Abstract
The changes in physiochemical properties of flours obtained from the four selected grains after germination were investigated. After germination, the sprout length of sorghum and millet was substantially larger than that of brown rice and oat. Germination led to a decrease in the apparent amylose content and swelling factor of flours. Gelatinization onset and peak temperatures increased after germination, while a slight decrease was found in conclusion temperature. Compared to the raw flours, the germinated flours derived from brown rice, sorghum, and millet had lower gelatinization enthalpy, whereas the germinated oat flour showed higher gelatinization enthalpy. Germination resulted in significant decrease in pasting parameters of the four flours. Amylose leaching of sorghum and millet flours increased after germination, while the brown rice and oat flours showed a significant decrease in amylose leaching. Results suggest that germination effectively altered the physicochemical properties of grain flours, which can be utilized as functional ingredient in the preparation of grain-based products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice, oat, sorghum, and millet play a critical role in daily human diet with providing a bulk nutrient and energy source. Brown rice (Oryza sativa L.) is dehulled from the whole grain rice, which contains nutrients including fatty acids, minerals, and vitamins (Komatsuzaki et al., 2007). Nonetheless, brown rice contains bran, which is not easy to cook and therefore, not considered superior to milled rice with a mild, nutty flavor, and chewier taste (Komatsuzaki et al., 2007). Oat (Avena sativa L.), an outstanding cereal, contains soluble dietary fiber and functionally active component of β-glucan with cholesterol-lowering and antidiabetic effects (Singh et al., 2013). Millet (Setaria italica) is healthy and all-value crop cultivated in the semi-arid tropics. The fermented sprouting millet is used to produce added-value foods and beverages (Sudha et al., 2016). Sorghum (Sorghum bicolor) has become increasingly popular to consumers due to its high level of nutrients and disease-prevention potential (Taylor et al., 2014). The potential benefits of ancient grains such as brown rice, oat, sorghum, and millet for food consumption were attributed to the presence of significant amounts of dietary fiber, bioactive compounds, vitamins, and minerals (Komatsuzaki et al., 2007; Sudha et al., 2016; Singh et al., 2013; Taylor et al., 2014).
Germination is an easily controlled and effective process to enhance the nutritional qualities of grains (Komatsuzaki et al., 2007). After soaking, grains in water are germinated in a humidity chamber under specific temperature. Germination leads to an increased activity of hydrolytic enzymes in seeds and disintegration of major compounds such as starch, fibers, and protein, which facilitates cooking of the cereal kernels and texture improvement (Kaneko et al., 2002). In addition, germination enhances the protein content, storage stability, bioactive compounds, antioxidant properties, and foaming capacity in grains. Thus, the consumption of germinated grains has potential benefits to human health (Chung et al., 2014; Suwanmanon and Hsieh, 2014). Compared to raw grains, germinated grains have received attention recently due to the increases in nutritional and functional qualities (Suwanmanon and Hsieh, 2014). In our previous study (Li et al., 2017), we investigated the structure and physicochemical properties of starches isolated from germinated grains (brown rice, oat, sorghum, and millet) and found that the starches from germinated grains had substantially decreased pasting viscosity possibly due to the breakdown of starch molecules by amylolytic enzymes. However, previous studies of using germination to modify grain starches are not very practical for industrial processing. There is growing interest from the food industry in utilizing the germinated grain flours and thus it is necessary to elucidate the physicochemical and functional properties of flours for better control of industrial manufacturing process (Falade and Christopher, 2015). However, the physicochemical and functional properties of germinated flour from various grains have yet to be fully investigated. Then, the aim of this study was to investigate the impact of germination process on the physicochemical characteristics of flours from brown rice, oat, sorghum, and millet. It is important to understand the impact of germination on the physicochemical properties of flours from various grains before they are incorporated to foods as ingredients and developed as added-value foods with potential health benefits.
Materials and methods
Preparation of germinated grain
Four grains (brown rice-Ilpum cultivar, oat-Choyang, sorghum-Sodamchal, and millet -Samdachal) were obtained from National Institute of Crop Science (Suwon, Korea) in 2016. The brown rice, oat, sorghum, and millet seeds (200 g) were sterilized in 1 L of 1% aqueous sodium hypochlorite for 30 min and then rinsed with enough distilled water at least 10 times before being soaked at 25 °C in 1 L of distilled water for 20 (brown rice), 12 (sorghum and millet) and 8 h (oat), respectively. The hydrated grains were allowed to germinate with layering over wet cellulose pads in a humid chamber (JSTH-8000CP, JS Research Inc., Gongju, Korea) for 60 h at 25 °C (oat seeds) or 30 °C (brown rice, sorghum, and millet seeds) with 95% relative humidity. Germinated grains were dried at 50 °C until reaching a moisture content of 10%. Sample seeds were milled to fine flour using a mechanical grinder (DA5500, Daesung Artlon Co., Seoul, Korea), screened through a 100-mesh sieve and stored at 4 °C for further analysis. The germination percentage of the grains was determined using the ratio of germinated seeds to the total seeds with 100 grains. The sprout length on the germinated grains was measured by a Vernier caliper with 30 sprouts after the specified germination times.
Apparent amylose content
The apparent amylose content of raw and germinated flours was measured colorimetrically by the iodine affinity method (William et al., 1970). Briefly, 5 mL of 0.5 N KOH was added to 10 mg of flour and mixed for 20 min. After neutralization by adding 0.5 N HCl, I2–KI solution immediately was added, and the absorbance was measured. The amylose content was determined from standard curve obtained with potato amylose and maize amylopectin (Sigma, St. Louis, MO, USA).
Physicochemical properties of flours
The color parameters of raw and germinated flours were determined using a colorimeter (Minolta JP/CM-3500D, Tokyo, Japan). The color parameters measured were L* (lightness), a* (redness), and b* (yellowness).
The thermal properties of flours derived from raw and germinated grains were measured by differential scanning calorimeter (DSC4000, PerkinElmer Inc., Waltham, MA, USA). Flour (6 mg, db) and distilled water (14 mg) were added into a stainless-steel pan (PerkinElmer Inc.), which was immediately sealed and allowed to stand for 2 h at room temperature. The sample pan was heated from 10 to 130 °C at a heating rate of 5 °C/min.
The pasting properties of raw and germinated flours were determined Rapid Visco-Analyzer (RVA-TecMaster, Newport Scientific Pty. Ltd., Warriewood, Australia). Flour (7% w/w, db) was dispersed in distilled water in the RVA canister with 30 g of total weight. Flour slurry was held at 50 °C for 1 min, heated to 95 °C at a rate of 6 °C/min, held at 95 °C for 5 min, cooled to 50 °C at a rate of 6 °C/min and then held at 50 °C for 2 min with constant stirring of 160 rpm.
Swelling factor of flours was measured following the method of Tester and Morrison (1990). Briefly, flour slurry (2%, w/w) was heated in a water bath at 80 °C for 30 min and cooled immediately at room temperature. One milliliter of blue dextran solution (0.5 mg/mL, Sigma) was added in the tube. After centrifugation at 1500 × g for 5 min, the absorbance of the supernatant was determined.
Amylose leaching of flours was determined by the method of Chung et al. (2008). Flour (20 mg, db) was mixed with distilled water (10 mL) in a sealed tube, which was heated at 80 °C for 30 min. The tube was cooled rapidly under cold running tap water and centrifuged at 2000 × g for 10 min. The amylose content of supernatant was determined.
Statistical analysis
The reported data were the means of triplicate measurements. Statistical analyses and least significant difference tests were carried out using the SPSS V. 22.0 software (SPSS Institute Inc., IL, USA) and p < 0.05 was considered to be statistically significant.
Results and discussion
Germination characteristics of seeds
The sprout length of brown rice, oat, sorghum, and millet seeds was evaluated to ensure germination (Fig. 1). With increased germination time, the sprout length of brown rice, oat, sorghum, and millet seeds was clearly increased after sufficient steeping. Proper steeping is essential to activate the enzymes needed for seed germination (Komatsuzakij et al., 2007). The germination percentage and sprout length of the tested grains differed during the germination periods in this study (Fig. 1). At 24 h of seed germination, the sprout length from sorghum and millet was substantially higher than that from brown rice and oat (Fig. 1). This trend in sprout length was more substantial with increase in germination time. After 60 h of germination, the sprout length in sorghum and millet ranged from 8 to 24 mm, while the sprouts obtained from brown rice and oat ranged from 3 to 6 mm. Interestingly, the germination percentage depended strongly on grain species. The germination percentage of sorghum and millet seeds after 48 h was 80% and 84%, respectively, while all seeds derived from brown rice and oat were germinated after 48 h in the humid chamber (data not shown). This result is in line with amylolytic enzyme activity reported by Dziedzoave et al. (2010) who found that the germinated rice (~ 200 units/g) had significantly higher α-amylase activity than sorghum (~ 20 units/g) and millet (~ 10 units/g) during germination periods. They claimed that the difference in the level of endogenous gibberellins could account for these differences in amylolytic enzyme activity behaviors since the gibberellins are the principal stimulators of enzyme development in germinated grains. The non-emergence of sprouts in some seeds of sorghum and millet indicates the inability for physical rupture of the cell wall.
Apparent amylose content
Apparent amylose content of raw and germinated four grain flours ranged from 15.8 to 11.3%, 17.2 to 14.3%, 4.0 to 3.0%, and 2.9 to 2.2%, respectively (Table 1). A significant decrease in amylose content occurred after germination in brown rice and oat. The sorghum and millet flours, which are waxy type species, also showed a slight decrease in amylose content after germination. These results are in accordance with previous studies reported by Zheng et al. (2006) and Wu et al. (2013) with brown rice. This result could be attributed that various stimulated enzymes during germination degraded amylose in raw starch constituents. The inflation in amylose content of raw sorghum and millet flours with waxy starch (4.0 and 2.9%, respectively) may result from the interaction between long-branched chains in amylopectin and iodine. Reduction in amylose content of sorghum and millet flours after germination may be due to the degradation of long-branched chains in amylopectin, which could form a helical complex with iodine.
Color properties
The germinated flours from brown rice, oat, sorghum, and millet differed in color properties during germination as shown in Table 1. The color of grain flours is an important physical property for utilization. The lightness (L*) was reduced whereas yellowness (b*) was increased after germination of grain seeds, while the redness (a*) was not significantly influenced (Table 1). Similarly, the decreased lightness and increased yellowness were reported by Wu et al. (2013) for germinated brown rice. Bhatty (1996) claimed that the oxidative enzymes catalyzing enzymatic browning were activated during germination, which resulted in browning of seeds. Chung et al. (2012) also suggested that the Maillard reaction between soluble sugars and amino acids released by enzymatic hydrolysis during germination resulted in color differences. The decrease in lightness during germination of brown rice and oat was more pronounced than that of sorghum and millet, which may be attributed to differences in amylose content. The greater decrease in amylose content of brown rice and oat produced higher levels of soluble sugars, which may be attributed to a more pronounced Maillard reaction.
Gelatinization properties
The gelatinization onset (To), peak (Tp), and conclusion (Tc) temperatures, temperature range (Tc–To), and melting enthalpy (ΔH) of germinated flours are presented in Table 2. A large endothermic peak around 60–90 °C was observed for all flours in our study, which may be attributed to the gelatinization of the starch in flours. The gelatinization temperatures, temperature range, and enthalpy of sorghum and millet flours were higher than those of brown and oat flours, probably due to the difference in amylose content of starch in grains. Amylopectin in starch may be responsible for starch crystallinity, while amylose acts to disrupt packing of double helices in crystalline lamellae. The sorghum and millet containing waxy starch may exhibit higher gelatinization enthalpy because the starch with larger amylopectin content has usually more double helices in crystalline regions.
The To and Tp for gelatinization of all flours increased after germination, while a slight decrease was found in Tc during germination (Table 2). Wu et al. (2013) and Xu et al. (2017) suggested a significant increase in gelatinization temperatures of brown rice and adlay flours after germination, respectively. During germination, the activated various enzymes such as amylase and protease may cause a significant increase in sugars, peptides, amino acids, and non-starch polysaccharide which may compete between starch and these materials for available water and a consequent reduction in water activity, resulting in an increase in the gelatinization temperature (Chung et al., 2012). It was reported that the amylase and protease activities of germinated rice were reached up to 200 units/g and 150 units/g, respectively (Dziedzoave et al., 2010; Li et al., 2011). Xu et al. (2017) also suggested that the increased protein hydrolysate such as peptides and amino acids after germination raised starch gelatinization temperature. Another potential mechanism for the increases in To and Tp for gelatinization might be the perfection of pre-existing crystallites in starch within germinated grains due to annealing by the applied thermal energy during germination process and drying of germinated grains. The reduction in Tc may be associated with the degradation of lipids and proteins derived by germination (Xu et al., 2012). The lipids and proteins in flour coat the starch surface with a film, resulting in restriction of granular swelling. In addition, the amylose–lipid complex in flour may increase the melting temperature of gelatinization (Wu et al., 2013). Thus, the degradation in interactions between starch and protein/lipid during germination may contribute to the decrease in Tc. Interestingly, the gelatinization temperature range of all flours decreased during the germination. The degree of decrease in gelatinization temperature range was much greater in sorghum and millet than in brown rice and oat, which may be attributed to the difference in amylose content of starch in grains.
The germinated flours from brown rice, sorghum, and millet showed a reduction in gelatinization enthalpy compared with those of raw flours, whereas an increase in the gelatinization enthalpy was observed until 60 h of germination in oat. Similarly, the decrease in gelatinization enthalpy during germination was reported for brown rice (Chung et al., 2012; Xu et al., 2012). Gelatinization enthalpy implies the extent of unravelling of the double helices in crystalline regions (Cooke and Gidley, 1992). During germination, enzymes are activated, catalyzing starch degradation, which may disrupt the double helical structure of starch. Consequently, less energy is required to unravel and melt double helices of starch in germinated flours. The increase in gelatinization enthalpy of germinated oat flour until 60 h may be due to dissolution of hydrolyzed starch granules during germination. The activated amylases preferentially may attack amorphous regions in starch and the degraded fragments could be discarded during prolonged germination and thereby residual starch could have more double helical structures.
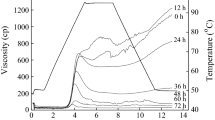
Pasting properties
The pasting properties of flours from raw and germinated grains are shown in Table 3 and Fig. 2. Germination significantly affected the pasting properties of selected grains. The pasting temperature of sorghum and millet flours was higher than that of brown rice and oat flours, which could be attributed to their lower amylose contents. Pasting temperature decreased with increased germination time. A similar trend in pasting temperature during germination was reported by Chinma et al. (2009) with tigernut flour. Pasting temperature is an index of initial viscosity increase by swelling of starch (Chinma et al., 2009). The decrease in pasting temperature during germination was consistent with the decrease in Tc of gelatinization.
A substantial reduction in peak viscosity and breakdown was observed for brown rice, oat, sorghum, and millet flours with increase in germination time (Fig. 2 and Table 3). Similar trend in reduction of peak viscosity during germination of brown rice was reported by Chung et al. (2012) and Xu et al. (2012). The significant decrease in peak viscosity and breakdown of germinated flours may be due to the degradation of protein and starch induced by the enzymes activated during germination (Chung et al., 2012). Final viscosity and setback significantly decreased with increase in germination time (Table 3). Setback and final viscosity are related to the aggregation of amylose molecules during cooling. Consequently, the decrease in amylose content during germination (Table 1) led to a low extent of recrystallization in leached amylose of gelatinized starch molecules during cooling.
In our previous study (Li et al., 2017) with same materials and under same germination conditions, the pasting viscosity of isolated starches derived from germinated brown rice and oat increased marginally, while sorghum and millet starches exhibited significant reduction in pasting viscosity, which was consistent with this study involving germinated flours. It was noteworthy that the impact of germination on pasting viscosity of flours and starches obtained from the four grains was varied. Varavinit et al. (2003) suggested that pasting properties of flours was highly influenced by starch, protein, and lipid as well as amylase activity. Because starch is the major component in the selected four grains, the degradation of starch chains during germination contributes to the reduction in pasting viscosity. Li et al. (2017) suggested that amylases activated during germination might cause the sharp decline in pasting viscosity of flours during germination. However, unlike germinated flour, amylase activity was substantially decreased during isolation of starches. Zhu et al. (2010) claimed that the protein was one of major factor underlying the differences in pasting viscosity of flour and isolated starch from germinated grains. The activated proteases hydrolyze proteins and disrupt disulfide linkages. The protein degradation and lack of protein network in flours increased mechanical fragility of the swollen starch granules, which resulted in substantial reduction in pasting viscosity. Consequently, the pasting properties of isolated starch from germination grains may be primarily influenced by the changes in amylose content and molecular structure of amylopectin during germination, while the overall changes in pasting parameters of the germinated flour were more pronounced due to the presence of protein, lipid, and residual enzymes.
Swelling factor and amylose leaching
The magnitude of swelling factor and amylose leaching of raw and germinated brown rice, oat, sorghum, and millet flours at 80 °C are shown in Fig. 3A and B, respectively. The swelling factor of raw sorghum and millet flours was greater than that of brown rice and oat flours, which could be associated with their lower amylose contents. Similarly, our previous report (Li et al., 2017) showed that the sorghum and millet starches exhibited significantly greater swelling factor than the brown rice and oat starches. A substantial reduction in swelling factor was observed in germinated flour compared with raw flour, and a profound reduction with increase in germination period was accordance with the results of peak viscosity in Table 3. Similarly, a reduction in swelling factor was observed for soybean flour, which was related to destruction of starch granules and protein structure via enzymatic hydrolyzation of glycosidic linkages and peptide bonds (Agume et al., 2017). Ilowefah et al. (2014) reported that reduction of swelling factor in rice flour after germination could be due to the reduced starch content and starch degradation. Tester and Morrison (1990) also reported that starch hydrolyzation contributed to the decrease in swelling factor. Swelling factor in flour is considered as the primary property of starch and influenced by interactions between starch and protein or lipid. Therefore, a significant decrease in swelling factor of grain flour after germination may be attributed to the degradation of starch and protein.
Amylose leaching of brown rice and oat flours decreased with an increase in germination time, whereas the sorghum and millet flours exhibited an increase in amylose leaching after germination (Fig. 3B). A similar trend in amylose leaching was found in our previous report (Li et al., 2017) involving the same four grain starches. We claimed that the degraded amylose structure derived during germination in brown rice and oat led to a decrease in amylose leaching, whereas the increase in amylose leaching of sorghum and millet during germination was associated with the linear long chains generated from amylopectin by debranching and hydrolyzing enzymes. However, the value of amylose leaching in brown rice and oat flours (3 ~ 5%) was much lower than in starches (6 ~ 10%) due to the interactions among the components including protein, fiber, and lipid in flour.
In conclusion, germination modified effectively the physicochemical properties of the four grains. Therefore, these germinated grain flours may be used as functional ingredients in the preparation of grain-based products.
References
Agume ASN, Njintang NY, Mbofung CMF. Effect of soaking and roasting on the physicochemical and pasting properties of soybean flour. Foods 6:12 (2017)
Bhatty RS. Production of food malt from hull-less barley. Cereal Chem. 73:75-80 (1996)
Chinma CE, Adewuyi O, Abu JO. Effect of germination on the chemical, functional and pasting properties of flour from brown and yellow varieties of tigernut (Cyperus esculentus). Food Res. Int. 42:1004-1009 (2009)
Chung HJ, Cho A, Lim ST. Utilization of germinated and heat-moisture treated brown rices in sugar-snap cookies. LWT-Food Sci. Technol. 57:260-266 (2014)
Chung HJ, Cho DW, Park JD, Kweon DK, Lim ST. In vitro starch digestibility and pasting properties of germinated brown rice after hydrothermal treatments. J. Cereal Sci. 56:451-456 (2012)
Chung HJ, Liu Q, Hoover R, Warkentin TD, Vandenberg B. In vitro starch digestibility, expected glycemic index and thermal and pasting properties of flours from pea, lentil and chickpea cultivars. Food Chem. 111:316-321 (2008)
Cooke D, Gidley MJ. Loss of crystalline and molecular order during starch gelatinization: Origin of the enthalpic transition. Carbohydr. Res. 227:103-112 (1992)
Dziedzoave NT, Graffham AJ, Westby A, Komlaga G. Comparative assessment of amylolytic and cellulolytic enzyme activity of malts prepared from tropical cereals. Food Control 21:1349-1353 (2010)
Falade KO, Christopher AS. Physical, functional, pasting and thermal properties of flours and starches of six Nigerian rice cultivars. Food Hydrocoll. 44:478-490 (2015)
Ilowefah,M, Bakar J, Ghazali HM, Mediani A.; Muhammad, K. Physicochemical and functional properties of yeast fermented brown rice flour. J. Food Sci. Technol. 52:534-5545 (2014)
Kaneko M, Itoh H, Ueguchi-Tanaka M, Ashikari M, Matsuoka M. The α-amylase induction in endosperm during rice seed germination is caused by gibberellins synthesized in epithelium. Plant Physiol. 128:1264-1270 (2002)
Komatsuzaki N, Tsukahara K, Toyoshima H, Suzuki T, Shimizu N, Kimura T. Effect of soaking and gaseous treatment on GABA content in germinated brown rice. J. Food Eng. 78:556-560 (2007)
Li C, Oh SG, Lee DH, Baik HW, Chung HJ. Effect of germination on the structure and physicochemical properties of starches from brown rice, oat, sorghum, and millet. Int. Biol. Macromol. 105:931-939 (2017)
Li, C, Cao X, Gu Z, Wen H. A preliminary study of the protease activities in germinating brown rice (Oryza sativa L.). J. Sci. Food Agric. 91:915-920 (2011)
Singh R, De S, Belkheir A. Avena sativa (Oat), a potential nutraceutical and therapeutic agent: An overview. Crit. Rev. Food Sci. Nutr. 53:126-144 (2013)
Sudha A, Priyenka Devi KS, Sangeetha V, Sangeetha A. Development of fermented millet sprout milk beverage based on physicochemical properties studies and consumer acceptability data. J. Sci. Ind. Res. 75:239-243 (2016)
Suwanmanon,K, Hsieh PC. Effect of γ-aminobutyric acid and nattokinase-enriched fermented beans on the blood pressure of spontaneously hypertensive and normotensive Wistar-Kyoto rats. J. Food Drug Anal. 22:485-491 (2014)
Taylor JRN, Belton PS, Beta T, Duodu KG. Increasing the utilisation of sorghum, millets and pseudocereals: Developments in the science of their phenolic phytochemicals, biofortification and protein functionality. J. Cereal Sci. 59:257-275 (2014)
Tester RF, Morrison WR. Swelling and gelatinization of cereal starches. I. Effects of amylopectin, amylose and lipids Cereal Chem. 67:551-557 (1990)
Varavinit S, Shobsngob S, Varanyanond W, Chinachoti P, Naivikul O. Effect of amylose content on gelatinization, retrogradation and pasting properties of flours from different cultivars of Thai rice. Starch 55:410-415 (2003)
William PC, Kuzina FD, Hlynka I. Rapid colorimetric procedure for estimating the amylose content of starches and flours. Cereal Chem. 47:411-420 (1970)
Wu F, Chen H, Yang N, Wang J, Duan X, Jin Z, Xu X. Effect of germination time on physicochemical properties of brown rice flour and starch from different rice cultivars. J. Cereal Sci. 58:263-271 (2013)
Xu J, Zhang H, Guo X, Qian H. The impact of germination on the characteristics of brown rice flour and starch. J. Sci. Food Agr. 92:380-387 (2012)
Xu L, Chen L, Ali B, Yang N, Chen Y, Wu F, Jin Z, Xu X. Impact of germination on nutritional and physicochemical properties of adlay seed (Coixlachryma-jobi L.). Food Chem. 229:312-318 (2017)
Zheng YM, He RG, Huang X, Zheng L, Hu QL, Hua P. Effects of germination on composition of carbohydrate and activity of relevant enzymes in different varieties of brown rice. Cereal Feed Ind. 5:1-3 (2006)
Zhu LJ, Liu QQ, Sang Y, Gu MH, Shi YC. Underlying reasons for waxy rice flours having different pasting properties. Food Chem. 120:94-100 (2010)
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2017R1D1A1B03034146).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have declared no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Li, C., Jeong, D., Lee, J.H. et al. Influence of germination on physicochemical properties of flours from brown rice, oat, sorghum, and millet. Food Sci Biotechnol 29, 1223–1231 (2020). https://doi.org/10.1007/s10068-020-00770-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-020-00770-2