Abstract
Effects of deuteron (D+) and electron donors on the oxidative stability in linoleic acid–water model systems were evaluated by analyzing headspace oxygen content and headspace volatiles. Acetic acid-d and tetrakis(dimethylamino)ethylene (TDAE) were selected as a deuteron and an electron donor, respectively. Samples containing acetic acid-d had significantly lower headspace oxygen content than controls while those containing TDAE had significantly higher headspace oxygen content (p < 0.05). Combination of acetic acid-d and TDAE accelerated the consumption of headspace oxygen. Volatiles including t-2-heptenal, 2-octenal, or 2,4-octadienal had higher mass to charge ratio (m/z) of (molecular weight +1)/molecular weight in samples with deuterium oxide than in samples with deuterium free water. However, no significant difference was observed in the m/z ratio of (molecular weight +1)/molecular weight of those volatiles among samples with or without deuteron or electron donors. Also, lipid hydroperoxides with deuterium, were not found in samples containing deuterium oxide and acetic acid-d. Therefore, added acetic acid-d may not be involved on the formation of lipid hydroperoxides and volatiles directly.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Lipid oxidation is a chemical reaction among unsaturated lipid and oxygen molecules. Besides unsaturated fatty acids and oxygen molecules, other compounds including prooxidative metal ions, antioxidative compounds, moisture content, and amphiphilic minor compounds including free fatty acid (FFA), monoacylglycerols (MAG), diacylglycerols (DAG), and phospholipid (PL) may play important roles in the rates of lipid oxidation [1,2,3,4]. Moisture can form association colloids with minor amphiphilic compounds and the interfaces of oil–water in association colloids has been proposed as key places for lipid oxidation [1, 4, 5]. During the lipid oxidation process, the moisture content in the lipids can change and the moisture can be transferred from the atmosphere to the oil [6, 7]. The antioxidant properties of chemical compounds are affected by the presence of moisture in the lipid. Kim et al. [8, 9] reported that antioxidant activities of α-tocopherol and ascorbic acid were greatly affected by the content of moisture in bulk oils. Also, aglycone quercetin and glycoside rutin showed various oxidative stability in vegetable oils depending on the moisture content and stripping process of the bulk oils [10].
Recently, the possibility of moisture as a substrate during the lipid oxidation has been reported by using analytical techniques for headspace volatiles in linoleic acid-deuterium oxide (D2O) model system [11, 12]. Headspace volatiles like pentane had more deuterium (D·) in linoleic acid with D2O than those in deuterium free water (H2O), which implies that moisture can participate in the formation of volatiles during lipid oxidation [11]. This phenomenon can be observed in linoleic acid–water model systems without air-tightness or different treatment temperature condition [12].
From previous studies, volatile compounds had higher ratios of D· when tested in lipid-D2O model compared to those of D· in lipid-H2O model from lipid oxidation. To produce D· from D2O, high energy should be applied to dissociate covalent bonding between deuterium and oxygen. However, previous studies used 30–90 °C, which may not provide enough energy to dissociate D2O into D·. Because D2O can generate deuteron (D+) due to the pD like ordinary water, D+ is likely to participate in the formation of volatiles from linoleic acid oxidation instead of D·. To evaluate this hypothesis, acetic acid-d and tetrakis(dimethylamino)ethylene (TDAE) were selected as a deuteron donor or electron donor, respectively. Acetic acid-d is an organic compound with the chemical formula CH3COOD and deuteron (D+) can be separated from the carboxyl group (–COOD) of acetic acid-d. TDAE is known as a very electron-rich and strong organic electron donor with an ionizing potential close to zinc (6.12 eV) [13]. Addition of deuteron or electron donors may influence the formation of certain oxidation products, which can be indirect evidence for the involvement of deuteron or electron donors on the steps of lipid oxidation.
The objectives of this study were to determine the effects of deuteron or electron donors on the oxidative stability in linoleic acid-D2O or -deuterium free water systems and to compare the increase in D· in headspace volatiles from oxidized model systems in the presence of acetic acid-d. Also, the presence of deuterium in lipid hydroperoxides (LOOD) was tested.
Materials and Methods
Materials
Deuterium free water (H2O), D2O, linoleic acid, acetic acid-d, and TDAE were purchased from Sigma-Aldrich (St. Louis, MO). Fiber of solid phase microextraction (SPME) [50/30 mm DVB/Carboxen/Polydimethylsiloxane (PDMS) StableFlex] was purchased from Supelco, Inc. (Bellefonte, PA). Vials, magnetic seals, and septa were purchased from Agilent technologies, Inc. (Santa Clara, CA). Other reagent grade chemicals were purchased from Daejung Chemical Co. (Seoul, Korea).
Sample Preparation
Acetic acid-d or TDAE were dissolved in D2O or H2O at the concentration of 416 or 1 mM, respectively. Preliminary study was conducted to find the concentration of acetic acid-d or TDAE to make detectable changes in the oxidative stability. The 0.2 g of linoleic acid was mixed with 0.6 g water containing acetic acid-d or TDAE in a 10-mL bottle. The sample bottles were sealed air-tight with rubber septa and aluminum caps and stored at 60 °C in a drying oven (HYSC, Seoul, Korea) for 6 h. Samples without adding acetic acid-d nor TDAE were prepared as controls. Controls in H2O or D2O were named as D0 or D1, respectively. Samples were prepared in triplicate and analyzed at 2, 4, and 6 h.
For the combination effects of mixtures of a deuteron donor and an electron donor, 0.6 g water containing both 416 mM acetic acid-d and 1 mM TDAE was mixed with 0.2 g of linoleic acid in 10-mL bottles, which were sealed air-tight with rubber septa and aluminum caps. Sample bottles were treated at 60 °C in the drying oven (HYSC, Seoul, Korea) for 7 h. Samples were prepared in triplicate. Samples containing TDAE, acetic acid-d, and a combination of acetic acid-d and TDAE were designated as TD, AD, and ADTD, respectively. Samples in H2O or D2O were differentiated adding 0 or 1 after their names like TD0 or TD1, respectively in the case of samples with TDAE.
Headspace Oxygen Analysis
A Hewlett-Packard 7890 gas chromatograph (GC) (Agilent Technologies, Inc., Santa Clara, CA) equipped with a 60/80 packed column (3.0 m × 2 mm ID, Restek Ltd.) and a thermal conductivity detector (TCD) from Agilent Technologies (Palo Alto, CA) was used to determine headspace oxygen content in air-tight sample bottles according to methods of Kim et al. [14]. The 30 μL headspace gas was transferred from a sample bottle using an air-tight syringe to a GC-TCD. The flow rate of helium gas was 200 mL/min. Temperatures of the oven, the injector, and the detector in GC were 60, 180, and 180 °C, respectively.
Identification of Lipid Hydroperoxides
Lipid hydroperoxides in samples of D1, D0, ADD1, and ADD0 were analyzed using a 5600 Q-TOF LC/MS/MS system (AB Sciex, Foster City, CA) using a Ultimate 3000 RSLC HPLC system (Dionex), including a degasser, an autosampler, diode array detector, and a binary pump. Stationary phase was a Hypersil GOLD column (2.1 × 50 mm, 1.9 μm, Thermo scientific) with a mobile phase A (0.1% formic acid in water) and a mobile phase B (0.1% formic acid in acetonitrile). The flow rate was 0.25 mL/min. The linear gradient was as follows. The injection volume was 5 μL. Mass spectra were acquired under negative or positive electrospray ionization (ESI) with an ion spray voltage of ±4500 V. The source temperature was 500 °C. The curtain gas, ion source gas 1, and ion source gas 2 were 35, 65, and 55 psi, respectively. Scan was conducted from 50 to 1500 (mass/charge). Mass spectra of peaks for hydroperoxides were analyzed to detect LOOD from LOOH by comparing the m/z (mass to charge ratio).
Analysis of Headspace Volatiles by Solid Phase Microextraction (SPME)
Analysis conditions of SPME for volatile compounds were adapted from the methods of Kim et al. [9]. For equilibration of the volatile compounds in the headspace, sample vials were placed in a water bath (RW-0525G; Lab Camp, Incheon, Korea) at 30 °C for 30 min, and then moved into an agitator of multipurpose sampler. Headspace volatiles were isolated and extracted for 30 min using a 50/30 mm DVB/Carboxen/PDMS StableFlex solid phase at 30 °C.
Volatiles attached in the solid phase of SPME were separated and identified using a Hewlett-Packard 6890 GC apparatus equipped with a 5971A mass selective detector (MS) (Agilent Technology) and a DB-5 ms column (30 m × 0.25 mm i.d., 0.25 μm film thickness, Agilent J & W, Folsom, CA). Solid phase of SPME was exposed in an injector for 3 min. Helium was carrier gas at 1.0 mL/min and the oven temperature was held at 40 °C for 3 min and increased from 40 to 150 °C at 4 °C/min and from 150 to 220 °C at a rate of 15 °C/min. All mass spectra were obtained at 70 eV and 220 °C ion source temperature with electron impact ionization mode. The temperature of the quadruple was 150 °C. The identification of compounds was made by a combination of NIST Mass Spectra version and gas chromatographic retention times of standard compounds.
Detection of Volatiles Containing Deuterium
The presence of D· in volatiles was analyzed by comparing m/z (mass to charge ratio) of each volatile [11, 12]. A peak representing molecular weight may not be the most abundant peak. If a D· is incorporated in a molecule, m/z will increase by 1 compared to those in a deuterium free molecule. Comparison of m/z (molecular weight +1)/molecular weight can show the presence of D· in each volatile. For example, in case of t-2-heptenal (molecular weight of 112.17) and 2-octenal (molecular weight of 126.20), the m/z of 113.17 from d 1-t-2-heptenal/112.17 from t-2-heptenal, and 127.20 from d 1-2-octenal/126.20 from 2-octenal, respectively were determined at each sampling time from the GC/MS ion chromatogram. The retention time of t-2-heptenal, 2-octenal, 2,4-octadienal, and 2,4-decadienal were 13.52, 16.03, 18.60, and 23.24 min, respectively.
Statistical Analysis
Data of the headspace oxygen content, total volatile contents, and the m/z of (molecular weight +1)/molecular weight of headspace volatiles were analyzed statistically by ANOVA and Duncan’s multiple range test using SPSS software program version 19 (SPSS Inc., Chicago, IL). A p value <0.05 was considered significant.
Results and Discussion
Effects of Deuteron Donor Acetic Acid-d
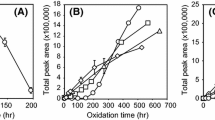
Changes of headspace oxygen content (a) and total peak areas of headspace volatiles (b) in a linoleic acid-deuterium free water model system containing acetic acid-d at 60 °C for 6 h are shown in Fig. 1. Preliminary study on the determination of acetic acid concentration showed that 416.32 mM acetic acid was needed to show its’ effects on the oxidative stability in the adapted model systems (data not shown). As oxidation time increased, headspace oxygen content in all the samples decreased gradually. After 6 h of treatment, ADD1 showed significantly low headspace oxygen content compared to other samples, which implies that acetic acid-d, a deuteron donor, acted as a prooxidant (Fig. 1a). Oxidative stability of edible oils is greatly influenced by the pH of the solution [15]. Oils with a non-buffered solution of pH 4 had low oxidative stability whereas oils with a buffered solution of pH 4 did not show any differences in their oxidative stability compared to oils with a pH of 7 [15]. Because non-buffered solution of acetic acid was added to the oil system, results of the current study agree with the previous report [15].
Changes of headspace oxygen content (a) and total peak areas of headspace volatiles (b) in linoleic acid-deuterium free water model system containing acetic acid-d at 60 °C for 6 h. Samples containing acetic acid-d in deuterium oxide or deuterium oxide free water were designated as ADD1 and ADD0, respectively while samples in deuterium oxide or deuterium oxide free water were designated as D1 and D0, respectively. Different lower case letters indicate significantly differences at 0.05
The total peak area of headspace volatile compounds was calculated excluding the peak area of acetic acid due to the high strength of acetic acid. Peak areas from D0 to D1 were significantly higher than ADD0 and ADD1 (p < 0.05) (Fig. 1b). Samples with deuterium oxide (D1 or ADD1) generated more total peak areas of volatiles than corresponding samples (D0 and ADD0), although no significant difference was observed (p > 0.05). Although addition of acetic acid-d accelerated the consumption of oxygen molecules based on Fig. 1a, some volatiles may be co-eluted with the large peak of acetic acid-d and results of total peak areas might not show prooxidative properties of acetic acid-d. Usually, presence of D2O significantly accelerated the consumption of headspace oxygen and generate more volatiles in samples compared to deuterium free H2O [11, 12]. However, prooxidative properties of D2O were not observed in current experimental conditions.
The mass to charge ratio (m/z) of (molecular weight +1)/molecular weight of t-2-heptenal (a), 2-octenal (b), and 2,4-octadienal (c) in linoleic acid-deuterium free water model system containing acetic acid-d at 60 °C for 6 h are shown in Fig. 2. t-2-Heptenal, 2-octenal, and 2,4-octadienal were reported as oxidation products of linoleic acid or trilinolein [16, 17]. For 2-octenal, the m/z ratios of 127.20/126.20 were significantly higher in linoleic acid samples containing D2O than in samples containing deuterium free water regardless of the addition of acetic acid-d (p < 0.05). This trend was observed for t-2-heptenal, and 2, 4-octadienal for linoleic acid samples containing either D2O or deuterium free water (Fig. 2). However, addition of acetic acid-d (ADD0) did not cause any significant changes in the m/z of (molecular weight +1)/molecular weight of t-2-heptenal, 2-octenal, and 2,4-octadienal in samples with deuterium free water (D0) (Fig. 2), which implies deuteron (D+) dissociated from acetic acid-d may not be major factors for the formation of volatiles during linoleic acid oxidation. The m/z of (molecular weight +1)/molecular weight of 2-octenal and 2,4-octadienal in ADD1 were not significantly different from those in D1 although t-2-heptenal had different result.
Ratios of peak abundance of t-2-heptenal (a), 2-octenal (b), and 2,4-octadienal (c) in linoleic acid-deuterium free water model system containing acetic acid-d at 60 °C for 6 h. Abbreviations were listed in Fig. 1. Different lower case letters indicate significantly differences at 0.05
Effects of Electron Donor TDAE
Changes of headspace oxygen content (a) and total peak areas of headspace volatiles (b) in linoleic acid-deuterium free water model system containing TDAE at 60 °C for 6 h are shown in Fig. 3. As oxidation time increased to 6 h, samples containing TDAE had significantly high headspace oxygen content compared to other control samples (p < 0.05), which implies that TDAE acted as an antioxidant (Fig. 3a). There was no significant difference in headspace oxygen content between TD0 and TD1 or D0 and D1, which implies that deuterium oxide did not give detectable changes in the headspace oxygen consumption under current experiment conditions (p > 0.05).
Changes of headspace oxygen content (a) and total peak areas of headspace volatiles (b) in linoleic acid-deuterium free water model system containing TDAE at 60 °C for 6 h. Samples containing TDAE in deuterium oxide or deuterium oxide free water were designated as TD1 and TD0, respectively. Samples in deuterium oxide or deuterium oxide free water were designated as D1 and D0, respectively. Different lower case letters indicate significantly differences at 0.05
Total peak areas from D0 to D1 were significantly higher than TD0 and TD1 since 2 h treatment (p < 0.05). D1 and TD1 had more total peak areas of volatiles than D0 and TD0, respectively, although there was no significant difference in the peak areas between D1 and D0 and between TD1 and TD0 at 6 h (p > 0.05) (Fig. 3b).
The mass to charge ratio (m/z) of (molecular weight +1)/molecular weight of t-2-heptenal (a), 2,4-octadienal (b), and 2,4-decadienal (c) in linoleic acid-deuterium free water model system containing TDAE at 60 °C for 6 h are shown in Fig. 4. 2,4-Decadienal is one of representative oxidation volatiles from linoleic acid or trilinolein [16, 17]. The m/z of (molecular weight +1)/molecular weight of 2,4-octadienal was significantly higher in linoleic acid samples containing D2O than in samples containing deuterium free water regardless of the addition of TDAE (p < 0.05). This trend was observed for t-2-heptenal, and 2, 4- decadienal for linoleic acid samples (Fig. 4), which agrees with the results of Fig. 2. TD1 with added TDAE did not increase the m/z of (molecular weight +1)/molecular weight of selected volatiles compared to D1 (Fig. 4), which implies electrons may not enhance the incorporation of deuterium in volatiles. On the contrary, the m/z of (molecular weight +1)/molecular weight of t-2-heptenal and 2-octenal in TD1 were significantly lower than those in D1 (p < 0.05). Electrons may interfere the incorporation of deuterium during the volatile generation.
Ratios of peak abundance of t-2-heptenal (a), 2,4-octadienal (b), and 2,4-decadienal (c) in linoleic acid-deuterium free water model system containing TDAE at 60 °C for 6 h. Abbreviations were listed in Fig. 3. Different lower case letters indicate significantly differences at 0.05
Combination Effects of Deuteron Donor Acetic Acid-d and Electron Donor TDAE
Changes of headspace oxygen content in linoleic acid-deuterium free water model system containing TDAE and acetic acid-d at 60 °C after 7 h of treatment are shown in Fig. 5. Generally, headspace oxygen content in deuterium oxide was lower than those in deuterium free water. Addition of acetic acid-d induced the consumption of headspace oxygen (ADD) and samples containing TDAE had higher headspace oxygen content than control samples (Fig. 5), which agrees with the results of deuteron or electron donor addition (Figs. 1, 3). In the case of combination of acetic acid-d and electron donor (ADTD), headspace oxygen content was significantly lower than controls or samples with TDAE (p < 0.05). However, no significant difference was observed between ADTD and ADD samples (p > 0.05), which implies prooxidative properties of acetic acid-d may overwhelm the antioxidant properties of TDAE. The presence of electron donor did not affect the consumption of headspace oxygen when acetic acid was present. If TDAE had stronger factor than acetic acid, combination of TDAE and acetic acid should have antioxidant properties.
Changes of headspace oxygen content in linoleic acid-deuterium free water model system containing TDAE and acetic acid-d at 60 °C after 7 h treatment. ADTD, TD, and ADD were samples containing combination of TDAE and acetic acid-d, TDAE, and acetic acid-d in deuterium oxide, respectively. Samples in deuterium oxide or deuterium oxide free water were designated as D1 and D0, respectively. ‘D’ indicate samples without addition of TDAE nor acetic acid-d. Different lower case letters indicate significantly differences at 0.05
The m/z of (molecular weight +1)/molecular weight of selected volatiles in linoleic acid-deuterium free water model system containing TDAE and acetic acid-d at 60 °C after 7 h treatment are shown in Fig. 6. Generally, samples with deuterium oxide produced more m/z of (molecular weight +1)/molecular weight of volatiles than samples with deuterium free water. The m/z of (molecular weight +1)/molecular weight of 2-octenal and 2,4-octadienal in D1, ADD1, TD1, and ADTD1 were significantly higher than those in D0, ADD0, TD0, and ADTD0, respectively (p < 0.05) although there were no significant difference among them (p > 0.05).
Ratios of peak abundance of selected volatiles in linoleic acid-deuterium free water model system containing TDAE and acetic acid-d at 60 °C after 7 h treatment. Samples containing acetic acid-d in deuterium oxide or deuterium oxide free water were designated as ADD1 and ADD0, respectively while samples in deuterium oxide or deuterium oxide free water were designated as D1 and D0, respectively. Samples containing TDAE in deuterium oxide or deuterium oxide free water were designated as TD1 and TD0, respectively. ADTD1 and ADTD0 are samples containing combination of TDAE and acetic acid in deuterium oxide or deuterium oxide free water, respectively. Different lower case letters indicate significantly differences at 0.05
Detection of LOOD by LC/MS/MS
Mass spectra of lipid hydroperoxides from linoleic acid-deuterium oxide model (D1) (a), linoleic acid-deuterium free water model (D0) (b), linoleic acid-deuterium oxide model with acetic acid-d (ADD1) (c), and linoleic acid-deuterium free water model containing acetic acid-d (ADD0) (d) at 60 °C after 7 h of storage are shown in Fig. 7. Four peaks of lipid hydroperoxides were eluted in LC/MS/MS chromatogram (data not shown) and mass spectra were the average of all the peaks of lipid hydroperoxides. Molecular weight of hydroperoxides from linoleic acid is 312.22 and that of lipid hydroperoxides containing deuterium should be 313.22. Because negative mode was used for collecting ions, mass to charge of hydroperoxides from linoleic acid without and with deuterium should be 311.22 and 312.22, respectively (Fig. 7). All the mass spectra from D1, D0, ADD1 and ADD0 showed peaks of 311.22 and 312.22 clearly, which came from lipid hydroperoxides (Fig. 7). Peak intensity of 312.22 mass to charge in D1 was similar to that in D0 (Fig. 7a, b) and that in ADD1 and ADD0 were also similar each other (Fig. 7c, d). Therefore, the addition of acetic acid-d (ADD1) or deuterium oxide (D1) did not detectably increase the concentration of deuterium containing lipid hydroperoxides.
LC/MS/MS chromatograms of lipid hydroperoxides from linoleic acid-deuterium oxide model or D1 (a), linoleic acid-deuterium free water model or D0 (b), linoleic acid-deuterium oxide model with acetic acid-d or ADD1 (c), and linoleic acid-deuterium free water model containing acetic acid-d or ADD0 (d) at 60 °C after 6 h storage
Lipid hydroperoxides from unsaturated double bonds can be formed using oxygen molecules and unsaturated double bonds. Also, addition of hydrogen donating antioxidants including α-tocopherol or quercetin could decrease the formation of lipid hydroperoxides [18]. If deuteron from acetic acid-d participates the formation of a lipid hydroperoxides peak of LOOD should increase. It seems that there is no step of inserting protons or deuteron from moisture into the formation of lipid hydroperoxide. Therefore, volatiles possessing deuterium may be generated during decomposition of lipid hydroperoxides.
Antioxidant properties of electron-donating compounds or reducing compounds have been documented in the literature. Hydrogen atom transfer (HAT) mechanisms or an electron transfer (ET) mechanism are typical theories to explain antioxidant properties of phenolic compounds. Bond dissociation enthalpy (BDE) and ionization potential (IP) could be good indicators for HAT or ET mechanisms of phenolic compounds, respectively [19,20,21]. Chemical compounds like curcumin with high IP should donate electrons to lipid radicals like peroxyl radicals (LOO·) and then lipid radicals will be anionic non-radicals (LOO−), which will receive protons (H+) and become lipid hydroperoxides (LOOH) [19, 22]. Since TDAE is a potent electron donating compound, the lipid radicals would be non-radical forms of lipids without sacrificing unsaturated fatty acids, which can explain the strong antioxidant properties of TDAE. Therefore, TDAE may be involved in the steps of formation of LOOH by protecting LH as a primary antioxidant. The added deuteron (D+) did not affect LOOD formation as shown in Fig. 7. Samples of ADD1 produced a lower content of deuterated volatiles compared to those of D1, which implies deuteron (D+) from acetic acid-d may not participate efficiently for the formation of volatiles. Deuterium from D2O may be involved in the β-scission steps of lipid hydroperoxides and may participate in the formation of volatiles, which needs further reseraches to confirm this specualtion.
Conclusion
Effects of deuteron and electron donors on the oxidative stability were evaluated in linoleic acid–water system. Acetic acid-d, a deuteron (D+) donor, accelerated the lipid oxidation rate while TDAE delayed the oxidation process. Oxidative stability in samples containing both acetic acid-d and TDAE in deuterium oxide was lower than that in deuterium free water. Under current experimental conditions, the addition of deuteron (D+) from acetic acid-d may not be involved in the formation of volatiles from linoleic acid oxidation. Lipid hydroperoxides containing deuterium (LOOD) were not detected in sample with deuteron (D+), which suggests that moisture could be involved in the lipid oxidation during the decomposition stage of lipid hydroperoxides rather than formation of lipid hydroperoxides. Providing a strong electron donor can be a practical strategy for controlling the rates of lipid oxidation.
References
McClements DJ, Decker EA (2000) Lipid oxidation in oil-in-water emulsions: impact of molecular environment on chemical reactions in heterogeneous food systems. J Food Sci 65:1270–1282
Choe E, Min DB (2006) Mechanisms and factors for edible oil oxidation. Compr Rev Food Sci F 5:169–186
Chaiyasit W, Elias RJ, McClements DJ, Decker EA (2007) Role of physical structures in bulk oils on lipid oxidation. Crit Rev Food Sci 47:299–317
Laguerre M, Bayrasy C, Panya A, Weiss J, McClements DJ, Lecomte J, Decker EA, Villeneuve P (2015) What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit Rev Food Sci Nutr 55:183–201
Frankel EN, Huang SW, Kanner J, German JB (1994) Interfacial phenomena in the evaluation of antioxidants: bulk oils vs emulsions. J Agric Food Chem 42:1054–1059
Park JW, Kim JY, Kim MJ, Lee JH (2014) Evaluation of oxygen limitation on lipid oxidation and moisture content in corn oil at elevated temperature. J Am Oil Chem Soc 91:439–444
Budilarto ES, Kamal-Eldin A (2015) Water content and micelle size change during oxidation of sunflower and canola oils. Eur J Lipid Sci Technol 117:1971–1977
Kim JY, Yi B, Kim MJ, Lee JH (2015) Effects of relative humidity on the antioxidant properties of α-tocopherol in stripped corn oil. Food Chem 167:191–196
Kim JY, Kim MJ, Yi BR, Oh SM, Lee JH (2015) Antioxidant properties of ascorbic acid in bulk oils at different relative humidity. Food Chem 176:302–307
Lee CK, Gim SY, Kim MJ, Lee JH (2017) Effects of quercetin or rutin on the oxidative stability of stripped or non-stripped oils containing α-tocopherol. Eur J Lipid Sci Technol 119:1600329
Kim JY, Kim MJ, Lee JH (2014) Role of moisture on the lipid oxidation determined by D2O in linoleic acid system. Food Chem 146:134–140
Kim JY, Kim MJ, Lee JH (2014) Effects of deuterium oxide on the oxidative stability and changes of headspace volatiles of corn oil. J Am Oil Chem Soc 91:623–628
Burkholder C, Dolbier WR, Medebielle M (1998) Tetrakis(dimethylamino)ethylene as a useful reductant of some bromodifluoromethyl heterocycles. Application to the synthesis of new gem-difluorinated heteroarylated compounds. J Org Chem 63:5385–5394
Kim JY, Yi BR, Lee JH (2014) Oxidative stability of solid fats containing ethylcellulose determined based on the headspace oxygen content. Food Sci Biotechnol 23:1779–1784
Kim JY, Yi BR, Lee CY, Gim SY, Kim MJ, Lee JH (2016) Effects of pH on the rates of lipid oxidative stability in oil-water model systems. Appl Biol Chem 59:157–161
Frankel EN (1985) In: Min DB, Smouse TH (eds) Flavor chemistry of fats and oils. American Oil Chemists’ Society Monograph, Champaign
Hwan HS, Winkler-Moser JK (2016) In: Min H, Jacobsen C (eds) Oxidative stability and shelf life of foods containing oils and fats. American Oil Chemists’ Society Monograph, Champaign
Nogala-Kalucka M, Kupczyk B, Polewski K, Siger A, Dwiecki K (2007) Influence of native antioxidants on the formation of fatty acid hydroperoxides in model systems. Eur J Lipid Sci Technol 109:1028–1037
Barzegar A (2012) The role of electron-transfer and H-atom donation on the superb antioxidant activity and free radical reaction of curcumin. Food Chem 135:1369–1376
Wright JS, Johnson ER, DiLabio GA (2001) Predicting the activity of phenolic antioxidants: theoretical method, analysis of substituent effects, and application to major families of antioxidants. J Am Chem Soc 123:1173–1183
Choe E, Min DB (2009) Mechanisms of antioxidants in the oxidation of foods. Compr Rev Food Sci F 8:345–358
Budilarto ES, Kamal-Eldi A (2015) The supramolecular chemistry of lipid oxidation and antioxidation in bulk oil. Eur J Lipid Sci Technol 117:1095–1137
Acknowledgments
This research was supported by a Grant (NRF-2017R1A2B4002613) and (NRF-2016R1E1A2A01939822) of the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Oh, S., Lee, C., Kim, S. et al. Oxidative Stability and Volatile Formations in Linoleic Acid-D2O Models in the Presence of Deuteron or Electron Donors. J Am Oil Chem Soc 94, 1385–1392 (2017). https://doi.org/10.1007/s11746-017-3044-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11746-017-3044-5