Abstract
Humans are characterized by complex social cognitive abilities that emerge early in development. Comparative studies of nonhuman primates can illuminate the evolutionary history of these social capacities. We examined the cognitive skills that rhesus monkeys (Macaca mulatta) use to follow gaze, a foundational skill in human social development. While rhesus monkeys can make inferences about others’ gaze when competing, it is unclear how they think about gaze information in other contexts. In study 1, monkeys (n = 64) observed a demonstrator look upwards either in a barrier condition where a box was overhead, so that monkeys could not see the target of her gaze, or a no barrier condition where nothing blocked her view. In study 2, monkeys (n = 59) could approach to observe the target of the demonstrator’s gaze when the demonstrator looked behind a barrier on the ground or, in the no barrier condition, behind a window frame in the same location. Monkeys were more likely to directly look up in study 1 if they could initially see the location where the demonstrator was looking, but they did not preferentially reorient their bodies to observe the out-of-view location when they could not see that location. In study 2, monkeys did preferentially reorient, but at low rates. This indicates that rhesus monkeys can use social cognitive processes outside of competitive contexts to model what others can or cannot see, but may not be especially motivated to see what others look at in non-competitive contexts, as they reorient infrequently or in an inconsistent fashion. These similarities and differences between gaze-following in monkeys and children can help to illuminate the evolution of human social cognition.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Many eyes are better than one: gaze-following, or looking in the direction that another individual is looking, can provide important information about the environment as well as about what others are seeing and thinking. In humans, gaze-following is an important social milestone that emerges early in development (Butterworth and Jarrett 1991; Flom et al. 2017). Gaze-following scaffolds the development of other important human social cognitive abilities such as language (Brooks and Meltzoff 2005; Morales et al. 1998) and theory of mind (the ability to ascribe subjective mental states to others; Baldwin and Moses 1994; Flom et al. 2017; Lee et al. 1998; Moll and Meltzoff 2011; Moll and Tomasello 2007). Yet gaze-following is also important for other species, as it can provide clues to the direction of food, predators, and mates in the external environment. Accordingly, basic co-orienting responses—where individuals match the head or eye position of others—are phylogenetically widespread (Rosati and Hare 2009; Shepherd 2010). Since other species do not display human-like social cognition or language, an important question concerns the differences between human and nonhuman gaze-following. We therefore examined whether rhesus monkeys (Macaca mulatta) can flexibly control their gaze-following responses, like humans.
A crucial distinction for considering comparative patterns of gaze-following concerns the psychological mechanisms different species use to follow gaze. One potential mechanism is termed ‘reflexive’ gaze-following: shifting where one is looking in response to external stimuli, such as simple directional eye and head cues that automatically capture attention, without further reasoning about the social context (Deaner and Platt 2003; Friesen and Kingstone 1998; Shepherd 2010). This kind of reflexive co-orienting has been documented in humans and many other species (Butterworth and Cochran 1980; Davidson et al. 2014; Friesen and Kingstone 1998; Ricciardelli et al. 2013; Ruiz et al. 2009). However, this reflexive mechanism breaks down in some contexts. For example, if the other individual’s line-of-sight is blocked by a barrier, reflexive matching of gaze direction will not allow individuals to actually perceive what the actor sees. Yet, humans and at least some other primate species can also engage in more ‘cognitive’ gaze-following responses, which involve reasoning about social or physical contexts to assess what the other agent actually sees. For example, humans and great apes habituate to repeated looks, reorient their body to observe the target of another’s gaze, and check back to reassess where that individual is looking—indicating that their responses are not purely reflexive (Bräuer et al. 2005; Butterworth and Jarrett 1991; Okamoto-Barth et al. 2007; Tomasello et al. 2001). This kind of mechanism enables individuals to accurately detect the location that others look at across a broader range of situations than purely reflexive gaze-following. In humans, more cognitive forms of gaze-following may further enable shared visual attention, a common frame-of-reference that is thought to promote the development of human social cognitive capacities in infancy (Tomasello 2014). Understanding what mechanisms different species use to follow gaze can, therefore, contextualize the patterns of human social cognitive development.
While basic gaze-following responses are common across species, the particular psychological mechanisms underlying these behaviors appear to vary (Rosati and Hare 2009; Shepherd 2010). A common test to distinguish between more reflexive versus more cognitive mechanisms for gaze-following comes from the studies of visual perspective-taking, generally involving competitive interactions. For example, chimpanzees (Pan troglodytes) can infer other’s visual perspective to outcompete both conspecifics and humans by targeting hidden food in scramble competition (Bräuer et al. 2005; Hare et al. 2000, 2001, 2006; Kaminski et al. 2008; Melis et al. 2006). Similarly, rhesus monkeys (Flombaum and Santos 2005; Lyons and Santos 2006; Santos et al. 2006) and ring-tailed lemurs (Lemur catta) (Bray et al. 2014; MacLean et al. 2014; Sandel et al. 2011) will preferentially try to steal food that a human competitor cannot perceive. In contrast, many of these same species do not use information about other’s perspective in ‘cooperative’ paradigms that involve similar inferences, but hinge on cooperative motives such as sharing food (Anderson et al. 1996; Anderson et al. 1995; Call et al. 1998, 2000, 2004; Itakura and Tanaka 1998; Vick and Anderson 2000). Thus, one proposal is that nonhuman primates can utilize ‘cognitive’ mechanisms to reason about what the others can see primarily in competitive contexts, either because they are more motivated during competition or because their abilities are actually constrained to competitive contexts (Byrne and Whiten 1989; Call et al. 2004; Hare 2001; Lyons and Santos 2006). In contrast, humans can use these abilities more flexibly across many contexts (Bettle and Rosati 2016; Rosati et al. 2016).
Another approach to understanding gaze processing in primates comes from work using ‘geometric’ gaze-following tasks. Here, a demonstrator looks at an object that is behind a barrier and thus outside of the subject’s line-of-sight, so individuals must actually reorient from their initial position, rather than just match their head to the demonstrator’s direction of gaze, to see what the demonstrator sees. In fact, both children and other great apes will actively move to look behind a barrier that a demonstrator is looking behind in this context (Bräuer et al. 2005; MacLean and Hare 2012; Moll and Tomasello 2004; Okamoto-Barth et al. 2007; Tomasello et al. 1999). Since children and apes move to look behind the barrier, their responses cannot stem from a purely reflexive co-orientation process; such a response would lead them to simply match the gaze direction of the demonstrator and, therefore, incorrectly look past the barrier and miss the true target location of the actor’s attention. Instead, these species exhibit a more cognitively controlled response by moving their bodies to see where the other is looking, accounting for some aspect of the other’s line-of-sight. Crucially, these interactions do not entail any obvious competitive motives like visual perspective-taking paradigms, such as contested food or initial agonistic displays. This suggests that apes can make inferences about where others are looking in a flexible manner, using cognitive gaze-following abilities even in the absence of competition. This aligns with accumulating evidence that great apes, like children, exhibit a broad spectrum of social cognitive abilities across competitive and non-competitive social contexts (Bulloch et al. 2008; Hopkins et al. 2007; Hostetter et al. 2007; Krupenye et al. 2016).
Can other primate species also reason about gaze across different social contexts, or do they show most robust skills specifically in competitive contexts (Byrne and Whiten 1989; Call et al. 2004; Hare 2001; Lyons and Santos 2006)? Rhesus macaques provide a strong test of this proposal. First, rhesus monkeys are characterized as a strongly despotic species, exhibiting high rates of agonism in their natural behavior. Long-term behavioral observations indicate the reduced levels of affiliative interactions compared to other macaque species (Brent et al. 2013; Thierry 2002; Widdig et al. 2002), and most clear examples of rhesus cooperation in natural interactions involve providing support during aggressive interactions or rank disputes (Cheney 2011; Higham and Maestripieri 2010; Widdig et al. 2006). Although there have been few experimental assessments of rhesus cooperation, comparisons with other closely related macaque species also reveal that dyads of rhesus monkeys collaborate much less frequently than more tolerant species (Petit et al. 1992). Thus, rhesus monkeys are characterized by extreme despotism, and competition is central to their natural social behaviors.
Currently, the strongest evidence that rhesus monkeys can make inferences about other’s gaze comes from competitive interactions. For example, rhesus monkeys will preferentially try to steal food from a human competitor that cannot see their approach, compared to one who can (Flombaum and Santos 2005), and will preferentially steal in a ‘quiet’ fashion to avoid alerting a human competitor to theft (Santos et al. 2006). This suggests that monkeys can deduce what others perceive and use it to outcompete them. Work using expectancy looking-time methodologies, involving measures of looking to index cognitive processes, has further shown that monkeys understand other’s knowledge states based on what the other individual previously saw (Marticorena et al. 2011; Martin and Santos 2014). For example, rhesus monkeys look longer when a demonstrator searching for hidden food searches in an empty box versus where the food is located—indicating surprise at this unexpected action (Marticorena et al. 2011; Martin and Santos 2016). While these looking-time tasks do not involve direct competition, the ability to track where individuals are searching for food is highly relevant to competitive scenarios. Finally, rhesus show basic co-orientation responses to both conspecifics and humans (Call et al. 1998; Emery et al. 1997; Itakura 1996; Rosati et al. 2016; Rosati and Santos 2017; Shepherd 2010; Shepherd et al. 2006; Tomasello et al. 2001), and are sensitive aspects of social context when doing so. For example, when a demonstrator looks at a distantly located object in a surprised manner while vocalizing, monkeys look to this object. In contrast, when she has previously seen this object, the monkeys search longer to identify an alternative target of gaze (Drayton and Santos 2017). However, rhesus macaques can use similar vocal signals in competitive contexts, such as during aggressive interactions (Lindburg 1971; Partan 2002). Thus, it is possible that they interpreted this as a competitive or agonistic situation. Consequently, evidence for cognitive gaze-following in rhesus comes primarily from interactions that are either clearly competitive or could be construed as so.
In the current work, we therefore examined rhesus monkeys’ abilities to use cognitive gaze-following mechanisms in neutral, non-competitive interactions. Across two experiments, rhesus monkeys saw a human demonstrator look in a particular direction. Monkeys could either see the target location of the actor’s attention by moving their head in the same direction as the actor, or it was necessary for the monkeys to reorient their bodies from their initial viewing position to see the target location, allowing us to distinguish reflexive responses (involving simple matching of head orientation) from more cognitive forms of gaze-following (involving reasoning about where others are specifically looking). In study 1, we examined monkeys’ responses when a human actor looked upwards (either at the sky or into an overhead box), and in study 2, we examined monkeys’ responses when a human actor looked down (either behind a window or behind a closed box). Importantly, these studies never involved contested food or agonistic emotional or vocal displays, key cues indicating a competitive context in prior work. These studies can shed light on how individuals from this highly competitive species use their social cognitive abilities across contexts.
Study 1: Overhead barrier
In study 1, we examined monkeys’ responses to a human demonstrator looking upwards. In the barrier condition, the human’s line-of-sight towards the sky was blocked by an overhead box, such that the target of her gaze was inside the box. In the no barrier condition, she produced the same action, but there was no box above her head. We predicted that if the monkeys understood the demonstrator’s line-of-sight, they should preferentially look up at the sky in the no barrier condition where they could also see the demonstrator’s target from their initial location compared to the barrier condition when the demonstrator looked inside the box, but should rather reorient by approaching the apparatus in the barrier condition in order to see where the demonstrator was looking. In contrast, monkeys using reflexive mechanisms should match their head direction to the demonstrator’s head direction similarly in both situations, and not reorient their body to see what the demonstrator was actually looking at in the barrier condition.
Methods
Subjects
Our final sample comprised 64 rhesus monkeys living at the Cayo Santiago Field Station (38 males and 26 females, ranging from 1.5 to 21.3 years). Cayo Santiago is a 38 acre island off the coast of Puerto Rico (Rawlins and Kessler 1987), with approximately 1500 semi-free-ranging monkeys that are highly habituated to humans and can be individually by unique chest tattoos and ear notches. While monkeys from this population have participated in prior studies of gaze-following (Drayton and Santos 2017; Rosati et al. 2016; Rosati and Santos 2017) and see a variety of natural barriers (such as trees and rocks), they were naïve to gaze-following tasks involving an overhead barrier used in the current study.
Apparatus and setup
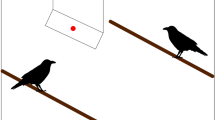
Monkeys experienced one of two conditions in a between-subjects design in which they saw a demonstrator look upwards by rotating both her head and eyes (see Fig. 1 and Movie S1 in the ESM for examples). In the barrier condition, the demonstrator looked upwards into a barrier above her head (a box 41 cm long, 31 cm wide, and 15 cm deep; propped onto a stick attached to a tripod of total height 1.77 m). The demonstrator could see into the box from her position, but the monkey could not (see Fig. 1a). In the no barrier condition, the demonstrator performed the same actions next to the tripod, but her line-of-sight was not blocked (the box was removed). As such, the monkeys could observe her visual target from their initial position in this condition.
Setup for study 1: overhead barrier. a In the barrier condition, the demonstrator’s line-of-sight was blocked by an overhead barrier. b In the no barrier condition, the demonstrator looked at the sky. c Diagram of setup. To see the target of the demonstrator’s gaze in the barrier condition, monkeys had to approach the apparatus. d Video still example of the subject looking at the demonstrator at the start of a trial. e Video still of a monkey looking upwards
Following the methods of prior work (Rosati and Santos 2017; Rosati and Santos 2017; Tomasello et al. 2001), there was no specific target stimulus in either condition that would cause the monkey to look up independently of the demonstrator’s actions; rather, the demonstrator either looked upwards with her line-of-sight unimpeded (no barrier condition) or she looked upwards into the box (barrier condition). To ensure that the gazing actions of the demonstrator appeared plausible, the apparatus was always set up near a tree, such that she could feasibly be gazing at something above (again following the methods of Rosati et al. 2016; Rosati and Santos 2017). We always checked that there were no other monkeys above the apparatus who might attract the subject’s attention. Finally, we used a human demonstrator to ensure tightly controlled behavior across conditions; the previous research shows that macaques robustly follow the gaze of both humans and conspecifics at similar rates (Ferrari et al. 2000; Rosati et al. 2016; Rosati and Santos 2017; Teufel et al. 2010; Tomasello et al. 2001).
Procedure
In sessions, Experimenter 1 (E1) identified a monkey who was sitting calmly. Condition was randomly assigned based upon a pre-designated list carried by Experimenter 2 (E2). Next, E1 placed the apparatus 2–3 m from the monkey and stood to the side of the apparatus (see Fig. 1). From their initial position, monkeys could observe the apparatus and E1, but could not see into the barrier. E2 stood approximately 6 m away from the monkey, to film the monkey’s response.
At the start of each trial, E1 attracted the monkey’s attention by calling to them and snapping her fingers. When the monkey attended, she said ‘now’ and looked directly upwards (into the box in the barrier condition, or parallel to the stick in the no barrier condition; see Fig. 1a, b). The 10 s looking phase, timed with a stopwatch by E1, began when she said ‘now’. This phase was used to assess if monkeys looked upwards after the demonstrator did (see Fig. 1d, e for examples). After E1 looked up for 10 s, the timer beeped and the approach phase began. This phase allowed us to assess if monkeys reoriented by approaching the apparatus, within a distance where they could see into the box in the barrier condition. Here, E1 turned around and walked approximately 7 m away from the monkey (to stand behind E2), so the monkey could approach without being in close proximity to E1. Monkeys had 1 min to approach. Subjects had to complete the initial 10 s looking phase to be included.
Exclusions
Occasionally, monkeys who were approached for testing failed to produce scorable responses. For example, they sometimes failed to observe the demonstrator look up in the looking phase, or were displaced by other monkeys before they could approach. To assess this, a blind coder scored which sessions should be excluded. For the looking phase, five individuals were scored as not observing the demonstrator’s looking demonstration (n = 5); an additional 2 monkeys were excluded at time of test because of apparatus failure or experimenter error (e.g., the apparatus fell down). For the approach phase, 15 additional individuals were excluded during video coding [following similar criteria described in Rosati and Santos (2016)], because another monkey displaced the subject before they made a response (n = 1), another monkey tampered with the apparatus (n = 11), or the subject left the testing area before the approach phase began (n = 1); additional 2 subjects were excluded due to apparatus failure. These individuals were included in the looking analyses. These exclusions resulted in a final sample of 31 subjects in the barrier condition and 33 in the no barrier condition for looking phase analyses; and 25 monkeys in the barrier condition and 24 in the no barrier condition for approach analyses.
Coding and reliability
Two independent coders scored responses of the final set of 64 subjects. We clipped out the 10 s looking phase and the minute-long approach phase to code them separately. Each video clip was assigned a random trial ID, so coders could score looks blind to condition. The barrier was inherently visible in the approach phase, but coders were blind to the monkey’s initial response in the looking phase. To code the looking phase responses (see ESM Movie S1), each coder marked the trial start (when E1 said ‘now’) and coded the subsequent 10 s frame-by-frame. Following the previous work (Rosati et al. 2016; Rosati and Santos 2017), we coded:
-
1.
whether the individual looked up towards the sky or not from their initial starting position, as a binomial response. This is based on changes in monkey’s head and/or eye direction (see Fig. 1d, e and Video S1 for examples). This was our key measure in the look phase. We predicted that the monkeys would look up more in the no barrier condition, because they could see the target location of the demonstrator’s gaze, but would be less likely to produce this response when it did not allow them to see the location where the demonstrator was looking.
-
2.
total duration of looking up in seconds; we predicted that monkeys would look up longer in the no barrier condition to better observe what the demonstrator was looking at.
-
3.
latency to look up in seconds; we predicted that the monkeys would look up sooner following the demonstrator’s movements in the no barrier condition where monkeys could see the target location of demonstrator’s gaze.
-
4.
the number of discrete looks up as a count response; we predicted that the monkeys would look up more times in the no barrier condition to better identify what E1 was looking at, since they could see the target location of the experimenter’s gaze in this condition.
The reliability coder had high reliability with the primary coder for these measures (looking up: Κ = 0.94; duration of looking: rp = 0.98; latency to look: rp = 0.89; number of looks: rs = 0.94).
For the approach phase, we examined whether the monkeys reoriented to a position where they could see what the demonstrator looked in the barrier condition. In this naturalistic context, it was difficult to assess whether the monkey actually looked into the barrier, so we used approaches within arm’s distance of the apparatus as a proxy for reorientation that could be scored in a comparable way in both conditions. We therefore coded each 1-min approach phase clip for:
-
1.
whether the monkey approached within arm’s distance of the apparatus as a binomial response (see Movie S1). This was our key measure in the approach phase; we predicted that the monkeys would preferentially approach the apparatus in the barrier condition, because reorientation was necessary to see the target location of the demonstrator’s gaze in this condition.
-
2.
the latency to approach within an arm’s distance of the apparatus in seconds; we predicted that if the monkeys’ approach response reflected attempts to look at the target of E1’s gaze, they would approach more quickly in the barrier condition. The reliability coder had high reliability with the primary coder for these measures (approaching: Κ = 1.0; latency to approach: rp = 0.98).
Statistical analyses
We analyzed the data in R v3.4.1 (R Development Core Team 2017). We used logistic regressions implemented with the glm function to examine propensity to look upwards or approach; linear regressions to examine total looking-time and latency to approach; and Poisson regressions to analyze number of looks, a count measure. The looking latency data were heavily right skewed, so we used an inverse Gaussian distribution (inverse link function) in accordance with recommendations for skewed reaction time data (Baayen and Milin 2010; Lo and Andrews 2015). For all analyses, we first constructed a base model that accounted for subject’s age and sex, which have been shown to affect gaze-following responses in prior studies (Rosati et al. 2016; Rosati and Santos 2017). We then added in condition as a predictor and examined whether model fit improved using likelihood ratio tests (Bolker et al. 2009); here, significant improvement in fit indicates that the predictor should be included in the model. We also report Akaike information criterion (AIC); lower AIC values indicate relatively better model quality.
Data availability
Data from both studies is available on Dryad Digital Repository: https://doi.org/10.5061/dryad.3g87c35.
Results
We examined monkeys’ propensity to look upwards across conditions. During the looking phase, M = 57.58% of monkeys looked up in the no barrier condition, whereas only 32.26% of monkeys looked up in the barrier condition (see Fig. 2a). Including condition significantly improved model fit (LRT: \(\chi^{2}\) = 4.31, df = 1, p = 0.04; AIC = 85.52 compared to 87.82 in base model). The full model also showed a non-significant trend that more males than females looked up, and more younger monkeys than older monkeys looked up (see Table 1). Importantly, in addition to accounting for age and sex in the models, there were similar numbers of males in both conditions (19 in each), and there was no difference in subjects’ age between conditions (mean age barrier = 7.69; mean age no barrier = 7.17; t62 = 0.39, p > 0.69). These demographic characteristics cannot account for the condition effect.
Looks upward and approaches in study 1 (overhead barrier). a Proportion of individuals who looked upward during the looking phase. b Proportion of monkeys who approached the apparatus during the approach phase. Total number of individual monkeys showing each response per condition is indicated above the relevant bar
Next, we looked at the total duration of time that monkeys spent looking up. We included only trials where the monkey did look up (10 in barrier and 19 in no barrier): in the barrier condition, monkeys looked up for 1.65 s on average, and for 2.52 s in the no barrier condition. Adding condition did not significantly improve model fit (LRT: \(\chi^{2}\) = 2.28, df = 1, p = 0.35; AIC = 116.21 compared to 115.20 for the base model; see ESM Table S1 for model parameters).
We found similar results for latency to look and number of total looks: including condition as a predictor did not improve model fit (latency to look: \(\chi^{2}\) = 0.53, df = 1, p = 0.21, AIC = 105.57 compared to 104.3 for the base model; number of looks: \(\chi^{2}\) = 0.00, df = 1, p = 0.98, AIC = 81.95 compared to 79.92 for the base model; see ESM Tables S2–S3). Overall, these results indicate that the key difference across conditions concerned the monkeys’ overall propensity to look up or not, not these other characteristics of their gazing response if they did look up.
We then analyzed the monkeys’ approaches. In the barrier condition, monkeys approached on 16.00% of trials; and on 20.83% of trials in the no barrier condition. Including condition did not significantly improve model fit (\(\chi^{2}\) = 0.19, df = 1, p = 0.66, AIC = 46.58 compared to 44.77 in base model; see Table 2). We also examined monkeys’ latency to approach. For this analysis, we only included those trials where the individual did approach: 4 in the barrier condition and 5 in the no barrier condition. On average, monkeys took 17.12 s to approach in the barrier condition, and 13.59 s in the no barrier condition. Including condition did not improve model fit (\(\chi^{2}\) = 0.00, df = 1, p = 1.00, AIC = 60.95 compared to 58.95 for the base model). Overall, monkeys did not preferentially reorient when their view of the demonstrator’s visual target was blocked.
Discussion
These results indicate that rhesus monkeys were more likely to look upwards when they could see the target location of the demonstrator’s attention from their initial position. This suggests that rhesus monkeys can utilize information about another individual’s line-of-sight to modulate gaze-following. However, the monkeys did not preferentially reorient to view the target location by approaching in the barrier condition, and did not approach very frequently. One possibility is that monkeys were not motivated to view what the demonstrator was looking at, unlike children and apes tested in similar paradigms (Bräuer et al. 2005; Moll and Tomasello 2004; Okamoto-Barth et al. 2007). However, note that our study differed from prior work using an overhead barrier. This allowed us to examine propensity to look up and approaches as two different dependent measures, but could have made it more difficult for the monkeys to reorient to locate the demonstrator’s visual target. From a motivational perspective, monkeys might be less likely to reorient to look into an overhead barrier, if they do not expect an interesting stimulus to be located there. They might expect any interesting stimuli to have ‘fallen out’ given the boxes’ orientation to the ground—although insects or hanging fruit could have been present, or items stuck to the inside of the box. In addition, the overhead setup made it challenging to code looks into the box if they approached. In study 2, we, therefore, developed a paradigm that was more analogous to prior work with apes.
Study 2: Reorienting around a barrier
In study 2, we examined monkeys’ responses to a human demonstrator looking downwards behind an apparatus. In the barrier condition, the monkey’s view of the demonstrator’s target of gaze was blocked by an occluder, whereas in the no barrier condition, the demonstrator looked behind a ‘window frame’ in the same location, so the monkeys also could already see where the demonstrator looked from their initial position. We predicted that if the monkeys understood the demonstrator’s line-of-sight, they should preferentially approach and look behind the apparatus in the barrier condition to observe the location where the demonstrator had looked.
Methods
Subjects
Our final sample was 59 rhesus monkeys from the same population as study 1 (41 males and 18 females, ranging from 0.84 to 18.41 years). Nine monkeys in our final sample also participated in study 1, but were naïve to the particular procedure used here. To our knowledge, there have been no prior studies of gaze-following utilizing these kinds of barriers in this population, although these monkeys have experienced studies involving boxes or other apparatuses placed on the ground (Marticorena et al. 2011; Martin and Santos 2014; Santos et al. 2006).
Apparatus and setup
Monkeys experienced one of two conditions in a between-subjects design in which they saw a demonstrator look behind an apparatus on the ground (see Fig. 3 and ESM Movie S2 for example.). The apparatus was a box (76 cm high, 27 cm wide, 28 cm deep; see Fig. 3). In the barrier condition, the apparatus blocked the monkey’s view of what she was looking at. In the no barrier condition, we removed the front panel of the apparatus facing the money, so they could directly see where the demonstrator looked from their starting position.
Setup for study 2: reorienting around a barrier. Demonstrator looking behind apparatus in the a barrier condition and b no barrier condition. c Diagram of setup. To look where the demonstrator had looked, the monkey has to approach behind the apparatus in the barrier condition. Monkeys in the no barrier condition could see the target location from their initial position, because the front panel of the box was removed (dashed lines). Video stills of monkeys a in their initial position and e reorienting to look behind the apparatus
Procedure
In sessions, experimenter 1 (E1) first identified a calm monkey for testing, and then, condition was randomly assigned by experimenter 2 (E2) based on a pre-assigned list. Next, E2 set up the apparatus approximately 2–3 m from the monkey, before standing approximately 6 m away from the monkey to film their response. E1 then approached the apparatus to stand beside it, such that the apparatus was close to E1 and oriented away from the subject. E1 then produced the looking demonstration. Each session then had two phases. In an initial 5 s looking phase, the demonstrator looked behind the apparatus; note that we did not code any responses from the monkey during this phase, unlike in study 1. This was followed by a 30 s approach phase. We used a 30 s long approach phase, rather than a minute-long approach phase, as in study 1, to minimize the possibility of interference by other monkeys. The average time to approach in study 1 was 15.16 s, so this gave sufficient time to approach. To start the trial, E1 attracted the monkey’s attention by calling and snapping her fingers. When the monkey attended to her, she turned her head and shoulders to gaze directly behind the apparatus, while bending at the waist (see Fig. 3). E1 held her pose for 5 s, and then turned around and walked to stand behind E2, so that the monkey could approach the apparatus. The approach phase began as she straightened up to turn around. After 30 s, E2 stopped filming and the trial ended. During the session, E2 filmed the monkey, so that the monkey and apparatus were both in the shot.
Exclusions
Some of the monkeys that were approached for testing were not included in the final data set, as in study 2. A blind coder, therefore, watched all the sessions from videotape and scored whether such interferences made the session unusable. In total, nine individuals were excluded from the approach measure, because other monkeys displaced the subject (n = 3) or tampered with the apparatus (n = 6). This resulted in a final sample of 31 monkeys in the barrier condition, and 28 in the no barrier condition.
Coding and analyses
Two coders scored the sessions from video, using similar procedures to study 1. We coded:
-
1.
whether the individual approached the apparatus (to within an arm’s distance of the apparatus) or not as a binomial response. This was our key measure; we predicted that the monkeys should preferentially approach behind the apparatus to observe the target of her gaze in the barrier condition.
-
2.
the latency to approach within an arm’s distance of the apparatus; we predicted that if the monkeys’ approach response reflected attempts to look at the target of E1’s gaze, they would approach more quickly (shortly after the looking phase demonstration) in the barrier condition.
-
3.
whether individuals looked behind the apparatus or not as a binomial response; we predicted that if monkeys understand the demonstrator’s line-of-sight, they would preferentially look behind—the apparatus in the barrier condition. We used looks behind the apparatus as a secondary code and approaches as our primary measure. This is because, sometimes, monkeys produced an approach response, but then another monkey interfered before they could produce a look response (two of ten trials with approaches).
The reliability coder had high reliability with the primary coder for all measures (approaching: Κ = 0.94; looking behind barrier: Κ = 0.91; latency to approach: rp = 1.0). We analyzed the data using the same general approach as for study 1.
Results
In the barrier condition, 25.81% of the monkeys approached the apparatus, whereas only 7.14% approached in the no barrier condition (see Fig. 4). Including condition as a predictor in our model significantly improved fit (LRT: \(\chi^{2}\) = 4.31, df = 1, p = 0.04; AIC = 56.52 compared to 58.83 for the basic model): more monkeys approached when they could not see the target of the demonstrator’s gaze from their initial position (see Table 3). In study 1, in addition to accounting for age and sex in the models, we also randomly assigned subjects to condition while accounting for sex and age cohort. There were similar numbers of males in both conditions (20 in barrier and 21 in no barrier), and no difference in subjects’ age between conditions (mean age barrier = 8.05, mean age no barrier = 8.31; t57 = 0.22, p > 0.82).
To analyze latency to approach, we only included trials where the individuals did approach. On average, monkeys took 12.66 s to approach in the barrier condition (n = 8), and 26.27 s to approach in the no barrier condition (n = 2). We found a non-significant trend for faster approaches in the barrier condition (LRT: \(\chi^{2 }\) = 221.63, df = 1, p = 0.051; AIC = 73.89 compared to 76.81 for the base model; see ESM Table S4).
Finally, we checked whether approaches did relate to looking behavior. For 7 of the 8 approach trials where looks were possible to code, the monkey clearly looked behind the apparatus, either by looking at this location from over the top of the apparatus (n = 3) or by walking to one side and looking back behind the apparatus (n = 3), or doing both (n = 1). The main condition effect from the analysis of approach responses held when we analyzed looks instead of approaches (LRT: \(\chi^{2 }\) = 4.98, df = 1, p = 0.03; AIC = 39.31 compared to 42.29 for the base model). This supports our interpretation of approaches as reflecting an attempt to observe the demonstrator’s visual target location behind the barrier.
Discussion
Monkeys were more likely to approach and look behind an apparatus in the barrier condition when they could not initially see where the demonstrator was looking, compared to the no barrier condition where they could. This shows that rhesus monkeys can model other’s the line-of-sight and use this information to reorient. Notably, the monkeys in study 1 did not preferentially approach the way which they did in study 2. One possibility is that reorienting in study 1 was more difficult, because that was overhead. By comparison, study 2 used an apparatus placed upon the ground, more similar to past work with apes and children (Bräuer et al. 2005; Moll and Tomasello 2004; Okamoto-Barth et al. 2007). Monkeys may also have more frequently experienced interesting stimuli upon the ground (such as food), so are subsequently more interested in approaching in that situation. Notably, however, approaches were infrequent in both studies. Thus, monkeys appear to understand that the target of the demonstrator’s gaze was hidden by the barrier in that situation and responded accordingly, but were overall relatively unmotivated to actually approach to observe the target of her gaze.
General discussion
Across two studies, we found that rhesus monkeys accounted for whether they could see where others are looking, exhibiting cognitive control over their gaze-following responses rather than just reflexively matching a shift in other’s attention. In study 1, more monkeys looked up in the no barrier condition when doing so enabled them to observe the same overhead location where the actor was looking, compared to a barrier condition where the actor’s line-of-sight upwards was blocked. In study 2, more monkeys approached an apparatus on the ground that a human had looked behind in the barrier condition when their initial view of the location was blocked, than a no barrier condition where the apparatus was a window frame. However, rhesus monkeys may not be especially motivated to see what other individuals are looking at in these contexts, as suggested by the low rates of approaches in both studies. Overall, these results show that gaze-following in rhesus macaques accounts for at least some aspects of what others actually see. The current work does not support the interpretation that monkeys engaged in reflexive gaze responses in response to simple directional head or eye cues (Friesen and Kingstone 1998; Hood et al. 1998; Shepherd 2010), because this would have produced identical responses regardless of whether the actor’s line-of-sight was blocked by a barrier. This builds on prior work, showing that rhesus monkeys exhibit basic co-orienting responses (Deaner and Platt 2003; Emery et al. 1997; Rosati et al. 2016; Rosati and Santos 2017; Tomasello et al. 2001).
As such, our results indicate that even extremely despotic primate species can use cognitive abilities outside of competitive situations to infer where others look, in contrast to some theoretical proposals (Byrne and Whiten 1989; Hare 2001; Hare and Tomasello 2004; Lyons and Santos 2006). Unlike prior work with rhesus monkeys, our paradigm did not involve competitive cues, did not involve food, and did not invoke a scenario where the subject needed to ‘outwit’ the demonstrator. This work, thus, adds to growing evidence that some primate species can use social cognitive abilities across many different social contexts (Bulloch et al. 2008; Crockford et al. 2012; Grueneisen et al. 2017; Hopkins et al. 2007; Hostetter et al. 2007; Melis and Tomasello 2013; Yamamoto et al. 2012). However, we also found that rhesus monkeys did not seem particularly motivated to see where others were looking in this non-competitive context. While rhesus monkeys adjusted their gaze-following responses depending on whether they could also see where the demonstrator had looked, they reoriented at low rates in both studies. Importantly, the monkeys clearly used information about the actor’s line-of-sight in both studies: more individuals looked up in the no barrier condition in study 1, and more approached to view behind the barrier in study 2. Thus, it is unlikely that they approached infrequently due to a (non-motivational) cognitive inability to assess where others look. Monkeys in this population also approach and search novel apparatuses at high rates when they think that there is hidden food (Flombaum and Santos 2005; Rosati and Santos 2016; Santos et al. 2006). Moreover, both infants and apes will approach to look behind a barrier that a demonstrator looks behind in a similar situation (Bräuer et al. 2005; Butler et al. 2000; Moll and Tomasello 2004; Okamoto-Barth et al. 2007). An important question is therefore why the rhesus monkeys were relatively unmotivated to approach and observe where the demonstrator was looking in these contexts.
One possibility is that monkeys were uninterested in approaching because the demonstrator was a human rather than a conspecific. However, this interpretation is inconsistent with the fact that monkeys did frequently follow the demonstrator’s gaze in study 1, demonstrating that they do pay attention to where humans look. More generally, macaques follow the gaze of both human and conspecific demonstrators (Ferrari et al. 2000; Rosati et al. 2016; Rosati and Santos 2017; Teufel et al. 2010; Tomasello et al. 2001), and most evidence that these rhesus can account for other’s visual perspective or knowledge states comes from studies involving interactions with a human (Drayton and Santos 2017; Flombaum and Santos 2005; Marticorena et al. 2011; Martin and Santos 2014; Santos et al. 2006). Moreover, prior work showing that great apes can follow gaze around barriers also involved human actors (Bräuer et al. 2005; Okamoto-Barth et al. 2007). Therefore, it is unlikely that the use of a human demonstrator alone can explain the rhesus monkeys’ low motivation to approach and observe where the actor had looked.
Another possibility is that rhesus monkeys’ low levels of social tolerance drive this response. Several proposals have argued that tolerant species exhibit more robust social cognitive abilities than do despotic species, the idea being that these social cognitive skills can facilitate cooperative or prosocial interactions (Burkart et al. 2009; Hare 2017; Hare and Tomasello 2005; Joly et al. 2017). For example, humans frequently use gaze as a cooperative cue to partake in joint attentional interactions (Tomasello 1995; Tomasello and Carpenter 2007) and when providing pedagogical information (Csibra and Gergely 2009; Senju and Csibra 2008). This raises the possibility that while competitive primate species can flexibly use social cognitive abilities, humans may be uniquely motivated to use these skills to engage in interactions where they share attention–for example, by looking at the same location or object. This shift may contribute to the development of human-unique social abilities.
One important question for future research, therefore, concerns how rhesus monkeys respond to the same gaze cues across different contexts. In this study, we tried to establish a neutral interaction, and, therefore, did not use cues that were either explicitly competitive or cooperative in nature. For example, the experimenters never handled food, and never produced emotional or vocal signals commonly used in either agonistic of affiliative contexts. Yet, previous work has established that closely related macaque species with different social style may respond differently to gaze cues depending on these kinds of signals, or have different reactions particular social contexts. For instance, competitive rhesus monkeys gaze-follow differentially according to rank (Shepherd et al. 2006), while more tolerant Barbary macaques (Macaca sylvanus) do not (Teufel et al. 2010). Crested macaques (Macaca nigra), another tolerant species, respond especially quickly to gaze cues from conspecific friends versus non-friends (Micheletta and Waller 2012). In contrast, long-tailed macaques (Macaca fascicularis), a competitive species, follow gaze more frequently when an actor exhibits a submissive facial expression versus an affiliative expression (Goossens et al. 2008). Future research should, therefore, compare how rhesus monkeys react to gaze cues in actively cooperative or affiliative situations, as well as how social tolerance across species may shape social cognitive processes more generally.
Finally, a key question invoked by our result is whether these kinds of gaze-following abilities scaffold later developing cognitive skills in primates: to what degree are the building blocks of human social cognitive development shared with other species? While rhesus macaques have demonstrated a sophisticated understanding of the mental states of other individuals (Drayton and Santos 2016, 2017; Flombaum and Santos 2005; Marticorena et al. 2011; Martin and Santos 2014; Santos et al. 2006), their social cognitive abilities appear to be different from that of humans. For instance, rhesus monkeys do not demonstrate false belief understanding (Marticorena et al. 2011; Martin and Santos 2014), suggesting that cognitive gaze-following is not a sufficient condition for the development of human-like social cognitive abilities. One possibility is that gaze-following follows a different developmental trajectory in rhesus monkeys, reflecting a different developmental role. While the previous work has shown that basic co-orienting follows a similar developmental trajectory in rhesus monkeys and humans (Rosati et al. 2016), it remains unclear whether this is also true for other social cognitive abilities such as inferring other’s line-of-sight, visual perspective-taking, or knowledge attribution. Analyzing the role of gaze-following in shaping the development of these abilities will shed light upon the origins of human-unique social cognitive abilities, and in particular how divergences in developmental trajectories influence mature social cognitive skills across species (Rosati et al. 2014).
In conclusion, rhesus monkeys can flexibly model a demonstrator’s line-of-sight in a neutral gaze-following context, and do not just reflexively shift attention to match the direction of other’s gaze. Our findings demonstrate that even highly despotic primate species are not constrained to using social cognitive processes in competitive contexts. However, rhesus monkeys show a low motivation to move to observe what others can see. This highlights that the ability to infer where others are looking, and the motivation to engage in social interactions using such skills, may be dissociated across species. This has important implications for our understanding of the interplay between tolerance and social cognition in the evolution of human-unique cognitive abilities that hinge on new forms of tolerant cooperative interactions in our own species.
References
Anderson JR, Sallaberry P, Barbier H (1995) Use of experimenter-given cues during object-choice tasks by capuchin monkeys. Anim Behav 49(1):201–208
Anderson JR, Montant M, Schmitt D (1996) Rhesus monkeys fail to use gaze direction as an experimenter-given cue in an object-choice task. Behav Proc 37(1):47–55
Baayen RH, Milin P (2010) Analyzing reaction times. Int J Psychol Res 3(2):12–28
Baldwin DA, Moses LJ (1994) Early understanding of referential intent and attentional focus: evidence from language and emotion. In: Lewis C, Mitchell P (eds) Children’s early understanding of mind: origins and development. Lawrence Erlbaum Associates Inc, Hillsdale, NJ, pp 133–156
Bettle R, Rosati AG (2016) Understanding human gaze. In: Encyclopedia of evolutionary psychological science, pp 1–4
Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MHH, White J-SS (2009) Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol 24(3):127–135
Bräuer J, Call J, Tomasello M (2005) All great ape species follow gaze to distant locations and around barriers. J Comp Psychol 119(2):145–154
Bray J, Krupenye C, Hare B (2014) Ring-tailed lemurs (Lemur catta) exploit information about what others can see but not what they can hear. Anim Cogn 17(3):735–744
Brent LJ, Heilbronner SR, Horvath JE, Gonzalez-Martinez J, Ruiz-Lambides A, Robinson AG, Platt ML (2013) Genetic origins of social networks in rhesus macaques. Sci Rep 3:1042
Brooks R, Meltzoff AN (2005) The development of gaze following and its relation to language. Dev Sci 8(6):535–543
Bulloch MJ, Boysen ST, Furlong EE (2008) Visual attention and its relation to knowledge states in chimpanzees, Pan troglodytes. Anim Behav 76(4):1147–1155
Burkart JM, Hrdy SB, Van Schaik CP (2009) Cooperative breeding and human cognitive evolution. Evol Anthropol Issues News Rev Issues News Rev 18(5):175–186
Butler SC, Caron AJ, Brooks R (2000) Infant understanding of the referential nature of looking. J Cogn Dev 1(4):359–377
Butterworth G, Cochran E (1980) Towards a mechanism of joint visual attention in human infancy. Int J Behav Dev 3(3):253–272
Butterworth G, Jarrett N (1991) What minds have in common is space: spatial mechanisms serving joint visual attention in infancy. Br J Dev Psychol 9(1):55–72
Byrne R, Whiten A (1989) Machiavellian intelligence: social expertise and the evolution of intellect in monkeys, apes, and humans. Oxford Science Publications, Oxford
Call J, Hare BA, Tomasello M (1998) Chimpanzee gaze following in an object-choice task. Anim Cogn 1(2):89–99
Call J, Agnetta B, Tomasello M (2000) Cues that chimpanzees do and do not use to find hidden objects. Anim Cogn 3(1):23–34
Call J, Hare B, Carpenter M, Tomasello M (2004) ‘Unwilling’ versus ‘unable’: chimpanzees’ understanding of human intentional action. Dev Sci 7(4):488–498
Cheney DL (2011) Extent and limits of cooperation in animals. Proc Natl Acad Sci 108(Supplement 2):10902–10909
Crockford C, Wittig RM, Mundry R, Zuberbühler K (2012) Wild chimpanzees inform ignorant group members of danger. Curr Biol 22(2):142–146
Csibra G, Gergely G (2009) Natural pedagogy. Trends Cogn Sci 13(4):148–153
Davidson GL, Butler S, Fernández-Juricic E, Thornton A, Clayton NS (2014) Gaze sensitivity: function and mechanisms from sensory and cognitive perspectives. Anim Behav 87:3–15
Deaner RO, Platt ML (2003) Reflexive social attention in monkeys and humans. Curr Biol 13(18):1609–1613
Drayton LA, Santos LR (2016) A decade of theory of mind research on Cayo Santiago: insights into rhesus macaque social cognition. Am J Primatol 78(1):106–116
Drayton LA, Santos LR (2017) Do rhesus macaques, Macaca mulatta, understand what others know when gaze following? Anim Behav 134:193–199
Emery NJ, Lorincz EN, Perrett DI, Oram MW, Baker CI (1997) Gaze following and joint attention in rhesus monkeys (Macaca mulatta). J Comp Psychol 111(3):286–293
Ferrari P, Kohler E, Fogassi L, Gallese V (2000) The ability to follow eye gaze and its emergence during development in macaque monkeys. Proc Natl Acad Sci 97(25):13997–14002
Flom R, Lee K, Muir D (2017) Gaze-following: Its development and significance. Psychology Press, London
Flombaum JI, Santos LR (2005) Rhesus monkeys attribute perceptions to others. Curr Biol 15(5):447–452
Friesen CK, Kingstone A (1998) The eyes have it! Reflexive orienting is triggered by nonpredictive gaze. Psychon Bull Rev 5(3):490–495
Goossens BM, Dekleva M, Reader SM, Sterck EH, Bolhuis JJ (2008) Gaze following in monkeys is modulated by observed facial expressions. Anim Behav 75(5):1673–1681
Grueneisen S, Duguid S, Saur H, Tomasello M (2017) Children, chimpanzees, and bonobos adjust the visibility of their actions for cooperators and competitors. Sci Rep 7(1):8504
Hare B (2001) Can competitive paradigms increase the validity of experiments on primate social cognition? Anim Cogn 4(3):269–280
Hare B (2017) Survival of the friendliest: homo sapiens evolved via selection for prosociality. Annu Rev Psychol 68:155–186
Hare B, Tomasello M (2004) Chimpanzees are more skilful in competitive than in cooperative cognitive tasks. Anim Behav 68(3):571–581
Hare B, Tomasello M (2005) Human-like social skills in dogs? Trends Cogn Sci 9(9):439–444
Hare B, Call J, Agnetta B, Tomasello M (2000) Chimpanzees know what conspecifics do and do not see. Anim Behav 59(4):771–785
Hare B, Call J, Tomasello M (2001) Do chimpanzees know what conspecifics know? Anim Behav 61(1):139–151
Hare B, Call J, Tomasello M (2006) Chimpanzees deceive a human competitor by hiding. Cognition 101(3):495–514
Higham JP, Maestripieri D (2010) Revolutionary coalitions in male rhesus macaques. Behaviour 147(13):1889–1908
Hood BM, Willen JD, Driver J (1998) Adult’s eyes trigger shifts of visual attention in human infants. Psychol Sci 9(2):131–134
Hopkins WD, Taglialatela JP, Leavens DA (2007) Chimpanzees differentially produce novel vocalizations to capture the attention of a human. Anim Behav 73(2):281–286
Hostetter AB, Russell JL, Freeman H, Hopkins WD (2007) Now you see me, now you don’t: evidence that chimpanzees understand the role of the eyes in attention. Anim Cogn 10(1):55–62
Itakura S (1996) An exploratory study of gaze-monitoring in nonhuman primates. Jpn Psychol Res 38(3):174–180
Itakura S, Tanaka M (1998) Use of experimenter-given cues during object-choice tasks by chimpanzees (Pan troglodytes), an orangutan (Pongo pygmaeus), and human infants (Homo sapiens). J Comp Psychol 112(2):119–126
Joly M, Micheletta J, De Marco A, Langermans JA, Sterck EH, Waller BM (2017) Comparing physical and social cognitive skills in macaque species with different degrees of social tolerance. Proc R Soc B 284(1862):20162738
Kaminski J, Call J, Tomasello M (2008) Chimpanzees know what others know, but not what they believe. Cognition 109(2):224–234
Krupenye C, Kano F, Hirata S, Call J, Tomasello M (2016) Great apes anticipate that other individuals will act according to false beliefs. Science 354(6308):110–114
Lee K, Eskritt M, Symons LA, Muir D (1998) Children’s use of triadic eye gaze information for “mind reading”. Dev Psychol 34(3):525–539
Lindburg DG (1971) The rhesus monkey in North India: an ecological and behavioral study. Primate Behav Dev Field Lab Res 2:1–106
Lo S, Andrews S (2015) To transform or not to transform: using generalized linear mixed models to analyse reaction time data. Front Psychol 6:1171
Lyons DE, Santos LR (2006) Ecology, domain specificity, and the origins of theory of mind: is competition the catalyst? Philos Compass 1(5):481–492
MacLean EL, Hare B (2012) Bonobos and chimpanzees infer the target of another’s attention. Anim Behav 83(2):345–353
MacLean EL, Hare B, Nunn CL, Addessi E, Amici F, Anderson RC, Barnard AM (2014) The evolution of self-control. Proc Natl Acad Sci 111(20):E2140–E2148
Marticorena DC, Ruiz AM, Mukerji C, Goddu A, Santos LR (2011) Monkeys represent others’ knowledge but not their beliefs. Dev Sci 14(6):1406–1416
Martin A, Santos LR (2014) The origins of belief representation: monkeys fail to automatically represent others’ beliefs. Cognition 130(3):300–308
Martin A, Santos LR (2016) What cognitive representations support primate theory of mind? Trends Cogn Sci 20(5):375–382
Melis AP, Tomasello M (2013) Chimpanzees’ (Pan troglodytes) strategic helping in a collaborative task. Biol Lett 9(2):20130009
Melis AP, Call J, Tomasello M (2006) Chimpanzees (Pan troglodytes) conceal visual and auditory information from others. J Comp Psychol 120(2):154–162
Micheletta J, Waller BM (2012) Friendship affects gaze following in a tolerant species of macaque, Macaca nigra. Anim Behav 83(2):459–467
Moll H, Meltzoff AN (2011) Joint attention as the fundamental basis of understanding perspectives. In: Seemann A (ed) Joint attention: new developments in psychology, philosophy of mind, and social neuroscience. MIT Press, Cambridge, MA, pp 393–413
Moll H, Tomasello M (2004) 12-and 18-month-old infants follow gaze to spaces behind barriers. Dev Sci 7(1):F1–F9
Moll H, Tomasello M (2007) How 14-and 18-month-olds know what others have experienced. Dev Psychol 43(2):309–317
Morales M, Mundy P, Rojas J (1998) Following the direction of gaze and language development in 6-month-olds. Infant Behav Dev 21(2):373–377
Okamoto-Barth S, Call J, Tomasello M (2007) Great apes’ understanding of other individuals’ line of sight. Psychol Sci 18(5):462–468
Partan SR (2002) Single and multichannel signal composition: facial expressions and vocalizations of rhesus macaques (Macaca mulatta). Behaviour 139(8):993–1027
Petit O, Desportes C, Thierry B (1992) Differential probability of “coproduction” in two species of macaque (Macaca tonkeana, M. mulatta). Ethology 90(2):107–120
Rawlins RC, Kessler MJ (eds) (1987) The Cayo Santiago macaques: history, behavior and biology. State University of New York Press, Albany
R Development Core Team (2017) A language and environment for statistical computing, Vienna, Austria. http://www.R-project.org. Accessed 9 Oct 2017
Ricciardelli P, Carcagno S, Vallar G, Bricolo E (2013) Is gaze following purely reflexive or goal-directed instead? Revisiting the automaticity of orienting attention by gaze cues. Exp Brain Res 224(1):93–106
Rosati AG, Hare B (2009) Looking past the model species: diversity in gaze-following skills across primates. Curr Opin Neurobiol 19(1):45–51
Rosati AG, Santos LR (2016) Spontaneous metacognition in rhesus monkeys. Psychol Sci 27(9):1181–1191
Rosati AG, Santos LR (2017) Tolerant Barbary macaques maintain juvenile levels of social attention in old age, but despotic rhesus macaques do not. Anim Behav 130:199–207
Rosati AG, Wobber V, Hughes K, Santos LR (2014) Comparative developmental psychology: how is human cognitive development unique? Evol Psychol 12(2):448–473
Rosati AG, Arre AM, Platt ML, Santos LR (2016) Rhesus monkeys show human-like changes in gaze following across the lifespan. Proc R Soc B 283:20160376
Ruiz A, Gómez JC, Roeder JJ, Byrne RW (2009) Gaze following and gaze priming in lemurs. Anim Cogn 12(3):427–434
Sandel AA, MacLean EL, Hare B (2011) Evidence from four lemur species that ringtailed lemur social cognition converges with that of haplorhine primates. Anim Behav 81(5):925–931
Santos LR, Nissen AG, Ferrugia JA (2006) Rhesus monkeys, Macaca mulatta, know what others can and cannot hear. Anim Behav 71(5):1175–1181
Senju A, Csibra G (2008) Gaze following in human infants depends on communicative signals. Curr Biol 18(9):668–671
Shepherd SV (2010) Following gaze: gaze-following behavior as a window into social cognition. Front Integr Neurosci 4:5
Shepherd SV, Deaner RO, Platt ML (2006) Social status gates social attention in monkeys. Curr Biol 16(4):R119–R120
Teufel C, Alexis DM, Clayton NS, Davis G (2010) Mental-state attribution drives rapid, reflexive gaze following. Attent Percept Psychophys 72(3):695–705
Thierry B (2002) Covariation of conflict management patterns across macaque species. In: Aureli F, de Waal FBM (eds) Natural conflict resolution. University of California Press, Berkeley, pp 106–128
Tomasello M (1995) Joint attention as social cognition. In: Moore C, Dunham PJ (eds) Joint attention: its origins and role in development. Lawrence Erlbaum Associates Inc, Hillsdale, NJ, pp 103–130
Tomasello M (2014) A natural history of human thinking. Harvard University Press, Cambridge
Tomasello M, Carpenter M (2007) Shared intentionality. Dev Sci 10(1):121–125
Tomasello M, Hare B, Agnetta B (1999) Chimpanzees, Pan troglodytes, follow gaze direction geometrically. Anim Behav 58(4):769–777
Tomasello M, Hare B, Fogleman T (2001) The ontogeny of gaze following in chimpanzees, Pan troglodytes, and rhesus macaques, Macaca mulatta. Anim Behav 61(2):335–343
Vick S-J, Anderson JR (2000) Learning and limits of use of eye gaze by capuchin monkeys (Cebus apella) in an object-choice task. J Comp Psychol 114(2):200–207
Widdig A, Nürnberg P, Krawczak M, Streich WJ, Bercovitch F (2002) Affiliation and aggression among adult female rhesus macaques: a genetic analysis of paternal cohorts. Behaviour 139(2):371–391
Widdig A, Streich WJ, Nürnberg P, Croucher PJ, Bercovitch FB, Krawczak M (2006) Paternal kin bias in the agonistic interventions of adult female rhesus macaques (Macaca mulatta). Behav Ecol Sociobiol 61(2):205–214
Yamamoto S, Humle T, Tanaka M (2012) Chimpanzees’ flexible targeted helping based on an understanding of conspecifics’ goals. Proc Natl Acad Sci 109(9):3588–3592
Acknowledgements
We thank Megan Cole, Francesca De Petrillo, Hayoung Chang, Megan Mulhinch, and Yijia Zheng for assistance with data collection and coding; Thore Bergman for helpful comments on an earlier version of the manuscript; and CSCAR at the University of Michigan for statistical advice. The authors are grateful to the Cayo Santiago Field Station and staff including Angelina Ruiz Lambides, Nahiri Rivera Barreto, Giselle Caraballo Cruz, and Bianca Giura for their research support.
Funding
This work was supported by a National Center for Research Resources CM-5-P40RR003640-13 award to the Caribbean Primate Research Center and the University of Puerto Rico, and an Office of Research Infrastructure Programs (ORIP) of the National Institutes of Health (NIH) through Grant Number 5P40OD012217 to the Caribbean Primate Research Center and the University of Puerto Rico. This research was supported in part by a Human Evolutionary Biology Early Training and Research Support Grant from Harvard University to RB. AR was supported by a Sloan Foundation fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All non-invasive behavioral tests were approved by the Institutional Animal Care and Use Committee (IACUC) for the University of Puerto Rico Medical Sciences Campus (protocol #A140116), and adhered to site guidelines for animal research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (MP4 119161 kb)
Supplementary material 3 (MP4 93662 kb)
Rights and permissions
About this article
Cite this article
Bettle, R., Rosati, A.G. Flexible gaze-following in rhesus monkeys. Anim Cogn 22, 673–686 (2019). https://doi.org/10.1007/s10071-019-01263-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10071-019-01263-4