13 subjects, only one dog produced only one false alarm on a neutral trial. Mean false alarm rate was significantly higher for no-go trials (0.24) than for neutral trials (0.00), indicating validity of the no-go versus neutral comparison (t = 4.47, P < 0.001, two-sided).
Group BOLD cluster for the [inhibit − neutral] contrast in the coronal, sagittal and transverse planes (left to right). Activations are thresholded at P FWE ≤ 0.05. Notable cortical frontal landmarks are indicated: Pr.G proreal gyrus, PrS.S. pre-sylvian sulcus, Orb.G. orbital gyrus, Cr.S. cruciate sulcus. Color indicates t statistic at each voxel against the null hypothesis of equal activity to inhibit and neutral conditions
Bold activation for each subject from the frontal brain cluster defined in the group analysis by the [inhibit − neutral] contrast showed a highly significant relationship with false alarm rate in a logit analysis (Table 2; Fig. 4). Subjects with a higher false alarm rate showed lower differential activation in the frontal region on no-go versus neutral trials than did subjects with a lower false alarm rate (logit regression, P < 0.001, residual deviance 39.1, df = 9, Chi-square test of model fit P < 0.001).
Logit regression line fitting activation in the group-defined frontal region (x axis, percent BOLD signal increase on successful no-go vs. neutral trials) against false alarm rate (y axis, proportion of false alarms on no-go trials) across subjects. The BOLD response was significantly correlated with false alarm rate such that subjects with higher BOLD activation in the frontal region made fewer false alarms (logit regression, P < 0.001, residual deviance 39.1, df = 9, Chi-square test of model fit P < 0.001)
Experiment 2
Methods
Subjects
Subjects were all 13 dogs who participated in fMRI scanning on the go/no-go task in Experiment 1.
Procedure
All subjects were tested in one session at our training facility or at our laboratory at Emory University with minimal pre-training. For testing, an enclosure 3 m × 5 m was constructed out of 1 m high plastic baby gates. At one end of the enclosure, three plastic food wells were suspended from the baby gates in a line with 1 m separating them. Height of the wells was adjusted for each dog such that they were able to obtain a food reward, but not easily see into the bottom of the well from a distance. Food reward for this task was Pup-Peroni® (Big Heart Pet Brands), and a piece was affixed out of sight directly behind each food well to control for olfactory cueing.
Testing sessions were very similar to those used by MacLean et al. (2014) and proceeded as follows (Supplemental Video S2). First, during the training phase, the dog was brought into the enclosure by a handler and stationed in a seated position facing the food wells, 2 m away from the wells. An experimenter was on the opposite side of the baby gates, facing the dog. One food well, either the leftmost or rightmost, was selected for each dog (this was randomly assigned so that left was selected for seven dogs and right for six). The experimenter placed a food reward in the pre-selected well (dubbed “A”) and then stood with her back turned behind the central well (which she did on all subsequent trials prior to the release of the dog). A handler then led the dog to the A well and prompted the dog to find and eat the food, following which they returned to their initial station. Next, the experimenter again placed a piece of food in the A well, and the handler released the dog. If the dog did not proceed directly to the food well to eat the treat, the handler took and led the dog to the well to receive the food. This was repeated until the dog promptly retrieved and ate the food on release (between 1 and 6 trials for each dog). Then, the testing phase began.
During testing, the experimenter placed the treat in the A well on three consecutive trials (“familiarization”). On the fourth trial, after placing the treat in the A well, the experimenter immediately retrieved the treat and moved it to the B well, the well farthest left or right from the A well, prior to the release of the dog. Departing from much of the prior comparative work using A-not-B testing, trials in this “switch” phase were repeated until the dog successfully retrieved the treat from the B well, allowing us to obtain a continuous subject-specific measure of perseveration. If he or she stopped first at the A or unused center well on these trials, the food was retrieved by the experimenter before the dog could access it, and the dog was returned to the starting point by the handler for another trial.
We used the total number of trials required to switch to the B well to get a continuous measure of inter-subject variability. Dogs showed variability in the number of training trials required (between 1 and 6) before they could reliably select the A well without experimenter guidance. It is well established that dogs prefer to follow previously learned navigational paths (Clarke et al. 1951) and that increased experience with a successful solution makes it more difficult for dogs to subsequently switch strategies (Pongrácz et al. 2003). This might suggest that dogs who required increased familiarization to the A location in the present experiment would consequently be more resistant to switching to the B location. However, Osthaus et al. (2010) have shown that number of prior navigational trials above two is not a linear predictor of subsequent difficulty of route switching for domestic dogs. Because, counting training and the familiarization phase, all of our dogs had at least 4 and no more than 10 trials to location A prior to switch, we used an uncorrected measure of number of trials to switch as representative of ability to inhibit response to A. To explicitly test for the influence of pre-switch trials on switch behavior in our sample, we used nonparametric correlation to compare number of training and familiarization trials with number of trials to switch. Due to its robustness with small sample size, we used Kendall’s tau (Gibbons and Chakraborti 2011).
Number of trials to switch in the A-not-B task was compared using linear regression to frontal activation defined by the [inhibit − neutral] contrast, and using logit regression to false alarm rate from the in-scanner go/no-go task.
Results
Number of pre-switch trials in our subjects did not predict number of trials to switch, justifying using a total-trials-to-switch measure (Table 2, Kendall’s tau = −0.34, P = 0.17).
Percent BOLD signal change in the group-defined frontal region specific to the [inhibit − neutral] contrast was not correlated with the number of trials to switch on the A-not-B task (Pearson’s R 2 = 0.09, P = 0.38; two-tailed, Table 2). However, logit regression of the number of trials to switch in the A-not-B task and false alarm rate in the fMRI task was highly significant (Fig. 5). Subjects with lower false alarm rates took fewer trials to switch (logit regression P < 0.001, residual deviance = 45.8, df = 11, Chi-square test of model fit P < 0.001).
Logit regression line fitting A-not-B errors (x axis, number of trials required to switch to the B location) against false alarm rate (y axis, proportion of false alarms on no-go trials) across subjects. On both axes, a higher score equates to worse task performance. False alarm rate from the in-scanner task was correlated with performance on A-not-B (logit regression P < 0.001, residual deviance = 45.8, df = 11, Chi-square test of model fit P < 0.001)
Discussion
Using a combination of awake neuroimaging and behavioral study with domestic dogs, we identified a frontal brain region involved in motor inhibition and demonstrated a clear relationship between increased activation in this region and false alarm rate from the in-scanner go/no-go task. In addition, false alarm rate predicted A-not-B task performance. Taken together, these findings suggest consistent individual differences in neurobehavioral mechanisms for cognitive control in the domestic dog, and they parallel robust evidence in humans that individual differences in control correlate with structural and functional measures in frontal brain regions (Miyake et al. 2000; Kane and Engle 2002; Forstmann et al. 2008).
Because fMRI measures could not be obtained from the dogs during movement, we were unable to measure brain response during either correct or incorrect go responses. Our solution to look for differential activation underpinning response inhibition was to compare brain activation on successful no-go trials with brain activation on neutral trials. In both trial types, the dog was rewarded for staying still for the duration of the no-go and neutral hand signals. It might be suspected that the dog did not differentiate between these two trial types, and might have ignored the whistle presented during no-go trials as irrelevant. Our results strongly argue against such an interpretation. Not only was the whistle go response highly trained and maintained throughout the experiment for all dogs, but there were almost no false alarm responses on neutral trials. Only one false alarm was produced by only one dog on these trials, versus robust and variable false alarm rates on no-go trials (ranging from 0 to 0.56). The remarkable performance of our subjects on neutral trials demonstrates both their extensive prior experience with the neutral hand signal and the strength of the conditioned go response to the whistle tone. Because performance was significantly impaired on no-go versus neutral trials, it is reasonable to assume that brain activation on successful no-go trials relative to successful neutral trials was indicative of increased inhibitory control.
The brain region we identified that showed greater mean activation at the group level on successful no-go versus neutral trials was in cortex along the ventrolateral margin of the pre-sylvian sulcus, and overlapped ventrally and rostromedially with the proreal and orbital cortex (Stepniewska and Kosmal 1986). Carnivore frontal cortical regions have not been comparatively mapped to those in humans, but dog proreal cortex includes granular layer IV (Tanaka 1987)—a hallmark of primate prefrontal cortex (Preuss and Goldman-Rakic 1991)—and may be comparable to frontal regions supporting inhibition in humans and other primates, such as inferior frontal cortex (Rubia et al. 2000; Aron et al. 2007, 2014). The ventrolateral pre-sylvian cortex has not been functionally defined in dogs, but tracing studies show it is connected with dorsal premotor regions (Kosmal et al. 1984), which, coupled with its proximity to frontal cortex, make it a likely candidate for an analog to human pre-supplementary motor area, also involved in inhibition (Sharp et al. 2010). Notably, in early dog lesion work, it was lateral frontal cortex that was most reliably associated with disinhibition (Dabrowska and Szafranska-Kosmal 1972).
Human individual differences in behavior and cognition can be predicted from structural and functional variation in frontal brain regions (Forstmann et al. 2008; Kanai and Rees 2011). In addition, impaired habituation and altered frontal and pre-SMA function are related to neuropathology including ADHD (Barkley 1997) and schizophrenia (Daskalakis et al. 2002) as well as maladaptive behavior including aggression (Horn et al. 2003) and addiction (Goldstein and Volkow 2002). Our findings suggest parity between human and canine neurobehavioral mechanisms for control and support the dog as a comparative model for better understanding maladaptive behavior in humans (cf., Overall 2000). In addition, there is extensive interest in the pet-owning and veterinary communities in predicting and mitigating impulsivity in dogs. Canine impulsivity has been related to behavioral phenotypes (Van den Berg et al. 2003) and broad changes in brain metabolism (Peremans et al. 2003), and, at the extreme, can result in dog–dog or dog–human aggression (Wright et al. 2012). Our findings present a specific neural target and combined behavioral suite for examining the neurobehavioral correlates with inhibition and impulsivity in dogs. Future studies with dogs may examine functional changes in both brain activity and behavior accompanying behavioral training interventions aimed at reducing impulsivity and increasing control.
Of note, inhibition in humans and other primates shows functional lateralization, with right inferior frontal gyrus most frequently implicated (Aron et al. 2014). The region we mapped in dogs was in left frontal cortex. Although there are limited data on functional brain lateralization in dogs and other carnivores, there is an extensive literature on canine behavioral lateralization. Dogs show some evidence of socioemotional lateralization, demonstrating a left gaze bias when looking at faces, as do humans and other primates (Guo et al. 2009), and directional biases in producing and interpreting tail wag direction (Quaranta et al. 2007; Siniscalchi et al. 2013; see Artelle et al. 2011). There is also evidence of motor lateralization in dogs, although as opposed to humans dogs are not predominantly right biased. Instead, female dogs tend to be right paw dominant and male dogs left paw dominant (Wells 2003; Poyser et al. 2006; see Tomkins et al. 2010). Our subject group was split between female (6) and male (7) dogs, and we did observe sub-threshold activation specific to inhibition in right frontal regions at the group level. Therefore, while our finding of potential left lateralization is intriguing, we cannot rule out the possibility of right frontal involvement in canine inhibition, meriting further study.
Dogs are increasingly touted as a promising emerging model for comparative cognition. This first canine fMRI experiment to use an active task-based protocol helps to establish parity with the neurobehavioral literature on human control and paves the way for further integration of neural measures into the increasingly rich canine behavior literature. The ability to conduct awake fMRI with dogs engaged in active task performance helps validate the dog as a humane and accessible comparative model for cognitive research.
References
Adams B, Chan A, Callahan H, Milgram NW (2000) The canine as a model of human cognitive aging: recent developments. Prog Neuro-Psychopharmacol Biol Psychiatry 24:675–692
Amici F, Aureli F, Call J (2008) Fission–fusion dynamics, behavioral flexibility, and inhibitory control in primates. Curr Biol 18:1415–1419
Andics A, Gácsi M, Faragó T, Kis A, Miklósi Á (2014) Voice-sensitive regions in the dog and human brain are revealed by comparative fMRI. Curr Biol 24:574–578
Andrews Espy K, Kaufmann P, McDiarmid M, Glisky M (1999) Executive functioning in preschool children: performance on A-not-B and other delayed response format tasks. Brain Cogn 41:178–199
Aron A, Durston S, Eagle D, Logan G, Stinear C, Stuphorn V (2007) Converging evidence for a fronto-basal-ganglia network for inhibitory control of action and cognition. J Neurosci 27:11860–11864
Aron A, Robbins T, Poldrack R (2014) Inhibition and the right inferior frontal cortex: one decade on. Trends Cogn Sci 18:177–185
Artelle KA, Dumoulin LK, Reimchen TE (2011) Behavioural responses of dogs to asymmetrical tail wagging of a robotic dog replica. Laterality 16:129–135
Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC (2011) A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage 54:2033–2044
Barkley RA (1997) Behavioral inhibition, sustained attention, and executive functions: constructing a unifying theory of ADHD. Psychol Bull 121:65
Baum CF (2008) Stata tip 63: modeling proportions. Stata J 8:299
Berns GS, Brooks AM, Spivak M (2012) Functional MRI in awake unrestrained dogs. PLoS ONE 7:e38027
Berns GS, Brooks AM, Spivak M (2014) Scent of the familiar: an fMRI study of canine brain responses to familiar and unfamiliar human and dog odors. Behav Process 110:37–46
Bray E, MacLean E, Hare B (2014) Context specificity of inhibitory control in dogs. Anim Cogn 17:15–31
Bray EE, MacLean EL, Hare BA (2015) Increasing arousal enhances inhibitory control in calm but not excitable dogs. Anim Cogn 18:1317–1329
Brutkowski S, Dabrowska J (1963) Disinhibition after prefrontal lesions as a function of duration of intertrial intervals. Science 139:505–506
Brutkowski S, Dabrowska J (1966) Prefrontal cortex control of differentiation behavior in dogs. Acta Biol Exp 26:425–439
Brutrowski S, Mempel E (1961) Disinhibition of inhibitory conditioned responses following selective brain lesions in dogs. Science 134:2040–2041
Casey B, Castellanos F, Giedd J, Marsh W, Hamburger S, Schubert A, Vauss Y, Vaituzis A, Dickstein D, Sarfatti S, Rapoport J (1997) Implication of right frontostriatal circuitry in response inhibition and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 36:374–383
Churchill NW, Oder A, Abdi H, Tam F, Lee W, Thomas C et al (2012) Optimizing preprocessing and analysis pipelines for single-subject fMRI. I. Standard temporal motion and physiological noise correction methods. Hum Brain Map 33:609–627
Clarke RS, Heron W, Fetherstonaugh ML, Forgays DG, Hebb DO (1951) Individual differences in dogs: preliminary report on the effects of early experience. Can J Psychol 5(4):150–156
Cook PF, Spivak M, Berns GS (2014) One pair of hands is not like another: caudate BOLD response in dogs depends on signal source and canine temperament. PeerJ 2:e596
Cook PF, Brooks AM, Spivak M, Berns GS (2015) Regional brain activations in awake unrestrained dogs. J Vet Behav 10:440
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Dabrowska J, Szafranska-Kosmal A (1972) Partial prefrontal lesions and go-no go symmetrically reinforced differentiation test in dogs. Acta Neurobiol Exp 32:817–834
Dale AM (1999) Optimal experimental design for event-related fMRI. Hum Brain Map 8:109–114
Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S (2002) Evidence for impaired cortical inhibition in schizophrenia using transcranial magnetic stimulation. Arch Gen Psychiatry 59:347–354
Datta R, Lee J, Duda J, Avants B, Vite C, Tseng B, Gee J, Aguirre G, Aguirre G (2012) A digital atlas of the dog brain. PLoS ONE. doi:10.1371/journal.pone.0052140
Dilks DD, Cook P, Weiller SK, Berns HP, Spivak MH, Berns G (2015) Awake fMRI reveals a specialized region in dog temporal cortex for face processing. Peer J 3:e1115. https://doi.org/10.7717/peerj.1115
Eagle D, Bari A, Robbins T (2008) The neuropsychopharmacology of action inhibition: cross-species translation of the stop-signal and go/no-go tasks. Psychopharmacology 199:439–456
Farah MJ (1994) Neuropsychological inference with an interactive brain: a critique of the locality assumption. Behav Brain Sci 17:43–61
Forstmann BU, Jahfari S, Scholte HS, Wolfensteller U, van den Wildenberg WP, Ridderinkhof KR (2008) Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: a model-based approach. J Neurosci 28(39):9790–9796
Friston KJ, Ashburner J, Frith CD, Poline JB, Heather JD, Frackowiak RS (1995) Spatial registration and normalization of images. Hum Brain Map 3:165–189
Gibbons JD, Chakraborti S (2011) Nonparametric statistical inference. Springer, Berlin, pp 977–979
Goldstein RZ, Volkow ND (2002) Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159:1642–1652
Guo K, Meints K, Hall C, Hall S, Mills D (2009) Left gaze bias in humans, rhesus monkeys and domestic dogs. Anim Cogn 12:409–418
Hagler DJ, Saygin AP, Sereno MI (2006) Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage 33:1093–1103
Horn NR, Dolan M, Elliott R, Deakin JFW, Woodruff PWR (2003) Response inhibition and impulsivity: an fMRI study. Neuropsychologia 41:1959–1966
Jia H, Pustovyy O, Waggoner P, Beyers R, Schumacher J, Wildey C, Barrett J, Morrison E, Salibi N, Denney T, Vodyanoy V, Deshpande G (2014) Functional MRI of the olfactory system in conscious dogs. Plos ONE. doi:10.1371/journal.pone.0086362
Jones AC, Gosling SD (2005) Temperament and personality in dogs (Canis familiaris): a review and evaluation of past research. Appl Anim Behav Sci 95:1–53
Kanai R, Rees G (2011) The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci 12:231–242
Kane M, Engle R (2002) The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual differences perspective. Pyschon B Rev 9:637–671
Kosmal A, Markow G, Stepniewska I (1984) The presylvian cortex as a transitional prefronto-motor zone in dogs. Acta Neurbiol 44:273–287
Logan G, Schachar R, Tannock R (1997) Impulsivity and inhibitory control. Psychol Sci 8:60–64
Logothetis N, Pauls J, Augath M, Trinath T, Oeltermann A (2001) Neurphysiological investigation of the basis of the fMRI signal. Nature 412:150–157
Long JS (1997) Regression models for categorical and limited dependent variables. Sage, London
MacLean E, Hare B, Nunn C, Addessi E, Amici F, Anderson R et al (2014) The evolution of self control. Proc Natl Acad Sci USA 111:E2140–E2148. doi:10.1073/pnas.1323533111
Miller H, Pattison K, DeWall C, Rayburn-Reeves R, Zentall T (2010) Self-control without a “self”? Psychol Sci 21:534–538
Miller H, DeWall C, Pattison K, Molet M, Zentall T (2012) Too dog tired to avoid danger: self-control depletion in canines increases behavioral approach toward an aggressive threat. Psychon B Rev 19:535–540
Miyake A, Friedman N, Emerson M, Witzki A, Howerter A, Wager T (2000) The unity and diversity of executive functions and their contributions to complex frontal lobe tasks: a latent variable analysis. Cogn Psychol 41:49–1000
Nichols T, Hayasaka S (2003) Controlling the familywise error rate in functional neuroimaging: a comparative review. Stat Methods Med Res 12:419–446
Osthaus B, Marlow D, Ducat P (2010) Minding the gap: spatial perseveration error in dogs. Anim Cogn 12:881–885
Overall KL (2000) Natural animal models of human psychiatric conditions: assessment of mechanism and validity. Prog Neuro-Psychopharmacol Biol Psychiatry 24:727–776
Peremans K, Audenaert K, Coopman F, Blanckaert P, Jacobs F, Otte A et al (2003) Estimates of regional cerebral blood flow and 5-HT2A receptor density in impulsive, aggressive dogs with 99mTc-ECD and 123I-5-I-R91150. Eur J Nucl Med Mol I 30:1538–1546
Piaget J (1954) The construction of reality in the child. Basic Books, New York
Pongrácz P, Miklósi Á, Kubinyi E, Topál J, Csányi V (2003) Interaction between individual experience and social learning in dogs. Anim Behav 65:595–603
Power JD, Schlaggar BL, Petersen SE (2015) Recent progress and outstanding issues in motion correction in resting state fMRI. Neuroimage 105:536–551
Poyser F, Caldwell C, Cobb M (2006) Dog paw preference shows lability and sex differences. Behav Proc 73:216–221
Preuss T, Goldman-Rakic P (1991) Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate galago and the anthropoid primate macaca. J Comp Neurol 310:429–474
Quaranta A, Siniscalchi M, Vallortigara G (2007) Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr Biol 17:199–201
Riemer S, Mills D, Wright H (2014) Impulsive for life? The nature of long-term impulsivity in domestic dogs. Anim Cogn 17:815–819
Rubia K, Russell T, Overmeyer S, Brammer M, Bullmore E, Sharma T, Simmons A, Williams S, Giampietro V, Andrew C, Taylor E (2000) Mapping motor inhibition: conjunctive brain activations across different versions of Go/No-Go and stop tasks. Neuroimage 13:250–261
Sagvolden T, Russell V, Aase H, Johansen E, Farshbaf M (2005) Rodent models of attention-deficit/hyperactivity disorder. Biol Psychiatry 57:1239–1247
Schoenbaum G, Nugent S, Saddoris M, Setlow B (2002) Orbitofrontal lesions in rats impair reversal but not acquisition of go, no-go odor discriminations. NeuroReport 13:885–890
Sharp D, Bonnelle V, De Boissezon X, Beckmann C, James S, Patel M, Mehta M (2010) Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci USA 107:6106–6111
Siniscalchi M, Lusito R, Vallortigara G, Quaranta A (2013) Seeing left-or right-asymmetric tail wagging produces different emotional responses in dogs. Curr Biol 23:2279–2282
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23:S208–S219
Stępień I, Stępień L, Kreiner J (1963) The effects of total and partial ablations of the premotor cortex on the instrumental conditioned reflexes in dogs. Acta Biol Exp 23:45–59
Stepniewska I, Kosmal A (1986) Distribution of mediodorsal thalamic nucleus afferents originating in the prefrontal association cortex of the dog. Acta Neurobiol 46:311–322
Tanaka D (1987) Neostriatal projections from cytoarchitectonically defined gyri in the prefrontal cortex of the dog. J Comp Neurol 261:48–73
Tapp PD, Siwak CT, Estrada J, Head E, Muggenburg BA, Cotman CW, Milgram NW (2003) Size and reversal learning in the beagle dog as a measure of executive function and inhibitory control in aging. Learn Mem 10:64–73
Thompson PM, Schwartz C, Lin RT, Khan AA, Toga AW (1996) Three-dimensional statistical analysis of sulcal variability in the human brain. J Neurosci 16:4261–4274
Tomkins LM, McGreevy PD, Branson NJ (2010) Lack of standardization in reporting motor laterality in the domestic dog (Canis familiaris). J Vet Behav 5:235–239
Topál J, Gergely G, Erdőhegyi Á, Csibra G, Miklósi Á (2009) Differential sensitivity to human communication in dogs, wolves, and human infants. Science 325:1269–1272
Van den Berg L, Schilder MBH, Knol BW (2003) Behavior genetics of canine aggression: behavioral phenotyping of golden retrievers by means of an aggression test. Behav Genet 33:469–483
Wayne RK (1986) Cranial morphology of domestic and wild canids: the influence of development on morphological change. Evolution 40:243–261
Wells DL (2003) Lateralised behaviour in the domestic dog, Canis familiaris. Behav Process 61:27–35
Wobber V, Hare B, Koler-Matznick J, Wrangham R, Tomasello M (2009) Breed differences in domestic dogs (Canis familiaris) comprehension of human communicative signals. Interact Stud 10:206–224
Wright HF, Mills DS, Pollux PM (2012) Behavioural and physiological correlates of impulsivity in the domestic dog (Canis familiaris). Physiol Behav 105:676–682
Acknowledgments
This work was supported by the Office of Naval Research (N00014-13-1-0253).
Author contributions
All authors contributed to study concept and design and data collection. P. F. Cook and G. Berns performed data analysis. P. F. Cook drafted the manuscript, and G. Berns and M. Spivak provided critical revisions. All authors approved the final version of the manuscript for submission.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
G Berns and M. Spivak own equity in Dog Star Technologies and developed technology used in some of the research described in this paper. The terms of this arrangement have been reviewed and approved by Emory University in accordance with its conflict of interest policies.
Ethical approval
This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study was approved by the Emory University IACUC (Protocol #DAR-2001274-120814BA). These guidelines are consistent with the Association for the Study of Animal Behaviour/Animal Behavior Society guidelines.
Informed consent
All dogs’ owners gave written consent for participation in the study. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
This video demonstrates successful neutral, no-go and go trials, as well as a typical false alarm response on a no-go trial. Here the dog (“Callie”) is performing the task in her custom-made chin rest in a training context. (MP4 6475 kb)
This video demonstrates the test phase of the A-not-B procedure with one dog (“Stella”). After three trials of familiarization to location A, the dog is tested in her ability to switch to location B. (MP4 18519 kb)
Rights and permissions
About this article
Cite this article
Cook, P.F., Spivak, M. & Berns, G. Neurobehavioral evidence for individual differences in canine cognitive control: an awake fMRI study. Anim Cogn 19, 867–878 (2016). https://doi.org/10.1007/s10071-016-0983-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-016-0983-4