Abstract
Onion and ginger are rich sources of bioactive compounds which are lost during conventional drying process. The present study was designed to optimize the novel Microwave Assisted Drying and Extraction technique (MADE) for simultaneous drying and extraction/recovery of bioactive compounds from model food products. The time required for drying of samples was 11 (onion) and 16 (ginger) minutes with recovery yield of 87% (onion) and 85% (ginger). The drying time was reduced to 100 times compared to hot air drying and moisture ratio of dried samples was best described by Midilli model. The diffusivities of onion and ginger slices were 1.27 e−11 and 1.43 e−11 m2/s, respectively. Moreover, microwave-based extraction was compared with conventional one. The results of antioxidant activity and total phenolic contents of condensates obtained through MADE were higher compared to conventional method. In short, MADE exhibited better yield of extraction and drying properties compared to conventional methods.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pakistan is among the top producers of fruits and vegetables in the world (FAO, 2018). However, 50% of these fresh produces are wasted due to improper handling and lack of proper processing technologies. Several processing techniques have been reported to extend the shelf life of fruits and vegetables. Among them, drying (dehydration) is adopted as an attractive option for a number of reasons, for instance, the dehydrated products are much easier to handle during storage and transportation. To date, various conventional and novel drying technologies are available for the dehydration of fruits and vegetables such as sun drying, hot-air drying, vacuum drying, freeze drying, osmotic drying, infra-red drying and microwave drying. These techniques have their own pros and cons; however, sun drying and hot air drying are the most commonly employed methods. These methods efficiently dry the fresh food items; however, a major apprehension is processing time and the loss of water-soluble bioactive components (Ferreira et al., 2018; Mongpraneet et al., 2002). Moreover, conventional drying methods significantly influence the texture and color of dried product. To overcome these issues, new dehydration technologies have emerged with an aim to make the drying process more sustainable and energy efficient together with an improved quality of the finished product.
Microwave-based drying is a promising technique that can efficiently dry the products in a shorter duration. Moreover, compared to other novel methods, the process is quite simple and much energy efficient (Khan et al., 2016; Therdthai and Zhou, 2009). The drying through microwave is not a latest concept and have already been investigated for dehydration of different fruits and vegetables (Darvishi et al., 2013; Khan et al., 2016). However, the loss of bioactive components (BACs) in vapors is still an unsolved problem in microwave-based drying.
Due to a distinct process design and rapid generation of vapors during this process, the existing design of microwave drying system can be modified to collect the vapors containing bioactive components. This would be an extra advantage i.e., extraction/recovery of bioactive compounds along with drying of the product. Hence, the process is regarded as Microwave Assisted Drying and Extraction (MADE) process (Khan et al., 2016). The present research was planned to optimize MADE technique for drying and simultaneous extraction of bioactive components from onion and ginger. Both, onion and ginger contain sufficient amounts of bioactive components exhibiting good anti-inflammatory, anti-septic and antipyretic activities (Ahmad et al., 2016; Mitra et al., 2012). In addition to this, onion and ginger vary in their cell structure and hence can provide better understanding of drying and extraction process using MADE technique.
Materials and methods
Procurement and preparation of raw materials
Fresh onions and ginger were purchased from local market of Faisalabad (Pakistan). The onion and ginger were manually cut into slices of equal thickness (3 mm) with a knife. All the chemicals were analytical grade and purchased from Merck (Germany).
Drying and extraction process
Drying of prepared samples was carried out with MADE technique. The experimental setup for MADE was a modified microwave oven HDG 236S (Homage, Korea) having capacity of 23 L (488W × 380H × 291D) and 50 Hz frequency with maximum power of 100 W. It contained a glass reactor (Pyrex, Japan) with area of 0.05 m2, connected to a condenser (Pyrex, Japan) which condensed the water vapors containing bioactive compounds (Fig. 1). The samples (100 g) were placed in glass reactor and microwave oven was operated at 100 W. The moisture loss was determined by weighing the samples at various intervals (1,2,3…15 min) with electronic weighing balance AP135W (Shimadzu, Japan) having accuracy of ± 0.01 mg. The drying process was stopped when sample color tended to change into brown or before burning of slices through visual observation.
The vapors were liquified through a condenser, collected in glass bottle and stored at 4 °C till further analysis. The extraction efficiency was calculated as follow;
where Cl, Wi and Wt represent the condensed liquid (g), weight of the sample before and after drying (g), respectively. For comparison purposes, a solvent based extraction (a conventional extraction, CE) was carried out. In this regard, 5 g sample was homogenized in 80% methanol solution at 8000 rpm for 45 min. The supernatant layer was separated and used for scavenging activity and total phenolic contents analysis as described in coming sections.
Drying rate (DR)
Drying rate, expressed as g water/(100 g.min), was calculated to estimate the drying efficiency using following equation:
where t is drying time in min, Mt and Mt+dt are the moisture contents (g water/100 g) at time t and t + dt, respectively (Darvishi et al., 2013; Khan et al., 2016).
Moisture ratio (MR)
The moisture ratio indicates the relative water removal from the food products. It was determined with following equation:
where Mt, Mo and Me are the moisture contents of sample at time t, zero and at equilibrium. The drying curves obtained were fitted with three different moisture ratio models (Eqs. 4, 5 and 6). Mathematical models were simulated by using Mathcad Prime 3 (PTC, London, UK) (Mahjoorian et al., 2017).
The experimental data was used to determine diffusivity coefficient by using Fick’s law of diffusion. As drying process is in unsteady state, the diffusion progresses through infinite slab, thus Fick’s law can be written as;
where Deff is the effective diffusion coefficient (m2/s), and L is the half-thickness of sample (m). Therefore, effective diffusion coefficient (Deff) was obtained by plotting ln MR versus time (Mahjoorian et al., 2017). From Eq. (7), a plot of ln MR versus time displayed a straight line with a slope of (α), in which (Eq. 8):
Free radical scavenging activity (DPPH assay)
The free radical scavenging or antioxidant activities of vegetable samples were determined by using the method as described in literature (Cheng et al., 2006; Sun et al., 2005) with some modifications. The samples were added in DPPH (4% w/v) and methanol mixture followed by vigorous shaking. Afterwards, the mixture was allowed to stand at ambient temperature in dark. Finally, absorbance of the samples was measured using a spectrophotometer (model U2020, IRMECO, Germany) at 515 nm. The results are reported in terms of percentage.
where, Ao and As are the absorbance values of blank and sample, respectively.
Total phenolic contents
Total phenolic compounds (TPC) were determined according to the method of Sun et al. (2005) with minor modifications (Lu et al., 2011). The Folin–Ciocalteu reagent was mixed with distilled water to make tenfold dilution. This solution was mixed with sample and incubated for ten minutes at ambient temperature. Afterwards, 0.75 mL of 2% (w/v) sodium carbonate solution was added into the mixture and stored in the darkness for 45 min. Sample absorbance was measured at 765 nm with spectrophotometer against a blank. TPC values were calculated from a calibration curve that was drawn against the different concentrations of gallic acid standards (0, 5, 10, 15, 20, 30 and 40 mg/L). The results were presented in terms of GAE mg/g.
Rehydration ratio
The dried slices were placed in hot distilled water (50 °C) in beaker for 20 min. Afterwards, samples were removed and put at tissue paper to absorb the free water from rehydrated slices (Markowski and Zielinska, 2013). Rehydration ratio was calculated by following equation:
where \({\text{w}}_{\text{rd}}\) is the weight of rehydrated sample and \({\text{w}}_{\text{d}}\) is the weight of dried sample before rehydration.
Statistical analysis
All the experiments were performed in triplicate and their results were reported in terms of mean along with standard error. Moreover, fitness of the models was evaluated through the comparison of the modeled and experimental data. In this regard, following statistical tests were performed.
where N represents the number of observations, np is the number of parameters, Vexp,i and Vmodel,i are the experimental and model values (of ith observation), respectively.
The coefficient of determination (R2) was the main criteria for selecting the best model. Additionally, RMSE, an RPD and CS indicate variation (goodness of fit) between experimental and model values. RMSE and CS values closer to zero indicate the closeness of model to experimental data. Similarly, RPD determines the absolute difference between model and experimental values. RPD value less than 10% indicate that fit is good (Mota et al., 2010; Roberts et al., 2008).
Results and discussions
Moisture ratio
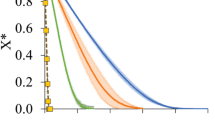
Moisture ratio (MR) was calculated according to Eq. 3 and values were compared to literature values for drying with hot air oven as can be seen in Fig. 2A (Arslan and Musa, 2010; Loha et al., 2012). Initially, the MR of onion decreased comparatively faster than ginger which indicates a rapid drying of onion slices compared to ginger. The duration required by hot air to dry the vegetables is about 100 times more compared to MADE method. It may be attributed to the distinct heating mechanism of microwave in which water molecules are excited by waves and generate heat. Moreover, heat and water vapors move in outer direction and prevent the crust formation that is observed in hot air heating systems. These results clearly indicated that MADE technique was more efficient than hot air and could help to save processing time and energy involved in drying process (shorter the time less energy will be required). It has already been reported in literature that microwave based drying consumes less energy (0.28 MJ) compared to hot air oven (3 MJ) and freeze (3.2 MJ) drying (Khan et al., 2016).
Dehydration of onion and ginger samples: A Moisture ratio as a function of time, B drying rate as a function of time, C drying rate as a function of moisture content, and D ln MR as a function of time. In all figures, circles represent onion samples and squares represent ginger samples. The filled symbols are results of present study, whereas, the empty symbols represent the data extracted from Arslan and Musa (2010) (for hot air drying of onion) and Loha et al. (2012) (for hot air drying of ginger)
According to model comparison, it is evident that Midilli model best described the drying of vegetables compared to single term model (Newton and page). This was supported by statistical analysis (Table 1). The R2 value was highest for Midilli model (0.9996) that indicates the best fit model. Moreover, lower RMSE and RPD values favour the fitness of model with experimental data of both vegetables. Thus, Midilli model was used to predict MR and determine the diffusivity of onion and ginger slices (Fig. 2D). The values of diffusivity are 1.27 e−11 and 1.43 e−11 m2/s for onion and ginger slices, respectively (Table 2). These values are comparable to the values reported in literature (Mahjoorian et al., 2017).
Drying rate
Results regarding drying time, drying rate and moisture ratio have been presented in Table 2 as well as in Fig. 2. It is obvious from the results that drying time varied with type of vegetable. Ginger required longer time (16.57 ± 2.07 min) for complete removal of moisture compared to onion (10.75 ± 0.36 min). The difference in drying time may be attributed to cell structure differences among vegetables as also reported in literature (Coolong et al., 2008; Özdemir et al., 2011).
Drying rates of ginger and onion slices were determined with Eq. 2 and results are plotted against time in Fig. 2B. Initially, the drying rate increased swiftly followed by a decrease, resulting in a parabolic curve. The higher drying rate may be due to the rapid heating and availability of the higher moisture contents in the food samples. This continued to increase till fifty percent of moisture contents had been removed. This was supported when drying rate was plotted against the moisture contents (Fig. 2C). The results indicated that the drying rate started to decline after 50% reduction in moisture content. Moreover, it was observed that drying curve was narrower for onion than ginger; indicating that onion has higher drying rate (11.78 g/100 g min) compared to ginger (10.95 g/100 g min). Lowering of the drying rate as a function of time can be attributed to the reduction of free moisture contents in the sample as dehydration proceeded. Initially, higher drying rate was due to higher moisture contents that absorbed more radiations resulting in a rapid increase in temperature. This resulted in higher evaporation of moisture from the product. After around 50% moisture removal, less moisture was available for evaporation that reduced the drying rate (Soysal et al., 2006; Therdthai and Zhou, 2009; Wang et al., 2007).
Condensate yield (CY)
The condensate yield (mL) is the measure of vapors converted into liquid in a graduated cylinder, and results are shown in Table 2. The results indicate that condensate yield of onion and ginger was 68.76 ± 3.91 and 69.26 ± 3.47 mL per 100 g of sample. Although, both differ in cell wall thickness, the yield is comparable due to the similar moisture contents. Similarly, extraction efficiency varied non-significantly (85 and 87% for ginger and onion, respectively) indicating that a significant amount of vapors were collected in the form of condensate liquid.
Free radical scavenging (DPPH) activity and total phenolic contents (TPC)
The results of DPPH and TPC of both condensates are presented in Fig. 3. The results indicate that TPC and DPPH activity values of onion condensate was higher than ginger. Similarly, MADE condensate exhibited better TPC and DPPH activities compared to methanol extraction methods. These results indicated that microwave-based extraction method successfully extracted the BACs Moreover, higher values of TPC, and DPPH compared with conventional extraction method indicated that this technique did not alter the effectiveness of BACs. These results are in line with the findings reported in literature (Ferreira et al., 2018; Nindo et al., 2003).
Rehydration ratio (RR)
The results indicated that RR was higher in ginger (3.59 ± 0.50) compared to onion (3.6 ± 0.65), which may be attributed to difference in cell structure (Fig. 4). Moreover, RR was influenced by drying methods; MADE dried samples had higher RR as compared to those dried with hot-air oven. This was also supported by previous studies which reported that rehydration ratio of vegetables dried with microwave is better than those dried with hot air drying (Kumar et al., 2006; Maskan, 2001; Nistor et al., 2017).
The drying time of MADE process was significantly lower (100 times) compared to convective drying method which ultimately saves time and energy. Moreover, the higher extraction efficiency (83–87%), acceptable quality characteristics of dried product as well as the simultaneous recovery/extraction of BACs makes it an efficient and sustainable approach.
Abbreviations
- MADE:
-
Microwave Assisted Drying and Extraction
- HA:
-
Hot air oven
- CE:
-
Conventional extraction (methanol-based extraction)
- RR:
-
Rehydration ration
- BACs:
-
Bioactive compounds/components
References
Ahmad A, Alghamdi SS, Mahmood K, Afzal M. Fenugreek a multipurpose crop: Potentialities and improvements. Saudi J. Biol. Sci. 23: 300-310 (2016)
Arslan D, Musa OM. Study the effect of sun, oven and microwave drying on quality of onion slices. LWT Food Sci. Technol. 43: 1121-1127 (2010)
Cheng Z, Moore J, Yu L. High-throughput relative DPPH radical scavenging capacity assay. J. Agric. Food Chem. 54: 7429-7436 (2006)
Coolong TW, Randle WM, Wicker L. Structural and chemical differences in the cell wall regions in relation to scale firmness of three onion (Allium cepa L.) selections at harvest and during storage. J. Sci. Food Agr. 88: 1277-1286 (2008)
Darvishi H, Azadbakht M, Rezaeiasl A, Farhang A. Drying characteristics of sardine fish dried with microwave heating. J. Saudi Soc. Agric. Sci. 12: 121-127 (2013)
FAO. FAO Stat. Retrieved from http://www.fao.org/faostat/en/#data (Dec. 24, 2018) (2018)
Ferreira SS, Passos CP, Cardoso SM, Wessel DF, Coimbra MA. Microwave assisted dehydration of broccoli by-products and simultaneous extraction of bioactive compounds. Food Chem. 246: 386-393 (2018)
Khan MKI, Ansar M, Nazir A, Maan AA. Sustainable dehydration of onion slices through novel microwave hydro-diffusion gravity technique. Innovative Food Sci. Emerging Technol. 33: 327-332 (2016)
Kumar DP, Hebbar HU, Ramesh M. Suitability of thin layer models for infrared–hot air-drying of onion slices. LWT Food Sci. Technol. 39: 700-705 (2006)
Loha C, Das R, Choudhury B, Chatterjee PK. Evaluation of Air Drying Characteristics of Sliced Ginger (Zingiber officinale) in a Forced Convective Cabinet Dryer and Thermal Conductivity Measurement. J. Food Process. Technol. 3: 130-134 (2012)
Lu X, Wang J, Al-Qadiri HM, Ross CF, Powers JR, Tang J, Rasco BA. Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chem. 129: 637-644 (2011)
Mahjoorian A, Mokhtarian M, Fayyaz N, Rahmati F, Sayyadi S, Ariaii P. Modeling of drying kiwi slices and its sensory evaluation. Food Sci. Nutr. 5: 466-473 (2017)
Markowski M, Zielinska M. Influence of drying temperature and rehydration on selected textural properties of carrots. Int. J. Food Prop. 16: 586-597 (2013)
Maskan M. Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. J. Food Eng. 48: 177-182 (2001)
Mitra J, Shrivastava S, Rao P. Onion dehydration: a review. J. Food Sci. Technol. 49: 267-277 (2012)
Mongpraneet S, Abe T, Tsurusaki T. Accelerated drying of welsh onion by far infrared radiation under vacuum conditions. J. Food Eng. 55: 147-156 (2002)
Mota C, Luciano C, Dias A, Barroca MJ, Guiné R. Convective drying of onion: Kinetics and nutritional evaluation. Food Bioprod. Process. 88: 115-123 (2010)
Nindo CI, Sun T, Wang S, Tang J, Powers J. Evaluation of drying technologies for retention of physical quality and antioxidants in asparagus (Asparagus officinalis, L.). LWT Food Sci. Technol. 36: 507-516 (2003)
Nistor OV, Seremet L, Andronoiu DG, Rudi L, Botez E. Influence of different drying methods on the physicochemical properties of red beetroot (Beta vulgaris L. var. Cylindra). Food Chem. 236: 59-67 (2017)
Özdemir C, Aktaş K, Altan Y. Morphological and anatomical investigations on three Allium L. (Liliaceae) species of east Anatolia, Turkey. Bangladesh J. Bot. 40: 9-15 (2011)
Roberts JS, Kidd DR, Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 89: 460-465 (2008)
Soysal Y, Oztekin S, Eren O. Microwave drying of parsley: Modelling, kinetics, and energy aspects. Biosyst. Eng. 93: 403-413 (2006)
Sun T, Tang J, Powers JR. Effect of pectolytic enzyme preparations on the phenolic composition and antioxidant activity of asparagus juice. J. Agric. Food Chem. 53: 42-48 (2005)
Therdthai N, Zhou WB. Characterization of microwave vacuum drying and hot air drying of mint leaves (Mentha cordifolia Opiz ex Fresen). J. Food Eng. 91: 482-489 (2009)
Wang ZF, Sun JH, Chen F, Liao XJ, Hu XS. Mathematical modelling on thin layer microwave drying of apple pomace with and without hot air pre-drying. J.Food Eng. 80: 536-544 (2007)
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Khan, M.K.I., Maan, A.A., Aadil, R.M. et al. Modelling and kinetic study of microwave assisted drying of ginger and onion with simultaneous extraction of bioactive compounds. Food Sci Biotechnol 29, 513–519 (2020). https://doi.org/10.1007/s10068-019-00695-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-019-00695-5