Abstract
Corn oil was heated using an infrared cooker, an air fryer, and a cooking oven at similar temperatures, and oxidative stability and physicochemical properties including moisture content, temperature change, the profile of headspace volatiles, formaldehyde and acetaldehyde of the heated oils were compared. Corn oil heated using the air fryer showed the lowest degree of oxidation, followed by that heated using the infrared cooker and the cooking oven. However, the content of headspace volatiles in 120 min heated oil using the infrared cooker was higher by 2.57 and 5.37 times than that in oil heated using the cooking oven and the air fryer, respectively. The profiles of formaldehyde and acetaldehyde in oils showed patterns inconsistent with those of headspace volatile and oxidation parameters. Generally, the air fryer-treated oil underwent slow lipid oxidation, whereas oil from the infrared cooker had more volatiles and imparted odor to foods.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fats and oils are important parts of daily diet and have been used as media for mass production in the food industry. Deep fat frying or shallow frying are good examples of food processing using fats and oils (Choe and Min, 2006). However, excessive heating of fats and oils can accelerate the rate of lipid oxidation, which deteriorates the sensory or nutritional quality of foods and leads to the formation of toxic chemicals.
Heating methods using infrared irradiation or hot-air circulation have been recently introduced, and the market share of cooking appliances based on these heating methods is increasing. An infrared cooker utilizes an electromagnetic spectrum with wavelengths ranging between 0.5 and 100 μm, which can be absorbed by water molecules and ions in foods, resulting in an increase in temperature (Rastogi, 2012a; Riadh et al., 2015). Foods subjected to infrared irradiation have a lower fat content than those subjected to deep fat frying. Infrared irradiation has several advantages, including efficient heat flux, uniform heating, and lower degradation of food ingredients, compared to the convective and conductive heat transfer, and it has been applied in diverse food processes (Bagheri et al., 2016; Rastogi, 2012b; Riadh et al., 2015; Yu et al., 2021).
An air fryer circulates a hot air stream in the fryer chamber, and oil and moisture from food may act as heat transfer media. Foods prepared via air frying have low acrylamide and absorbed oil contents (Rahman et al., 2016; Teruel et al., 2015). Air-fried foods have 70–80% lower oil content than that of deep fat-fried foods, partly due to the removal of excess oil from foods (Sansano et al., 2015). The cooking time and temperature of an air fryer can be decreased owing to the continuous flow of hot air (Sani et al., 2014). The degree of lipid oxidation in air-fried foods is high, partly due to the denaturation of heme proteins caused by continuous oxygen exposure and high temperature (Cropotova et al., 2019).
Recently, demand for an infrared cooker and air fryer is increasing among consumers who want to cook meals at home (Lang et al., 2022; Song et al., 2020). As an infrared cooker and air fryer deliver thermal energy through air, the degree of lipid oxidation or formation of oxidized volatiles may be different in bulk oil compared to that in a conventional cooking oven. Limitation of oxygen molecules in an air fryer may play an important role in reducing the degree of lipid oxidation in oils. Moisture content is another important factor responsible for lipid oxidation in oils (Budilarto and Kamal-Eldin, 2015; Park et al., 2014). Moisture and amphiphilic minor compounds may form association colloids in edible oils, and the interface of the association colloids may act as a major oxidation site (Chaiyasit et al., 2007; Grosshagauer et al., 2019; Kim et al., 2018). Generally, moisture content increases as the degree of oxidation increases because amphiphilic compounds can carry water molecules in the headspace (Budilarto and Kamal-Eldin, 2015; Park et al., 2014). In addition, moisture can act as a substrate for the formation of volatiles via lipid oxidation (Lee et al., 2018; Oh et al., 2017).
Although diverse cooking devices have replaced the conventional cooking oven, information on the degree of lipid oxidation in edible oils is scarce.
The objectives of this study were to evaluate the degree of lipid oxidation in edible oils heated using an infrared cooker, air fryer, and a conventional cooking oven, and to compare the contents of volatiles, including formaldehyde (FA) and acetaldehyde (AA), in oils subjected to different cooking methods.
Materials and methods
Materials
Corn oil was purchased from a local grocery market (Suwon, Korea). AA, FA, and 50/30 mm Divinylbenzene/Carboxen/Polydimethylsiloxane (DVB/CAR/PDMS) StableFlex were purchased from Sigma Aldrich (St. Louis, MO, USA). 1,2-13C2-acetaldehyde was purchased from Toronto Research Chemicals (Toronto, Canada). O-(2,3,4,5,6-Pentafluoro-benzyl)-hydroxylamine hydrochloride (PFBHA) and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Aldrich. Isooctane and methanol were purchased from Daejung Chemical Co. (Seoul, Korea). Other chemicals were of reagent grade and obtained from Daejung Chemical Co.
Sample preparation
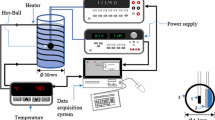
To determine the effects of infrared cooking on oxidative stability of corn oil, 6 g of corn oil was placed in a 30 mL vial, and the vial was placed in a round-shaped rack under an infrared cooker (L9282; Convex, Seoul, Korea). The distance between the infrared light source and vials was maintained at 5 cm, and vials were removed from the deck every 20 min for 120 min. Temperature of the infrared cooker was set at 180 °C.
The vials containing 6 g of corn oil were placed in an air fryer (Simeo, Seoul, Korea) and heated for 20, 40, 60, 80, 100, and 120 min. Temperature of the air fryer was set at 200 °C, consistent with the preliminary studies.
Control vials, containing 6 g of corn oil, were placed in a conventional cooking oven (EON-C500F; SK magic, Seoul, Korea) at 160 °C and sampled every 20 min for 120 min. All samples were collected in triplicate at each time point.
The heating temperature of the three devices was adjusted based on preliminary studies. Although temperature setting was different in cooking devices, actual temperature of all the sample oils was measured about 160-169.2 °C. Oil samples from the infrared cooker, air fryer, and cooking oven were denoted as IR, AF, and CO, respectively.
Moisture content and temperature analyses
The moisture content of oil was determined using a coulometric Karl Fischer titrator (C20; Mettler-Toledo Intl., Columbus, OH, USA) according to the previous study (Lee et al., 2018). The temperature of oil was determined using a digital thermometer TP-300 (Zhejiang, China).
Analysis of conjugated dienoic acid (CDA) and p-anisidine value (p-AV)
The CDA and p-AV of oil samples were measured according to the AOCS methods Ti 1a-64 and Cd 18–90, respectively (AOCS, 2006).
DPPH loss for antioxidants and oxidation products in oils
DPPH radicals were dissolved in methanol and isooctane at a final concentration of 0.1 mM. Oil samples were mixed with methanol at a final concentration of 40,000 mg/L (w/v). The mixture of corn oil and methanol was vortexed for 1 min and centrifuged at 12,225×g for 3 min. The supernatant (0.25 mL) was allowed to react with 0.75 mL of 0.1 mM DPPH in methanol. For preparing isooctane solvent, 0.75 mL of 0.1 mM DPPH in isooctane was mixed with 0.25 mL of the mixture containing 40,000 mg/L (w/v) oil. The absorbance of the sample mixture was measured using a UV/VIS-spectrometer (Genesys 10UV; Thermo Fisher Scientific Inc., Waltham, MA, USA) after allowing it to react in the dark for 30 min. The absorbance of DPPH dissolved in methanol and isooctane was determined at 517 and 509 nm, respectively. The absorbance of DPPH was expressed as DPPH loss, according to previous reports (Song et al., 2016, 2017).
Analysis of headspace volatiles via solid-phase microextraction (SPME)
The SPME conditions for analysis of volatile compounds were adapted from the method described by Kim et al. (2021). To equilibrate volatile compounds in the headspace, sample vials were placed in a water bath (RW-0525G; Lab Camp, Incheon, Korea) at 30 °C for 30 min and then shifted to the agitator of a multipurpose sampler (MPS; Gerstel, Mülheim, Germany). Headspace volatile compounds were isolated and extracted for 30 min using a 50/30 mm DVB/CAR/PDMS StableFlex solid phase at 30 °C.
Volatile compounds attached to the solid phase of SPME equipment were separated and identified using Hewlett-Packard 6890 GC (Agilent Technology, Palo Alto, CA, USA) equipped with a 5971 A mass selective detector (MSD; Agilent Technology, Palo Alto, CA, USA) and a DB-5ms column (30 m ⋅ 0.25 mm i.d., 0.25 μm film thickness; Agilent J&W, Folsom, CA, USA). The solid phase of SPME equipment was exposed in an injector port for 3 min. Helium was used as a carrier gas at a flow rate of 1.0 mL/min; oven temperature was held at 40 °C for 3 min and increased gradually to 150 °C at a rate of 4 °C/min and then to 220 °C at a rate of 15 °C/min. All mass spectra were obtained at 70 eV and an ion source temperature of 220 °C. The Kovat index of volatile compounds was obtained using a mixture of alkane standards (C8-C20). Compounds were identified based on a combination of NIST mass spectra and gas chromatographic retention times of some standard compounds.
Analysis of acetaldehyde and formaldehyde
AA and FA were analyzed using the method reported by Kim et al. (2021), with some modifications. Briefly, 1 g of oil was placed in a 10 mL vial along with 50 µL of 1,2-13C2-acetaldehyde (as an internal standard) and 4.95 mL of a 30% NaCl solution. Then, 100 mg of potassium hydrogen phthalate and 0.05 mL of PFBHA (10 mg/mL) were added for derivatization, and the samples were air-tight sealed. Sample vials were placed in a shaking water bath (Lab Companion, Seoul, Korea) at 45 °C for 40 min at 75 rpm. SPME-GC/MS was used to analyze volatile compounds. Data were acquired in the selected ion monitoring mode (SIM mode). AA and FA were quantified by calibration curves, using standard materials of analytical grade. The derivatives of AA, FA, and 1,2-13C2-acetaldehyde were quantified at m/z 209, 195, and 211, respectively. The coefficient of determination (r2) of the calibration curves for AA and FA was 0.9996 and 0.9969, respectively.
Statistical analysis
Data on physicochemical properties and oxidation parameters were analyzed with ANOVA and Duncan’s multiple range test using the SPSS software program (version 19; SPSS Inc., Chicago, IL, USA). Statistical significance was set at p < 0.05.
Results and discussion
Physicochemical properties and degree of oxidation of oils
Changes in moisture content and temperature of corn oil depending on the treatment duration in infrared cooker, air fryer, and cooking oven are shown in Table 1.
The temperature of oil from infrared cooker, air fryer, and cooking oven was in the range of 160–169.2 °C, 161–164.7 °C, and 163.5–168.1 °C, respectively. This means that the temperature of oil in this study was not significantly different with respect to heating duration (p > 0.05) (Table 1).
Moisture content decreased in corn oil, irrespective of the device type. For the first 20 min, AF had the lowest moisture content, followed by IR and CO, in a decreasing order. Moisture content in CO remained high after 120 min; moreover, it did not differ significantly in AF and CO after 80 min (p > 0.05). The high moisture content in CO implied that heat treatment using a conventional oven did not effectively reduce the moisture content of corn oil, even when the temperature of oil was not lower than that of AF and IR. Heat energy can be transferred through convection, conduction, and radiation (Riadh et al., 2015). Although both air fryer and cooking oven can evaporate moisture from oils through conduction and convection, hot air circulation in an air fryer may reduce the moisture content of oil more efficiently than the cooking oven. As infrared radiations can penetrate the food, lower moisture content through superior thermal efficiency can be achieved in an oil matrix (Riadh et al., 2015).
Effects of heating oil in an infrared cooker, air-fryer, and a cooking oven on the changes in CDA (a) and p-AV (b) in corn oil are shown in Fig. 1. After 20 min of treatment, the CDA values of corn oil were not significantly different irrespective of the cooking device used (p > 0.05). After 40 min of treatment, CO had significantly higher CDA values than AF and IR (p < 0.05). AF showed the lowest CDA value, followed by IR and CO, in an increasing order (Fig. 1a). For secondary oxidation products, CO showed a significantly higher p-AV, than IR and AF, after 40 min of treatment (p < 0.05). The p-AV value of AF was higher than that of IR after the first 20 min; however, this trend changed after 80 min of treatment (Fig. 1b). Overall, CO showed the highest degree of lipid oxidation. Infrared irradiation accelerated the rate of lipid oxidation compared to hot-air circulation in air fryer, which could be partly due to the limited oxygen molecules under air-tight conditions of the air fryer.
Effects of an infrared cooker, an air-fryer, and a cooking oven treatment on the changes of conjugated dienoic acid (CDA) (A) and p-anisidine value (p-AV) (B) in corn oil. IR, AF, and CO were corn oils from the infrared cooker, the air-fryer, and the cooking oven, respectively. Different letters at 120 min were significant different at 0.05
Changes in DPPH loss in methanol (a) and DPPH loss in isooctane (b) in edible oils heated in different cooking devices are shown in Fig. 2. DPPH loss in methanol provides information on the quantity of radical-scavenging antioxidants in oils (Song et al., 2016, 2017). DPPH loss in AF was significantly higher than that in IR and CO, in a decreasing order, which implies that AF contained the highest amount of antioxidants, while CO had the lowest antioxidant content (Fig. 2a). DPPH loss in isooctane indicates the contents of antioxidants and radical-scavenging oxidized lipids in oils (Song et al., 2016, 2017). DPPH loss in isooctane from CO was the lowest, followed by that in isooctane from IR and AF, in an increasing order. Interestingly, the first 20 min treatment resulted in a difference between DPPH loss in methanol and isooctane. Approximately 15% of DPPH loss in isooctane decreased, while less than 3% of DPPH loss in methanol decreased, which implies that more amount of oxidation products was generated than the amount of depleted inherent antioxidants in oil.
Changes in DPPH loss in methanol (A), and DPPH loss in isooctane (B) in edible oil by an infrared cooker, an air-fryer, and a cooking oven. IR, AF, and CO were corn oils from the infrared cooker, the air-fryer, and the cooking oven, respectively. Different letters at 120 min were significant different at 0.05
Headspace volatile profile analysis
Changes in the total volatile content (a) and selected groups of volatile compounds (b) in edible oil with respect to heating duration in different cooking devices are shown in Fig. 3. IR had the highest total volatile content, followed by CO and AF, in a decreasing order, irrespective of the treatment time (Fig. 3a). After 40 min of treatment, total peak area of IR was 1.55 and 2.49 times higher than that of CO and AF, respectively. Differences in the total peak area among the heated oils increased further, and after 120 min, the total peak area of IR was 2.57 and 5.37 times higher than that of CO and AF, respectively (Fig. 3a). Although CO underwent oxidation to a greater extent, as shown by the primary and secondary oxidation products (Fig. 1), the total headspace volatile content was the highest in IR. In general, as the more oxidation proceeds, the more volatile compounds are generated. This unexpected result could be due to the differences in lipid oxidation and heat delivery mechanisms among different cooking devices.
Changes in total volatiles (A) and selected groups of volatiles (B–D) in edible oil depending on the treatment time of an infrared cooker, an air-fryer, and a cooking oven. IR, AF, and CO were corn oils from the infrared cooker, the air-fryer, and the cooking oven, respectively. Different letters on the bar were significant different at 0.05
The major volatile compounds present in corn oil heated using different cooking devices are shown in Table 2. Before heat treatment, the presence of all lipid oxidation products, including hexane, 2-octene, t-2-heptenal, 1-pentanol, heptane, and heptanal, was observed in corn oil. After 40 min of heat treatment, a total of 14 oxidation volatile compounds were identified in the heated oil; however, the volatile content profile was slightly different. Hexanal was the most detected volatile compound, followed by t-2-heptenal, irrespective of the cooking device used. After 80 and 120 min, hexanal was the dominant volatile compound in CO and AF, respectively, while 2-heptenal was the most abundant compound in IR. Moreover, after the heating duration increased to 120 min, the contents of 2-pentyl furan, 2,4 decadienal, and 2-octenal increased substantially.
After 120 min, hexanal content was approximately 18–19% in CO and IR and approximately 30% in AF. In addition, after 120 min, the contents of 2-pentyl furan and 2,4 decadienal were approximately 10.7% and 13% of the total volatile content in CO and IR, respectively, and 8.8% and 10.5%, respectively, in AF.
Hexanal and 2,4 decadienal are formed from 13- and 9-carbon hydroperoxides in linoleic acid, respectively, while t-2-heptenal and 1-octen-3-ol are formed by singlet oxygen oxidation of linoleic acid (Choe and Min, 2006; Oh et al., 2015). Generally, corn oil contains 53.6% linoleic acid and 1.6% linolenic acid, and hexanal has been used as an indicator to determine the rate of lipid oxidation in oils containing linoleic acid (Chen et al., 2012; Lee and Decker, 2011). In case of t-2-heptenal and 1-octen-3-ol, which are major volatile compounds, singlet oxygen oxidation is likely to occur in the system. IR had relatively high contents of t-2-heptenal and 1-octen-3-ol compared to those in CO and AF, which implies that oxidation by reactive oxygen species, including singlet oxygen, may occur during infrared irradiation. Therefore, more volatiles in infrared irradiated oils could be partly due to the singlet oxygen oxidation.
Analysis of acetaldehyde and formaldehyde
Changes in AA and FA concentrations in oils, depending on the type of heating appliance, are shown in Fig. 4. AA and FA were not detected in unheated corn oil (data not shown). AA is the secondary product of lipid oxidation of polyunsaturated fatty acids (Hsieh and Kinsella, 1989). AA is naturally present in fruits, vegetables, dairy products, fruit drinks, meat, and fish (Miyake and Hibamoto, 1993). FA is naturally present in foods such as fruits, vegetables, meat, fish, and crustaceans. FA is used as a preservative, reducing agent, fumigant, or sterilizing agent in various foods (Norliana et al., 2009). Strecker degradation of glycine and lipid oxidation of polyunsaturated fatty acids can generate FA (Jung et al., 2021; Velíšek et al., 1989). FA has been classified as carcinogenic to humans (Group 1) by the International Agency for Research on Cancer (IARC 2016).
Interestingly, AA and FA were formed differently depending on the type of heating appliance used. After heating for 20 and 40 min, the content of AA in IR was the lowest, followed by that in AF and CO, in an increasing order. However, this trend was not observed after 80 min of treatment. The content of AA in AF was lowest after 80 min of treatment, which might be due to the limited amount of oxygen molecules and low degree of lipid oxidation, as shown in Figs. 1 and 3. Generally, IR had a low AA content, whereas CO had the highest AA content. AA content seems to be closely correlated with the degree of lipid oxidation.
A slightly different profile was observed for FA compared to that of AA. The lowest FA content was observed in IR after treatment for 20–100 min compared to that observed in other samples. AF had a significantly higher FA content than IR after 20 min of treatment (p < 0.05); however, CO and AF did not show a uniform trend with regard to FA content (Fig. 4b).
Formation of AA and FA from decomposition of aldehydes, including 2-pentenal, 2-octenal, 2,4-heptadienal, and 2,4-decadienal, during thermal treatment has been reported (Zamora et al., 2015). Considering profile changes, AA is more correlated with the degree of lipid oxidation than FA. AA can be formed as a secondary oxidation product or via decomposition directly from other long-chain aldehydes. Interestingly, oxygen molecules and other factors seemed to limit the formation of FA in AF and IR.
Infrared irradiation mainly utilizes thermal energy, whereas an air-fryer circulates heated air inside the mantle using ‘rapid air technology’. Due to the limited amount of oxygen molecules and moisture content, relatively high oxidative stability of edible oils was expected. However, presence of moisture in the headspace of edible oils, which may migrate into the oil matrix, could be another factor determining the degree of lipid oxidation. Moisture can act as an interface for lipid oxidation through formation of association colloids and a substrate for volatile formation after β-scission of lipid hydroperoxides (Laguerre et al., 2015; Lee et al., 2018; Park et al., 2014). For the first 20 min, AF had a higher degree of lipid oxidation, as determined from CDA and p-AV, than IR. The limited amount of oxygen molecules in an air fryer could be a crucial factor in enhancing the oxidative stability of heated bulk oils. Considering volatile profiles and other oxidation parameters, edible oil heated using an air fryer had the highest oxidative stability compared to oils heated using an infrared cooker and a cooking oven.
An infrared cooker provides thermal energy primarily by irradiation, while a conventional oven provides both thermal energy and the driving force for air circulation. IR had relatively low CDA and p-AV and high contents of headspace volatiles compared to AF, which implies that infrared irradiation rather than thermal energy plays an important role in the formation of volatiles in oxidized oils. In addition, oxidation by reactive oxygen species, including singlet oxygen, is likely to be involved in the oxidation of IR mainly through formation of t-2-heptenal and 1-octen-3-ol, as shown in Table 2.
In conclusion, edible oils containing unsaturated fatty acids undergo lipid oxidation after heat stimulation, which causes the formation of volatiles, including aldehydes, alcohols, and ketones. Heating in conventional oven resulted in a high degree of lipid oxidation in edible oils, followed by that in infrared cooker and air fryer, in a decreasing order. However, more volatiles, which may be formed by reactive oxygen species, including singlet oxygen, were detected in the oil heated using an infrared cooker. In an air fryer, oxygen molecules could be a rate-limiting factor, resulting in a low degree of lipid oxidation and volatile content. Oxidation products from bulk oils differed depending on the type of cooking device. Infrared cooker promoted the formation of odor-active volatiles, while air fryer reduced the degree of oxidation due to limited amount of oxygen molecules.
References
Abd Rahman NA, Abdul Razak SZ, Lokmanalhakim LA, Taip FS, Mustapa Kamal SM. Response surface optimization for hot air-frying technique and its effects on the quality of sweet potato snack. Journal of Food Process Engineering. 40: e12507 (2019)
AOCS. Official methods and recommended practices of the American Oil Chemists’ Society (4th ed.). Champaign. IL.: AOCS Press. (2006).
Bagheri H, Kashaninejad M, Ziaiifar AM, Aalami M. Novel hybridized infrared-hot air method for roasting of peanut kernels. Innovative Food Science and Emerging Technologies. 37: 106-114 (2016)
Budilarto ES, Kamal-Eldin A. The supramolecular chemistry of lipid oxidation and antioxidation in bulk oils. European Journal of Lipid Science and Technology. 117: 1095-1137 (2015)
Chaiyasit W, Elias RJ, McClements DJ, Decker EA. Role of physical structures in bulk oils on lipid oxidation. Critical Reviews in Food Science and Nutrition. 47: 299-317 (2007)
Chen B, Panya A, McClements DJ, Decker EA. New insights into the role of iron in the promotion of lipid oxidation in bulk oils containing reverse micelles. Journal of Agricultural and Food Chemistry. 60: 3524-3532 (2012)
Choe E, Min DB. Mechanisms and factors for edible oil oxidation. Comprehensive Reviews in Food Science and Food Safety. 5: 169-186 (2006)
Cropotova J, Mozuraityte R, Standal IB, Rustad T. Assessment of lipid oxidation in Atlantic mackerel (Scomber scombrus) subjected to different antioxidant and sous-vide cooking treatments by conventional and fluorescence microscopy methods. Food Control. 104: 1-8 (2019)
Grosshagauer S, Steinschaden R, Pignitter M. Strategies to increase the oxidative stability of cold pressed oils. LWT-Food Science and Technology. 106: 72-77 (2019)
Hsieh RJ, Kinsella JE. Lipoxygenase generation of specific volatile flavor carbonyl compounds in fish tissues. Journal of Agricultural and Food Chemistry. 37: 279-286 (1989)
Jung HJ, Kim SH, Yoo KC, Lee JH. Changes in acetaldehyde and formaldehyde contents in foods depending on the typical home cooking method. Journal of Hazardous Materials. 414: 125475 (2021)
Kim JS, Kim MJ, Lee JH. The critical micelle concentration of lecithin in bulk oils and medium chain triacylglycerol is influenced by moisture content and total polar materials. Food Chemistry. 261: 194-200 (2018)
Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA, Villeneuve P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Critical Reviews in Food Science and Nutrition. 55: 183-201 (2015)
Lang GH, Timm NS, Neutzling HP, Ramos AH, Ferreira CD, Oliveira M. Infrared radiation heating:A novel technique for developing quick cooking rice. LWT. 154:112758 (2022)
Lee CK, Yi BR, Kim SH, Choi HS, Kim MJ, Lee JH. Volatile profiles and involvement step of moisture in bulk oils during oxidation by action of deuterium oxide (D2O). Food Science and Biotechnology. 27: 1327-1332 (2018)
Lee JH, Decker EA. Effects of metal chelators, sodium azide, and superoxide dismutase (SOD) on the oxidative stability in riboflavin photosensitized O/W emulsion systems. Journal of Agricultural and Food Chemistry. 59: 6271-6276 (2011)
Min DB, Boff JM. Chemistry and reaction of singlet oxygen in foods. Comprehensive Reviews in Food Science and Food Safety. 1: 58-72 (2002)
Miyake T, Hibamoto T. Quantitative analysis of acetaldehyde in foods and beverage. Journal of Agricultural and Food Chemistry. 41: 1968-1970 (1993)
Norliana S, Abdulamir AS, Abu BF, Salech AB. The health risk of formaldehyde -to human beings. American Journal of Pharmacology and Toxicology. 4: 98-106 (2009)
Oh SM, Lee CK, Kim SH, Choi HS, Kim MJ, Lee JH. Oxidative stability and volatile formations in linoleic acid-D2O models in the presence of deuteron or electron donors. Journal of the American Oil Chemists’ Society. 94: 1385-1392 (2017)
Oh SM, Yi BR, Kim MJ, Lee JH. Effects of deuterium oxide on formation of volatiles in linoleic acid model systems at different temperatures and oxygen limitation conditions. Food Science and Biotechnology. 24: 41-46 (2015)
Park JW, Kim JY, Kim MJ, Lee JH. Evaluation of oxygen limitation on lipid oxidation and moisture content in corn oil at elevated temperature. Journal of the American Oil Chemists’ Society. 91: 439-444 (2014)
Rastogi N. Infrared Heating of Fluid Foods. pp.411-418 In: Novel Thermal and Non-Thermal Technologies for Fluid Foods. Cullen PJ, Tiwari BK, Valdramidis VP (eds). Academic Press. (2012a)
Rastogi NK. Recent trends and developments in infrared heating in food processing. Critical Reviews in Food Science and Nutrition. 52: 737-760 (2012b)
Riadh MH, Ahmad SAB, Harhaban MH, Soh AC. Infrared heating in food drying: An overview. Drying Technology. 33: 322-335 (2015)
Sansano M, Juan-Borrás M, Escriche L, Andrés A, Heredia A. Effect of pretreatments and air‐frying, a novel technology, on acrylamide generation in fried potatoes. Journal of Food Science. 80: T1120-T1128 (2015)
Song G, Li L, Wang HX, Zhang M, Yu X, Wang J, Xue J, Shen Q. Real-time assessing the lipid oxidation of prawn (Litopenaeus vannamei) during air-frying by iKnife coupling rapid evaporative ionization mass spectrometry. Food Control. 111: 107066 (2020)
Song JH, Jang EY, Kim MJ, Kim YJ, Lee JH. Development of a spectroscopic method to determine the content of free radical scavenging compounds and oxidation products in thermally oxidised oils. International Journal of Food Science and Technology. 51: 2424-2432 (2016)
Song JH, Kim MJ, Kim YJ, Lee JH. Monitoring changes in acid value, total polar material, and antioxidant capacity of oils used for frying chicken. Food Chemistry. 220: 306-312 (2017)
Velíšek J, Davidek T, Davídek J, Víden I, Trška P. Some formaldehyde reaction products in non-enzymatic browning reactions. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 188: 426-429 (1989)
Yu J, Wang M, Zhang M, Liu Y, Li J. Effect of infrared ray roasting on oxidation stability and flavor of virgin rapeseed oils. Journal of Food Science. 86: 2990-3000 (2021)
Zamora R, Navarro JL, Aguilar I, Hidalgo FJ. Lipid-derived aldehyde degradation under thermal conditions. Food Chemistry. 174: 89-96 (2015)
Acknowledgements
This research was supported by Ottogi HamTaeHo Foundation and the Basic Science Research Program through the National Research Foundation of Korea (NRF-2020R1A2C2006600), funded by the Ministry of Education, Science and Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, Y., Kim, MJ. & Lee, J. Physicochemical properties and oxidative stability of corn oil in infrared-based and hot air-circulating cookers. Food Sci Biotechnol 31, 1433–1442 (2022). https://doi.org/10.1007/s10068-022-01127-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-022-01127-7