Abstract
Although primates have often been found to co-orient visually with other individuals, members of these same species have usually failed to use co-orientation to find hidden food in object-choice experiments. This presents an evolutionary puzzle: what is the function of co-orientation if it is not used for a function as basic as locating resources? Co-orientation responses have not been systematically investigated in object-choice experiments, and requiring co-orientation with humans (as is typical in object-choice tasks) may underestimate other species’ abilities. Using an object-choice task with conspecific models depicted in photographs, we provide experimental evidence that two lemur species (Eulemur fulvus, n = 4, and Eulemur macaco, n = 2) co-orient with conspecifics. Secondly, by analysing together two measures that have traditionally been examined separately, we show that lemurs’ gaze following behaviour and ultimate choice are closely linked. Individuals were more likely to choose correctly after having looked in the same direction as the model, and thus chose objects correctly more often than chance. We propose a candidate system for the evolutionary origins of more complex gaze following: ‘gaze priming.’
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Following the gaze of another individual offers many opportunities for a social animal: to locate food sources, to detect predators, and to witness important social interactions (Emery 2000; Zuberbühler 2008). For humans, visual co-orientation is recognised as a crucial component of language and social learning (Csibra and Gergely 2006; Bruner 1983) and is also thought to be important in development of theory of mind, including the ability to deceive intentionally (Whiten and Byrne 1988) and to attribute intentions to others (Santos and Hauser 1999).
Researchers tracing the evolution of visual co-orientation among primates have typically asked whether subjects were able to follow the line of gaze of a human experimenter, as advertised through cues such as head orientation and eye gaze direction. Great apes (Tomasello et al. 1999, 2001; Itakura 1996; Povinelli and Eddy 1996; Brauer et al. 2005) and Old World monkeys (Tomasello et al. 2001; Anderson and Mitchell 1999; Ferrari et al. 2000; Goossens et al. 2008) have been shown able to follow human gaze, whereas prosimian primates have failed at this task (Itakura 1996; Anderson and Mitchell 1999). Fewer studies have investigated following a conspecific’s gaze, but these have reported a similar ability to follow gaze in New World monkeys (Burkart and Heschl 2007; Neiworth et al. 2002) as well as Old World monkeys and apes (Tomasello et al. 1998), even when they have used photographs instead of live models (Lorincz et al. 1999; Horton and Caldwell 2006; Scerif et al. 2004). In addition, some non-primate species also show evidence of visual co-orientation (e.g. dogs, Hare and Tomasello 1999, goats, Kaminski et al. 2005, and ravens Bugnyar et al. 2004). These results, given the apparent absence of gaze following in prosimians (Itakura 1996; Anderson and Mitchell 1999), raise the possibility that the cognitive skills allowing gaze following have evolved independently in different taxa. However, if instead gaze following were found in prosimians, then it would most likely be primitive in mammals, rather than derived independently in dogs, goats, and simians. Unfortunately, systematic evidence is very meagre for prosimian primates, just the species whose evolutionary history is key to understanding the phylogenetic pattern. Recent observational evidence suggests male ring-tailed lemurs may engage in some co-orientation during everyday interactions (Shepherd and Platt 2008), but what significance this has for gaze following has not been systematically explored. The competence of prosimians in gaze following remains, therefore, to be determined.
Most puzzling, non-human primates have often seemed unable to use the information provided by others’ gaze for any practical purpose, such as locating a hidden object, even where there is positive evidence that they can follow gaze. The task generally used to assess use of gaze is the object-choice paradigm. In object-choice tasks, subjects must follow visual cues provided by an experimenter when choosing one of two (or more) potential hiding places in which a food item has been placed. Evidence that any non-human primate possesses this ability has been inconsistent. Even though chimpanzees have been shown to co-orient with humans (Itakura 1996; Brauer et al. 2005; Povinelli and Eddy 1996; Tomasello et al. 1999, 2001), subjects typically fail to reliably select the correct location (Hare and Tomasello 2004; Call et al. 2000), and only perform successfully under certain limited circumstances (Barth et al. 2005; Call et al. 1998). Orangutans and gorillas also follow human gaze (Brauer et al. 2005; Itakura 1996), and similarly appear unable spontaneously to use the cues provided in object-choice tasks, although some improvement with extensive training has been reported (Byrnit 2004; Peignot and Anderson 1999; Byrnit 2008). A recent study by Hauser et al. (2007) reported that rhesus macaques could use a conspecific-like communicative head gesture or a pointing gesture, both provided by a human experimenter, when choosing to search for a food reward in one of two boxes. However, this paper also reported that rhesus failed to use human head orientation and gaze cues to solve the task, even though this species has been shown to follow human gaze cues in previous studies (Ferrari et al. 2000; Anderson and Mitchell 1999; Tomasello et al. 2001). Possession of a valuable cognitive skill without the ability to use it for such an adaptive purpose as finding hidden food resources presents an evolutionary paradox. One possible resolution of this paradox might be that gaze following has evolved for some other function than foraging and, in non-human primates, it remains dissociated from foraging capabilities.
Some of these results, on the other hand, could reflect motivational rather than cognitive deficits (Tomasello et al. 1998). Simply, primates may be most interested in what other individuals of their own species are looking at and, as a result, might not reliably interpret human gaze as conveying information even when they automatically follow human gaze. Moreover, it is difficult to reconcile the apparent paradox when the different tasks have never been combined in a single experiment.
We therefore modified the traditional object-choice paradigm in order to study both gaze following and object choice within the same experiment, using prosimian primates as subjects and photographs of conspecific as stimuli.
In particular, we asked whether brown lemurs, Eulemur fulvus, (n = 4) and black lemurs, Eulemur macaco, (n = 2) would co-orient with a photograph of a conspecific and, if so, would they also go on to use that information when locating a hidden food item. We predicted that by analysing these two measures in tandem we would be able to determine whether co-orientation has any direct bearing on the evolution of attention understanding. If lemurs are able to co-orient but fail to incorporate this information in choosing a search location, this could suggest that gaze following evolved for another function altogether. A correlation between co-orientation and choice, however, would be testament to the evolutionarily adaptive value of gaze following, as a simple way of reading the attentional focus of others.
Methods
Subjects
Subjects were four brown lemurs (Eulemur fulvus) and two black lemurs (Eulemur macaco) at the Centre de Primatologie de l’Université Louis Pasteur, Strasbourg, France. Each species was socially housed in its own enclosure, consisting of both an outdoor (8.0 × 2.9 × 2.6 m) and indoor (4.9 × 2.1 × 2.6 m) compartment furnished with tree trunks and shelters. A tunnel between these two sections could be closed in order to isolate subjects for testing. All lemurs had been previously trained to enter the inside compartment individually, and had participated in several cognitive studies in this manner. Except during test sessions, lemurs could move freely between the two sections.
The lemurs were provided with commercial primate pellets each day and with fresh fruit and vegetables once a week. Water was available ad libitum. The lemurs were not food- or water-deprived for testing.
Training
To accustom subjects to the experimental apparatus, training sessions were first given in which the location of the raisin was revealed before the subject made its choice. A pivoting platform (70 × 16 × 25 cm) was presented, with a small opaque barrier on either end (15 × 8 cm), just as subjects would later experience in the test procedure. The experimenter placed both her hands behind the barriers, one behind each, while maintaining a neutral expression and looking straight ahead, and surreptitiously deposited a raisin behind only one. During these training sessions the barriers were lifted to reveal the raisin’s location and then replaced. The subject was then allowed to indicate one barrier to be removed by extending its arm toward one or the other barrier. The chosen barrier was lifted and that side of the platform was moved toward the subject, allowing the subject to retrieve the raisin or to see that no raisin was available. All subjects had been trained in a previous experiment to show their choices by reaching (Genty et al. 2004). In order to train subjects to attend to the full presentation, a trial was aborted and no reward was given if subjects reached before presentation was complete.
Training sessions consisted of ten trials, and each subject was permitted to complete one session per day. The location in which the raisin was hidden was pseudo-randomized so that five trials in a session were to the right, and five were to the left, with the raisin hidden in the same location in no more than three consecutive trials. Aborted trials were given again at the end of the session in order to maintain this balance. When a subject performed at 80% correct for two consecutive sessions, it was switched to the test procedure for subsequent trials.
Testing
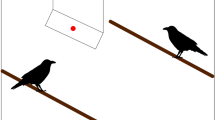
The test procedure was similar to the training phase, except that the barriers were not first lifted to reveal the correct choice. Instead, a full-color photo (15 × 15 cm) of the adult male of the group, with head and eyes oriented to the right or to the left, was placed in the center of the platform (Fig. 1). The apparent gaze of this photographic model was always oriented to the barrier behind which the experimenter placed a raisin at the start of the trial. The subject was then permitted to indicate one or other barrier. The experimenter lifted this barrier, revealing a raisin if the subject had chosen correctly or no raisin if the subject had chosen incorrectly. In either case, the indicated side of the platform was rotated toward the subject, allowing retrieval of the reward or showing the subject that no reward was available. The experimenter then reoriented the platform to its starting position and removed the model. The next trial began after the subject had consumed its reward (if it had chosen correctly), and only when the subject was sitting attentively in front of the apparatus, equidistant from each end.
Apparatus and experimental stimuli. A representation of the apparatus and stimuli presented to brown (top) and black (bottom) lemur subjects. An opaque barrier was sited on either end of a pivoting platform. After surreptitiously depositing a raisin behind only one, the experimenter placed in the centre of the platform a photograph of a known conspecific whose head and eyes were oriented towards the baited barrier
As in the training phase, a trial was aborted and no reward was given if the subject did not attend to the full presentation before reaching. Also, trials in which subjects were distracted by outside stimuli (e.g. the vocalizations of other animals) were aborted during testing and given again at the end of the session.
Test sessions consisted of ten trials, and each subject was permitted to complete one session per day. As some individuals were more willing to participate than others, the number of trials completed for each individual differs.
The direction in which the model was looking in a given trial was pseudo-randomized so that five trials in a session were to the right, and five were to the left, with no more than three consecutive trials cueing the same direction. Before each session, a sliced raisin was rubbed on either side of the platform to ensure that lemurs could not use olfactory cues to locate the food reward.
Video coding
All trials were recorded using a Sony DCR-HC19E miniDV camcorder situated behind the experimenter. Each test session was uploaded to a PC and analysed using Microsoft Windows Movie Maker version 2.1. For each test session, the experimenter provided the date, subject, and trial numbers during recording. As the direction of the model’s attention was not visible on film records, blind coding was possible. For each trial, we recorded the direction of the subject’s first inspection upon seeing the model of at least 80 ms duration (Horton and Caldwell 2006), and the subject’s subsequent choice of barrier. Trials in which the video revealed that the subject did not attend to the full presentation before reaching were discarded from the analysis.
To assess inter-observer reliability, a researcher unassociated with the project coded 5% of trials (n = 56). In judging the lemurs’ choices, the primary coder (AR) and secondary coder agreed on 100% of these trials (Cohen’s K = 1). In judging the location of the subject’s first visual inspection, the primary and secondary coders agreed on 94.6% of trials (Cohen’s K = 0.89).
Results
Lemurs can co-orient with conspecifics
Videos of each trial were coded for the location of subjects’ first visual inspection. If lemurs are able to follow gaze, they should look towards the same location as the model. We found that this was the case. Upon seeing the model, all subjects were significantly more likely to look at the barrier on the side to which the model was attending than to look at the other barrier (Binomial probability, one-tailed: Hy, Hu, Ho P < 0.001, He P = 0.043, Ro P = 0.016, Ru P = 0.002; Fig. 2).
First visual inspection and ultimate choice. Data showing both the location of first visual inspections and ultimate choice of barrier, grouped by subject. Trials in which the subject first looked to the same location as the model (“target”) are on the left, and trials in which the subject first looked to the location opposite to that of the model’s gaze (“anti-target”) are on the right. All subjects were significantly more likely to look to the target than the anti-target location. These data are further divided into correct (black) and incorrect (grey) choices
Lemurs are able to use gaze to find objects of interest
Because the lemurs did not always follow gaze, and because they did not always act upon the first target their gaze fell upon, their overall pattern of choices superficially appeared to be random. Indeed, if analyzed simply for each individual’s performance in object-choice, our data would resemble those chance performances reported by other object-choice experiments, with the lemurs’ success ranging anywhere from 10% to 100% in a given session, and each subject’s overall performance at chance levels (mean 53.8%, SD ± 16.8%). However, overall performance was not below 50% for any subject (Fig. 3). In other words, although subjects did not appear to solve the task, all six seemed to be choosing correctly more often than chance. As such, we continued our analysis by using a replicated goodness of fit test (Sokal and Rohlf 1995) for observed ratios of correct to incorrect choices (null hypothesis 1:1). A test for heterogeneity showed that the data were homogeneous (G = 1.39, df = 5, P = 0.93), allowing us to conduct our analysis on the pooled data. The outcome of this test was still significant (G = 6.78, df = 1, P = 0.009), meaning that, even though no subject did so individually, as a group subjects chose the correct search location more often than they did the incorrect search location.
To assess whether lemurs were learning over the course of the study, we examined each individual’s performance using trend analyses (Sheskin 2004; Howell 2001; Lane 2008). Five subjects did not show any significant upward trend across sessions (Hy: t = 0.48, df = 21, P = 0.64; Hu: t = 0.37, df = 17, P = 0.72; He: t = 1.79, df = 13, P = 0.10; Ro: t = 1.52, df = 20, P = 0.15; Ru: t = 0.93, df = 20, P = 0.36), while one subject’s performance did improve (Ho: t = 2.86, df = 20, P = 0.0097). This raised the possibility that Ho’s performance might be the cause of the finding that, as a group, subjects were able to choose the correct target more often than chance. We therefore repeated the replicated goodness of fit test (Sokal and Rohlf 1995) for observed ratios of correct to incorrect choices (null hypothesis 1:1), excluding Ho from this analysis. A test for heterogeneity showed that the data were homogeneous (G = 1.34, df = 5, P = 0.85), allowing us to conduct our analysis on the pooled data. The outcome of this test was significant (G = 5.20, df = 1, P = 0.02), implying that, although Ho’s performance improved over the course of the experiment, this trend was not driving the effect.
When examining choice behaviour together with gaze following for each subject, we found that a subject’s visual co-orientation and its ultimate choice of search location were closely linked. Using a chi-squared test, we found that when a lemur successfully co-oriented with the model, it was significantly more likely to choose the correct location. When, instead, it looked to the location opposite to the model’s gaze, it was more likely to choose the incorrect location (Hy: χ2 = 96.23, df = 1, P < 0.001; Hu: χ2 = 25.77, df = 1, P < 0.001; Ho: χ2 = 100.77, df = 1, P < 0.001; He: χ2 = 83.71, df = 1, P < 0.001; Ro: χ2 = 40.82, df = 1, P < 0.001; Ru: χ2 = 39.13, df = 1, P < 0.001; Fig. 2). We also explored the connection between visual co-orientation and ultimate choice by conducting a Pearson correlation, which yielded similar results, indicating that the location of lemurs’ first visual inspection is a reliable predictor of ultimate choice (Hy: R = 0.69, df = 1, P < 0.001; Hu: R = 0.38, df = 1, P < 0.001; Ho: R = 0.71, df = 1, P < 0.001; He: R = 0.79, df = 1, P < 0.001; Ro: R = 0.46, df = 1, P < 0.001; Ru: R = 0.43, df = 1, P < 0.001).
So, when paired with the information provided by the lemurs’ first visual inspections upon seeing the model, our analysis shows that when they follow gaze, lemurs do preferentially act upon the co-oriented target.
Discussion
We provide the first experimental evidence that two species of prosimian primates are able to follow the gaze of conspecifics. Further, by integrating two experimental paradigms that traditionally have been used separately, we show that lemurs preferentially act upon locations to which other individuals are attending and thereby locate hidden resources.
Visual co-orientation
Because some non-primate mammals and birds show evidence of gaze following (Kaminski et al. 2005; Hare and Tomasello 1999; Bugnyar et al. 2004; Ittyerah and Gaunet 2008), evidence of the presence or absence of this skill in prosimian primates is needed to indicate whether this ability evolved independently in different taxa, or once in a single common ancestor. Prosimian data thus hold the key to the possibility that positive results in non-primate mammals (Hare and Tomasello 1999; Kaminski et al. 2005) are due to ancient adaptation instead of convergent evolution. Prosimian species had failed the few visual co-orientation tasks given to them (Itakura 1996; Anderson and Mitchell 1999), but these tasks only explored lemurs’ ability to follow human gaze and, as such, may have underestimated their true abilities. Moreover, recent observational evidence has shown that male ring-tailed lemurs engage in some visual co-orientation when navigating their environment (Shepherd and Platt 2008), suggesting the need for further investigation.
Our results provide experimental evidence that prosimian are indeed able to follow conspecific gaze as expressed through the orientation of head and eyes jointly, implying that this cognitive skill is, at the very least, primitive for all primates. We suggest, therefore, that gaze following might have evolved only once among mammals. The abilities of birds such as ravens (Bugnyar et al. 2004), however, may reflect independent evolution of the same skill, though further exploration into the abilities of non-mammal species is needed.
It is important to note, however, that the ability to follow the gaze direction of another individual does not necessarily imply any ability to follow the eye-gaze of another individual, as head orientation plays an important role in gaze direction processing (Langton et al. 2000). As such, future work into eye-gaze direction processing in prosimian species is also needed. Like Old World monkeys (Scerif et al. 2004; Lorincz et al. 1999) and apes (Horton and Caldwell 2006), lemurs are able to co-orient with a static image of a conspecific, indicating that properties of the face are sufficient in eliciting a gaze-following response, without the presence of other cues (e.g. motion).
Object-choice
Up to now, the abilities of non-human primates to interpret gaze have seemed puzzling: many species have been shown to look in the same direction as others, yet in object-choice tasks they failed to use this information to access hidden resources. This contrast presented a paradox: what is the function of gaze following if it is not used for such a basic function as locating resources? Clear conclusions have been hindered because object-choice tasks and studies exploring visual co-orientation, though investigating different aspects of the same ability, have remained separate. Although chimpanzees were shown to follow an experimenter’s gaze in a sampling of trials in one object-choice experiment (Povinelli et al. 1999), neither this nor other studies have systematically explored visual co-orientation behaviour in tandem with subjects’ responses. Our results show that, when both factors are analysed together, there is indeed a connection between visual co-orientation and foraging choices. While the mean object-choice performance of our subjects hovered at chance levels, much like that of other species tested in similar experiments, this is because neither gaze following nor object choice work like a reflex. Lemurs do not always follow gaze, and they do not always choose the object at which they are looking. If their gaze following and object choice were independent, then one would expect a random distribution of choices whether or not the subject had followed the gaze of the model. What we find is precisely the opposite: that the lemurs’ response to the model’s gaze closely influenced their choice behaviour. When they followed gaze, they tended to choose the cued object; if they did not follow gaze, they tended to choose the non-cued object.
This link between gaze-following and ultimate choice is one that would have gone unnoticed without analysing together gaze following and choice. We therefore argue that other non-human primate subjects’ failure to perform at a high level of correct choices in an object-choice task does not imply they are incapable of using gaze at all. We encourage other researchers to re-examine the details of their subjects’ behaviour during these tasks in order to explore fully the link between gaze-following behaviour and the ultimate choice.
While our results resolve the apparent evolutionary paradox, at least for lemurs, they do not indicate that lemurs are capable of full mentalistic attribution. In other words, the link between the lemurs’ successful visual co-orientation and their above chance choice behaviour is not evidence that they understand gaze at the level of mental perspective taking––representing others as having the inner experience of seeing or attending. Instead, it is possible that lemurs interpret gaze functionally without understanding the mental states involved, simply by tending to act upon objects at which they happen to be looking, and reliably looking at objects to which other individuals are attending––a phenomenon we call ‘gaze priming.’ Gaze priming is defined as the process by which an object or location becomes more salient for an observer, as a result of its following another individual’s attention to that object or location. When an object is made more salient to an observer, this individual acts differentially in relation to that object, depending on the social and environmental context, and the information gathered as a result of following gaze. Consequently, the resulting behavioural response is flexible and can be appropriately different for dangerous or positive stimuli. On this hypothesis, visual co-orientation is indeed used to locate objects of interest in the environment, as has been postulated (Emery 2000; Zuberbühler 2008), but this orientation response need not involve reasoning about unobservable mental states.
Responding to a relation between gaze and targets may already, however, be an adaptation to one primitive, but key, feature of mentalism––so-called “intentionality,” defined as the property of mental states to be directed at or point to something other than themselves (Dennett and Haugeland 1987). Adaptations to “see” others’ gaze (an overt behaviour) as directed to targets may have been a starting point for more complex adaptations to code intentional relations in terms of covert mental states (Gómez 2008). What we report here is a candidate system for the evolutionary origins of more complex gaze following, as found in humans.
References
Anderson JR, Mitchell RW (1999) Macaques but not lemurs co-orient visually with humans. Folia Primatol 70:17–22
Barth J, Reaux JE, Povinelli DJ (2005) Chimpanzees’ (Pan troglodytes) use of gaze cues in object-choice tasks: different methods yield different results. Anim Cogn 8:84–92
Brauer J, Call J, Tomasello M (2005) All great ape species follow gaze to distant locations and around barriers. J Comp Psychol 119:145–154
Bruner J (1983) Play, thought, and language. Peabody J Educ 60:60–69
Bugnyar T, Stowe M, Heinrich B (2004) Ravens, Corvus corax, follow gaze direction of humans around obstacles. Proc R Soc Lond B Biol 271:1331–1336
Burkart JM, Heschl A (2007) Understanding visual access in common marmosets, Callithrix jacchus: perspective taking or behaviour reading? Anim Behav 73:457–469
Byrnit JT (2004) Nonenculturated orangutans’ (Pongo pygmaeus) use of experimenter-given manual and facial cues in an object-choice task. J Comp Psychol 118:309–315
Byrnit JT (2008) Gorillas’ (Gorilla gorilla) use of experimenter-given manual and facial cues in an object choice task. Anim Cogn. doi:10.1007/s10071-008-0200-1
Call J, Hare B, Tomasello M (1998) Chimpanzee gaze following in an object-choice task. Anim Cogn 1:89–99
Call J, Agnetta B, Tomasello M (2000) Cues that chimpanzees do and do not use to find hidden objects. Anim Cogn 3:23–34
Csibra G, Gergely G (2006) Social learning and social cognition: the case for pedagogy. Attention Perform XXI:249–274
Dennett DC, Haugeland JC (1987) Intentionality. In: Gregory RL (ed) The Oxford companion to the mind. Oxford University Press, Oxford, pp 383–386
Emery NJ (2000) The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev 24:581–604
Ferrari PF, Kohler E, Fogassi L, Gallese V (2000) The ability to follow eye gaze and its emergence during development in macaque monkeys. Proc Natl Acad Sci USA 97:13997–14002
Genty E, Palmier C, Roeder JJ (2004) Learning to suppress responses to the larger of two rewards in two species of lemurs, Eulemur fulvus and E-macaco. Anim Behav 67:925–932
Gómez JC (2008) Pretence in non-human primates: from intentional availability to intentional non-existence. Mind Lang (in press)
Goossens BMA, Dekleva M, Reader SM, Sterck EHM, Bolhuis JJ (2008) Gaze following in monkeys is modulated by observed facial expressions. Anim Behav 75:1673–1681
Hare B, Tomasello M (1999) Domestic dogs (Canis familiaris) use human and conspecific social cues to locate hidden food. J Comp Psychol 113:173–177
Hare B, Tomasello M (2004) Chimpanzees are more skilful in competitive than in cooperative cognitive tasks. Anim Behav 68:571–581
Hauser MD, Glynn D, Wood J (2007) Rhesus monkeys correctly read the goal-relevant gestures of a human agent. Proc R Soc B Biol Sci 274:1913–1918
Horton KE, Caldwell CA (2006) Visual co-orientation and expectations about attentional orientation in pileated gibbons (Hylobates pileatus). Behav Process 72:65–73
Howell, DC (2001) Statistical methods for psychology, 5th edn. Wadsworth Publishing, Belmont
Itakura S (1996) An exploratory study of gaze-monitoring in nonhuman primates. Jpn Psychol Res 38:174–180
Ittyerah M, Gaunet F (2008) The response of guide dogs and pet dogs (Canis familiaris) to cues of human referential communication (pointing and gaze). Anim Cogn. doi:10.1007/s10071-008-0188-6
Kaminski J, Riedel J, Call J, Tomasello M (2005) Domestic goats, Capra hircus, follow gaze direction and use social cues in an object choice task. Anim Behav 69:11–18
Lane DM (2008) Trend analysis. In: HyperStat online statistics textbook. http://davidmlane.com/hyperstat/index.html. Accessed 30 July 2008
Langton SRH, Watt RJ, Bruce V (2000) Do the eyes have it? Cues to the direction of social attention. Trends Cogn Sci 4:50–59
Lorincz EN, Baker CI, Perrett DI (1999) Visual cues for attention following in rhesus monkeys. Cah Psychol Cogn 18:973–1003
Neiworth JJ, Burman MA, Basile BM, Lickteig MT (2002) Use of experimenter-given cues in visual co-orienting and in an object-choice task by a new world monkey species, cotton top tamarins (Saguinus oedipus). J Comp Psycho 116:3–11
Peignot P, Anderson JR (1999) Use of experimenter-given manual and facial cues by gorillas (Gorilla gorilla) in an object-choice task. J Comp Psychol 113:253–260
Povinelli DJ, Eddy TJ (1996) Factors influencing young chimpanzees’ (Pan troglodytes) recognition of attention. J Comp Psychol 110:336–345
Povinelli DJ, Bierschwale DT, Cech CG (1999) Comprehension of seeing as a referential act in young children, but not juvenile chimpanzees. Br J Dev Psychol 17:37–60
Santos LR, Hauser MD (1999) How monkeys see the eyes: cotton-top tamarins’ reaction to changes in visual attention and action. Anim Cogn 2:131–139
Scerif G, Gómez JC, Byrne RW (2004) What do Diana monkeys know about the focus of attention of a conspecific? Anim Behav 68:1239–1247
Shepherd SV, Platt ML (2008) Spontaneous social orienting and gaze following in ringtailed lemurs (Lemur catta). Anim Cogn 11:13–20
Sheskin DJ (2004) Handbook of parametric and nonparametric statistical procedures. CRC Press, Boca Raton
Sokal RR, Rohlf FJ (1995) Biometry: The principles and practice of statistics in biological research, 3rd edn. W.H. Freeman and Company, New York
Tomasello M, Call J, Hare B (1998) Five primate species follow the visual gaze of conspecifics. Anim Behav 55:1063–1069
Tomasello M, Hare B, Agnetta B (1999) Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Anim Behav 58:769–777
Tomasello M, Hare B, Fogleman T (2001) The ontogeny of gaze following in chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Anim Behav 61:335–343
Whiten A, Byrne R (1988) The manipulation of attention in primate tactical deception. In: Byrne R, Whiten A (eds) Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford University Press, Oxford, pp 211–223
Zuberbühler K (2008) Gaze following. Curr Biol 18:R453–R455
Acknowledgments
Ruiz’s postgraduate study is funded by the Jack Kent Cooke Foundation and the Overseas Research Student Award Scheme provided by the British Council. We are grateful to the staff of the Centre de Primatologie for enthusiasm and support. Many thanks to Klaus Zuberbühler, David Perrett, and Robert S. Kelly for providing feedback and discussion that contributed to this project, to Simon W. Townsend for inter-observer reliability, and to three anonymous reviewers for helpful comments. The authors attest that the above study complies with the current French laws.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ruiz, A., Gómez, J.C., Roeder, J.J. et al. Gaze following and gaze priming in lemurs. Anim Cogn 12, 427–434 (2009). https://doi.org/10.1007/s10071-008-0202-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-008-0202-z