Abstract
Ginger extracts (GEs) are antioxidant, antimicrobial, and anti-inflammatory. Their bioactivity can benefit foods and active packaging by extending shelf life, enhancing safety, and providing health benefits. Highly bioactive GEs are crucial to formulating potent active products and avoiding negative effects on their properties. Sesquiterpenes and phenolics are the main bioactives in ginger, but drying and extraction affect their composition. GEs are usually obtained from dry rhizomes; however, these operations have been studied independently. Therefore, a combined study of innovative drying and extraction technologies to evaluate their influence on extracts’ composition will bring knowledge on how to increase the bioactivity of GEs. The effects of an emergent drying (vacuum microwave, VMD) followed by an emergent extraction (ultrasound, UAE, 20 or 80 °C) were investigated in this work. Microwave extraction (MAE) of fresh ginger was also studied. Convective oven drying and Soxhlet extraction were the references. Drying kinetics, powder color, extract composition, and antioxidant activity were studied. While MAE preserved the original composition profile, VMD combined with UAE (20 °C) produced extracts richer in phenolics (387.6 mg.GAE/g) and antioxidant activity (2100.7 mmol.Trolox/mL), with low impact in the sesquiterpenes. VMD generated shogaols by its high temperatures and facilitated extracting bioactives by destroying cellular structures and forming pores. UAE extracted these compounds selectively, released them from cell structures, and avoided losses caused by volatilization and thermal degradation. These findings have significant implications, as they provide an opportunity to obtain GE with tailored compositions that can enhance the formulation of food, active packaging, and pharmacological products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Ginger (Zingiber officinale Roscoe) is a highly relevant herb due to its bioactivity that comprises antimicrobial [1], antiviral [2, 3], anti-inflammatory, antioxidant [2, 4], antitumorigenic [5, 6] effects. It is a result of their rich composition, which makes them appropriate to be used as natural active ingredients or additives to foods and packaging, such as bio-based films and coatings, replacing artificial compounds.
Due to the potential of ginger in those applications, increased concentrations of bioactive compounds have been sought [7, 8] as they enable higher bioactivity in the final formulations and the requirement of lower extract volumes, leading to lower impact in their physicochemical and sensory properties. The main bioactives of ginger are phenolic compounds and sesquiterpenes. Among these, the phenolics gingerols and shogaols stand out [9, 10]. Gingerols are responsible for the pungent taste of fresh ginger. However, high temperature, low pH, or long storage cause the dehydration of these compounds, converting them to shogaols [10]. Several studies have shown that shogaols are more bioactive than gingerols [4, 11]. However, conditions to produce shogaols are harsh and can cause the loss of other phenolics and terpenes. Thus, besides attaining high extraction yields, finding ways to promote the production of shogaols while avoiding the loss of other bioactives is fundamental to obtaining more potent ginger extracts and high extraction yields.
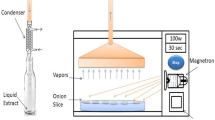
The conversion of gingerols to shogaols can occur, intentionally or not, during drying [8, 9] and extraction [12, 13]. With adequate knowledge of how those affect the extract’s composition, they can be optimized to produce components with higher bioactivity and in higher concentrations. Innovative methods have been applied to ginger aiming to improve its bioactivity and get more sustainable, environmental-friendly, safe, time- and cost-effective processing. Microwave drying has shown the potential to increase the shogaols content [12, 14]. Adding a vacuum to this process would avoid the degradation of other thermolabile compounds in the extract due to the lower water vapor pressure and short processing time [15]. Reports on how vacuum microwave drying (VMD) affects the antioxidant activity of ginger are scarce in the literature [7], and it would be expected that lower pressures could have optimistic results. The association of VMD followed by ultrasound-assisted extraction (UAE) is promising as it could enable the obtention of extracts rich in phenolic compounds, gingerols, and shogaols [12, 16, 17], but yet unexplored. Both those technologies have been scaled recently for industrial-scale production, with favorable results [15, 18, 19].
Commercial ginger extracts are usually obtained from dried ginger, to extend the rhizomes’ shelf-life. However, conventional drying and extraction processes can affect the composition and bioactivity of the extract [8, 9, 12, 13]. The lack of studies on how the combination of drying and extraction technologies and conditions influence ginger extracts’ composition is a gap in the literature. Therefore, studies evaluating the combined effect of drying and extraction of conventional and innovative trending technologies are highly relevant in the scientific and technological fields. In addition, this approach can bring better knowledge on how to obtain valuable extracts with enhanced composition, high concentration, and better bioactivity to be used as functional additives in food, active packaging, and pharmacological fields.
The objective of this study was to investigate the influence of the combination of drying and extraction techniques on the composition and bioactivity of ginger, enabling it to produce extracts with high antioxidant activity and rich in phenolic and other active compounds with potential food, packaging, and pharmaceutical applications. For that, VMD, UAE, and microwave-assisted extraction (MAE) were studied, and the results were compared to the conventional methods of convective oven drying (OD) and Soxhlet extraction (Sox). As not yet verified in the literature, combinations of drying and extraction processes were evaluated by the global extraction yields, antioxidant activity, total phenolic content (TPC), and volatile compounds composition. In addition, drying processes were studied regarding drying kinetics, including the drying rate, drying time, final moisture content, water activity, and ginger powder color.
Materials and Methods
The material and methods section are presented as Online Resource 1.
Results and Discussion
Ginger Drying Kinetics
Dry ginger particles showed an average size of 35 ± 12 mm. Initial moisture content (Heq) and water activity (aw) of fresh ginger were 93.6 ± 0.7 and 0.97 ± 0.2%, respectively. Ginger drying kinetics (Fig. 1) and the related results are shown in Table S1 (Online Resource 2). Online Resource 2 presents the supplementary tables of this study. The aw of all the dried samples indicated microbiological stability. OD curves showed typical behavior in which a high and constant drying rate period caused by the evaporation of free water is followed by a falling rate, which is limited by the internal diffusion mechanism caused by the bound water in the system. Between those, the maximum drying rates (Vsecmax) were directly proportional to temperature [20,21,22]. Consequently, drying time (Td) was the opposite. VMD showed the highest Vsecmax and lowest Td, which were 8.1–21.1 folds higher and 10.6–35.7 folds lower than OD samples. That was probably due to a high energy transfer promoted by the microwaves along with the high moisture removal rate by the vacuum. Besides, microwaves can increase cell pressure by producing intracellular vapor, inducing the formation of pores on the surface and moisture migration channels for the vapor release [23, 24]. An et al. [14] used microwaves and their association with forced convection at 60 °C, which reduced by 6.67 and 8.00 folds, respectively, the drying time compared to OD at 60 °C.
Effects of Drying Over Ginger Powder
Color
Color changes can indicate nutritional, compositional, and flavor quality alterations [22, 25, 26]. Also, they affect consumers’ acceptance and can cause restrictions when food applications are aimed [27, 28]. The results for the color of ginger powder obtained by different drying conditions are shown in Table S2 (Online Resource 2). Images are presented in Fig. 2. Chroma (C*) was not significantly affected by drying type. h0 was lower for OD samples dried at 120 than 80 °C. That indicates a trend to red color (+ a*, h0 = 0) and a detachment from the yellow quadrant (+ b*, h0 of 1.571) related to the browning. The higher browning of F120 can also be evidenced by the lower L* and higher a* (redness) compared to F80. That effect may be caused by Maillard’s reaction, which produces brown products such as melanoidins. The reactivity of the amine groups and the concentration of sugars increases with the temperature, and it is enough for Maillard’s reaction to occur, potentializing the browning [25]. Furthermore, higher temperatures may induce the degradation of thermolabile yellow compounds of ginger, such as curcumin, demethoxycurcumin, and 6-dehydrogingerdione [22, 29], and the browning of ascorbic acid [30]. Within the OD conditions, F60 showed lower L*, a*, and h0, suggesting an intermediary browning. Even though it was subjected to a lower temperature, that level of browning may be justified by the long exposure (14 h) of the samples to the drying conditions compared to the other treatments, F80 (6 h) and F120 (3 h), which showed lower browning. Besides, the slightly higher moisture content (5.68%) reached in equilibrium after this treatment may also contribute to its darkening. Izli and Polat [25] verified that the increment of OD temperature (60–80 °C) reduced L* and b* and increased a* values, resulting in higher overall color changes compared to fresh ginger (∆E from 12.70 to 17.75), similarly to this study. Mahayothee et al. [22] observed that OD promoted yellowish and brownish colors in relation to fresh cassumunar ginger, evidenced by 1ower C* values. However, no significant difference was detected between OD samples at tested temperatures (40–80 °C).
MD induced intermediary browning level compared to OD, despite the maximum temperatures near 100 °C. In this case, the result may be related to the short exposure time to the high temperatures, thus reducing the effects of Maillard’s reaction and thermal degradation.
Effects of Drying and Extraction Over Ginger Extracts
The results for global extraction yield (Yg), total phenolic compounds (TPC), and antioxidant activity (AA) are shown in Table 1.
Global Extraction Yield (Yg)
Fresh-extracted ginger had substantially higher Yg, which could be related to residual water in the extracts. There was no significant difference between fresh ginger extracted by Soxhlet or by MAE, indicating that MAE was highly efficient in extracting ginger in a short time (15 min) compared to Sox (6 h). Yg of VMD (MicSox) was not significantly different from OD at 120 °C (F120Sox), but those were lower than F60Sox and F80 Sox. This result may be related to the starch gelatinization over 70 °C and the consequent reduction of Yg due to the entrapment of ginger extract compounds inside the starch gel matrix, as reported by Huang et al. [21]. An et al. [14] found similar Yg for MD and convective OD (60 °C for 12 h) ginger. Yg of Ult20 was lower than Sox when fixing the drying method (F80, F120, or Mic). Increasing the temperature (Ult80) reduced this effect, promoting Yg values near Sox despite the shorter processing time (3 h) than Sox (6 h). That could be due to a reduction in the solubility of the ginger components in ethanol at the lower temperature (20 °C), consequently reducing the mass transfer rates and Yg. Furthermore, ultrasonic waves and higher temperatures could increase the Yg due to the solubilization of degradation products of non-bioactive macromolecules, such as starch and fibers. Similarly, Mallavadhani and Panigrahi [12] observed a reduction in Yg for methanol UAE (room temperature) compared to a Soxhlet extraction (65 °C) from ginger, but the application of the UAE at 50 °C led to results similar to those of Soxhlet extraction.
Total Phenolic Content (TPC)
Extracts of fresh ginger showed TPC values between 202.8–207.3 mg.GAE/mL of extract (or 5.25–6.20 mg.GAE/g of dry ginger), similar to OD samples at 60 °C (Table 1). Comparatively, dried ginger extracts were richer in TPC but had the lowest Yg, indicating a higher extraction selectivity. VMD resulted in slightly higher TPC concentrations between the drying methods coupled with conventional extraction (Sox) but without significant difference compared to OD samples. Opposite, Ghafoor et al. [31] and An et al. [14] observed reductions in TPC of microwave-dried ginger of 20.3 and 13.2%, respectively, compared to OD at 60 °C. These results could be due to the vacuum absence and a consequent increase in the temperature above 120 °C. Also, inadequate sample homogenization could have generated hotspots that favored the degradation of phenolics.
UAE at 20 °C or 80 °C promoted different TPC concentrations between themselves for fixed drying methods (F80, F120, or Mic). There is a trend that UAE promotes an increase in TPC concentration compared to Soxhlet when the drying method is fixed. This increase was more pronounced for UAE at 20 °C than for 80 °C and led to a significant rise in TPC between F80Ult20 and F80Sox. As Yg presents the opposite behavior of TPC, it evidences the selectivity of Ult20. VMD associated with UAE at 20 °C (MicUlt20) produced the extract with the highest TPC value of 387.6 ± 0.1 mg.GAE/mL of extract (19.4 ± 0.5 mg.GAE/g of dry ginger), which was significantly higher than the treatments F60Sox, F80Sox, and F80Ult80. After water removal and cellular disruption by MAE drying, UAE at 20 °C extracted phenolics, avoiding unwanted molecules such as starch and fibers and their degradation products. Furthermore, ultrasonic cavitation destroys cellular structures of ginger releasing phenolic compounds. Also, the lower temperature of this treatment avoids the thermal degradation of phenolic compounds [32].
Antioxidant Activity (AA)
MicUlt20 showed the highest AA among the samples, which was 2100.7 ± 110.3 mmol.Trolox/mL of extract (or 105.4 ± 0.9 mmol.Trolox/g of dry ginger) for DPPH scavenging activity, representing an increase of 187% compared to F60Sox that had an AADPPH of 731.8 ± 46.2 3 mmol.Trolox/mL of extract (or 162.3 ± 3.3 mmol.Trolox/g of dry ginger) (Table 1). All the Sox samples did not show significant differences in AAABTS and AADPPH between themselves or fresh-extracted samples. As phenolic compounds are the main antioxidants in ginger, it was verified that the AA was proportional to TPC. Cherrat et al. [20] observed an increase of AADPPH (73.47–78.23%) of OD with the temperature. Considering MAE, the increase of AADPPH of MicUlt80 and MicUlt20 (43 and 99%, respectively) compared to MicSox indicates that UAE was more efficient in recovering antioxidants from ginger than Sox. Similarly. An increase of 82.74% in the AADPPH can be observed for F80Ult20 compared to F80Sox. Ultrasonic energy could increase the extraction of phenolic compounds, as observed at TPC concentration, thus increasing the AA [19, 32]. Furthermore, ultrasound could increase the formation of shogaols, which are more antioxidant than its precursors, and reinforce the AA [11, 32].
Components Profile
The compositions of the ginger extracts are shown in Table S3 (Online Resource 2). In fresh ginger (FreSox), 22 compounds were identified. The major constituents were the sesquiterpenes, followed by alkenes, phenolic compounds, a monoterpene, an aldehyde, and a fatty acid. In FreMic, 17 compounds were identified, and the contents of major sesquiterpenes (α-Zingiberene, α-Farnesene, β-Sesquiphellandrene, and α-Curcumene) were not significantly different from FreSox. Higher differences in the total alkenes (-135.1%) and phenolics (+ 78.3%) were observed. Phenolics in FreMic were not significantly different from FreSox. FreMic’s temperature (150 °C) is enough to produce 6-shogaol from 6-gingerol, but the production was not significant, probably due to the short exposure time. Jacotet-Navarro et al. [19] verified increases of 5.20 and 450% in the concentration of 6-gingerol and 6-shogaol, respectively, by microwave treatment of ginger press cake, but no statistical analysis was carried out.
OD at any temperature did not affect the main volatile compounds compared to the fresh ginger (FreSox). F60Sox, F80Sox, and F120Sox showed 14, 16, and 18 sesquiterpenes, respectively, and only 13 compounds were identified in the fresh sample (FreSox). The content of sesquiterpenes in F60Sox (77.1%) was higher than FreSox (71.2%). The monoterpenes eucalyptol, α-terpineol, (R)-( +)-β-citronellol, and β-citral were identified in OD samples. Those were absent in fresh-extracted samples. The water removal during drying may favor the liberation of low-polarity compounds to extraction, such as mono- and sesquiterpenes. Besides, higher drying temperatures may convert sesquiterpenes to monoterpenes [14]. However, while the monoterpenes in dried samples could have been produced from sesquiterpenes, there was no significant proportional reduction in sesquiterpenes.
MicSox had a lower content of α-zingiberene and β-sesquiphellandrene (lower at 26.72 and 26.15%, respectively) than F60Sox. That may be caused by thermal degradation by the microwaves. Besides, as VMD samples were submitted to low pressure, these could have a higher loss of sesquiterpenes by volatilization compared to less volatile components, such as phenolics. This hypothesis is supported by the sum of phenolic compounds identified in MicSox (18.75%), which was higher by 35.3, 2.6, and 24.0% than the samples F60Sox, F80Sox, and F120Sox, respectively. Simultaneously, the sum of identified sesquiterpenes of MicSox (60.80%) was lower by 20.7, 13.2, and 14.1% than using the same respective treatments. MicUlt20 and MicUlt80 had contents of α-curcumene lower by 43.21 and 43.79%, respectively than F60Sox. That indicates that the combined effect of microwaves and ultrasound can reduce the content of this compound due to volatilization and degradation. Losses caused by vacuum drying were reported by Osae et al. [33], but the association of this technique to MD reduced the processing time and consequently lowered the losses by volatilization to acceptable levels.
The highest contents of monoterpenes were shown by F80Ult20 and MicUlt20. That suggests that OD at 80 °C or VMD reduced monoterpenes loss, and their association with UAE at 20 °C promoted a higher extraction due to ultrasonic cavitation while avoiding thermal degradation and volatilization at the extraction stage. OD at 120 °C associated with hot extraction (F120Sox, F120Ult80) led to a lower content of monoterpenes, possibly caused by thermal degradation and volatilization during drying.
The contents of phenolics in dried ginger compounds comprised 13.86–22.35% of the extracts. The molecules were: 6-shogaol, 6-paradol, zingerone, and its stereoisomer butan-2-one, 4-(3-hydroxy-2-methoxyphenyl)-. These gingerol-derived molecules are all bioactive compounds with several benefits for human health and food preservation and safety [4, 10]. The highest content of 6-gingerol was detected in FreMic, while in FreSox it was about half that value. The high temperature (150 °C) and pressure (11.8 bar) reached by FreMic may have destroyed the cellular components to which 6-gingerol is attached, enabling its extraction. And temperatures higher than 80 °C can increase the conversion of 6-gingerol to 6-shogaol, as discussed by Cherrat et al. [20] and Huang et al. [21]. In another research, Cherrat et al. [20] reported that heat energy contributes to the destruction of cellular constituents to which phenolic compounds are attached, releasing them and making them available for extraction. That could explain the lower contents of identified phenolic compounds OD at 60 °C in comparison to the other dried samples. Those results are consistent with the contents of 6-shogaol observed. The highest contents of 6-shogaol were shown by F120Ult80 (3.95%), MicSox (3.49%), F120Ult20 (2.72%), F80Ult80 (2.52%), F120Sox (2.01%), and MicUlt20 (1.77%). Therefore, hot drying was crucial to increase shogaol production. That was expected, since the formation of 6-shogaol is triggered from 80 °C, and those processes subjected the samples to high temperatures during both drying and extraction. It could be expected that an increase in 6-shogaol by the temperatures from 80 °C would lead to an increase in the antioxidant activity of the extract, as it has proved to have higher antioxidant activity than gingerol [4, 11]. However, thermal degradation and volatilization during heated extractions can lead to the loss of other relevant antioxidants, such as sesquiterpenes. Consequently, the mildest temperature condition of Ult20 extractions was adequate to avoid thermal and volatilization losses of other antioxidant compounds such as sesquiterpenes, monoterpenes, and phenolics, making MicUlt20 the extract richest in antioxidants.
Minor compounds found in the ginger extracts were aldehydes, ketones, alkenes, and one pyrone. The aldehydes and ketones are degradation products of phenolic compounds produced by higher processing temperatures [34]. 4H-pyran-4-one,2,3-dihydro-3,5-dihydroxy-6-methyl- is a bioactive pyrone decurrent from Maillard’s reaction of hexoses [35]. This compound was detected for F120Sox, F120Ult80, and MicSox. The higher temperatures of those processes induced Maillard’s reaction, generating this compound.
Conclusion
The combination of emerging drying and extraction technologies has been proven effective in enhancing the bioactive composition and concentration of ginger extracts. Drying increased the concentration of bioactive compounds in the extracts by water removal, lowering global extraction yields. On the other hand, MAE promoted high yields and preserved the original bioactive compounds, such as gingerol, being an adequate method for the recovery of this compound. In addition, this process is achieved in shorter process times than conventional processes and is highly energy-efficient. When aiming for higher phenolics, shogaols, or antioxidant activity, drying is required. Both OD at 120 °C and VMD increased the release of phenolics and induced the production of shogaols, consequently increasing the antioxidant activity in comparison to fresh ginger. However, VMD produced extracts with a higher concentration of phenolics and antioxidant activity than conventional OD, with a process 9 to 42 times shorter. The association of VMD with UAE at 20 °C showed good compatibility to improve the extract composition. UAE promoted higher concentrations of phenolics and antioxidants, avoiding thermal degradation, volatilization loss of terpenes and phenolics, and favoring the formation of shogaols compared with the conventional Sox method. Extracts with the highest TPC and antioxidant activity were obtained by VMD coupled with UAE at 20 °C. Thus, this method is indicated to produce highly bioactive and concentrated extracts, which can be further used as active food ingredients or additives and incorporated in active food packaging or in the medical field.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Abbreviations
- Bioactives :
-
Bioactive compounds
- GEs :
-
Ginger extracts
- MAE :
-
Microwave-assisted extraction
- MD :
-
Microwave drying
- OD :
-
Oven drying
- Phenolics :
-
Phenolic compounds
- UAE :
-
Ultrasound-assisted extraction
- VMD :
-
Vacuum microwave drying
References
Oyedemi BO, Kotsia EM, Stapleton PD, Gibbons S (2019) Capsaicin and gingerol analogues inhibit the growth of efflux-multidrug resistant bacteria and R-plasmids conjugal transfer. J Ethnopharmacol 245:111871. https://doi.org/10.1016/j.jep.2019.111871
Zhu H, Hu M, Wang D et al (2020) Mixed polysaccharides derived from shiitake mushroom, poriacocos, ginger, and tangerine peel enhanced protective immune responses in mice induced by inactivated influenza vaccine. Biomed Pharmacother 126:110049. https://doi.org/10.1016/j.biopha.2020.110049
Chang JS, Wang KC, Yeh CF et al (2013) Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J Ethnopharmacol 145:146–151. https://doi.org/10.1016/j.jep.2012.10.043
Dugasani S, Pichika MR, Nadarajah VD et al (2010) Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol 127(2):515–520. https://doi.org/10.1016/j.jep.2009.10.004
Saha A, Blando J, Silver E et al (2014) 6-Shogaol from dried ginger inhibits growth of prostate cancer cells both in vitro and in vivo through inhibition of STAT3 and NF-κB signaling. Cancer Prev Res 7(6):627–638. https://doi.org/10.1158/1940-6207.CAPR-13-0420
Isa Y, Miyakawa Y, Yanagisawa M et al (2008) 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-α mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem Biophys Res Commun 373(3):429–434. https://doi.org/10.1016/j.bbrc.2008.06.046
An K, Wei L, Fu M et al (2020) Effect of carbonic maceration (CM) on the vacuum microwave drying of chinese ginger (Zingiber officinale Roscoe) slices: drying characteristic, moisture migration, antioxidant activity, and microstructure. Food Bioproc Tech 13:1661–1674. https://doi.org/10.1007/s11947-020-02504-y
Ghasemzadeh A, Jaafar HZE, Rahmat A (2015) Optimization protocol for the extraction of 6-gingerol and 6-shogaol from Zingiber officinale var. rubrum Theilade and improving antioxidant and anticancer activity using response surface methodology. BMC Complement Altern Med 15:258. https://doi.org/10.1186/s12906-015-0718-0
Ghasemzadeh A, Jaafar HZE, Baghdadi A, Tayebi-Meigooni A (2018) Formation of 6-, 8- and 10-shogaol in ginger through application of different drying methods: Altered antioxidant and antimicrobial activity. Molecules 23(7):1646. https://doi.org/10.3390/molecules23071646
Semwal RB, Semwal DK, Combrinck S, Viljoen AM (2015) Gingerols and shogaols: important nutraceutical principles from ginger. Phytochemistry 117:554–568. https://doi.org/10.1016/j.phytochem.2015.07.012
Kou X, Li X, Rahman MRT et al (2017) Efficient dehydration of 6-gingerol to 6-shogaol catalyzed by an acidic ionic liquid under ultrasound irradiation. Food Chem 215:193–199. https://doi.org/10.1016/j.foodchem.2016.07.106
Mallavadhani UV, Panigrahi R (2013) UPLC-Q-TOF-MS based studies to evaluate the effect of extraction methodology on the yield of 6-shogaol, a biomarker of Z. officinale. Ind Crops Prod 50:821–827. https://doi.org/10.1016/j.indcrop.2013.08.059
Dalsasso RR, Valencia GA, Monteiro AR (2022) Impact of drying and extractions processes on the recovery of gingerols and shogaols, the main bioactive compounds of ginger. Food Res Int 154:111043. https://doi.org/10.1016/j.foodres.2022.111043
An K, Zhao D, Wang Z et al (2016) Comparison of different drying methods on Chinese ginger (Zingiber officinale Roscoe): changes in volatiles, chemical profile, antioxidant properties, and microstructure. Food Chem 197(B):1292–1300. https://doi.org/10.1016/j.foodchem.2015.11.033
González-Cavieres L, Pérez-Won M, Tabilo-Munizaga G et al (2021) Advances in vacuum microwave drying (VMD) systems for food products. Trends Food Sci Technol 116:626–638. https://doi.org/10.1016/j.tifs.2021.08.005
Cheng XL, Liu Q, Peng YB et al (2011) Steamed ginger (Zingiber officinale): changed chemical profile and increased anticancer potential. Food Chem 129(4):1785–1792. https://doi.org/10.1016/j.foodchem.2011.06.026
Guo JB, Zhang WJ, Wu H, Du LM (2015) Microwave-assisted decomposition coupled with acidic food condiment as an efficient technology for ginger (Zingiber officinale Roscoe) processing. Sep Purif Technol 146:219–226. https://doi.org/10.1016/j.seppur.2015.03.049
Chemat F, Rombaut N, Sicaire AG et al (2017) Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason Sonochem 34:540–560. https://doi.org/10.1016/j.ultsonch.2016.06.035
Jacotet-Navarro M, Rombaut N, Deslis S et al (2016) Towards a “dry” bio-refinery without solvents or added water using microwaves and ultrasound for total valorization of fruit and vegetable by-products. Green Chem 18(10):3106–3115. https://doi.org/10.1039/c5gc02542g
Cherrat S, Boulkebache-Makhlouf L, Iqbal J et al (2019) Effect of different drying temperatures on the composition and antioxidant activity of ginger powder. Ann Univ Dunarea de Jos Galati Fascicle VI: Food Technol 43(2):125–142. https://doi.org/10.35219/foodtechnology.2019.2.09
Huang TC, Chung CC, Wang HY et al (2011) Formation of 6-shogaol of ginger oil under different drying conditions. Dry Technol 29(16):1884–1889. https://doi.org/10.1080/07373937.2011.589554
Mahayothee B, Thamsala T, Khuwijitjaru P, Janjai S (2020) Effect of drying temperature and drying method on drying rate and bioactive compounds in cassumunar ginger (Zingiber montanum). J Appl Res Med Aromat Plants 18:100262. https://doi.org/10.1016/j.jarmap.2020.100262
Zeng S, Wang B, Lv W et al (2022) Dynamic analysis of moisture, dielectric property and microstructure of ginger slices during microwave hot-air flow rolling drying. Food Control 134:108717. https://doi.org/10.1016/j.foodcont.2021.108717
Lin X, Xu JL, Sun DW (2020) Evaluating drying feature differences between ginger slices and splits during microwave-vacuum drying by hyperspectral imaging technique. Food Chem 332:127407. https://doi.org/10.1016/j.foodchem.2020.127407
Izli N, Polat A (2019) Effect of convective and microwave methods on drying characteristics, color, rehydration and microstructure properties of ginger. Food Sci Technol 39(3):652–659. https://doi.org/10.1590/fst.04518
Kutlu N, Pandiselvam R, Kamiloglu A et al (2022) Impact of ultrasonication applications on color profile of foods. Ultrason Sonochem 89:106109. https://doi.org/10.1016/j.ultsonch.2022.106109
Liu SQ, Wu LL, Yu X, Huang H (2022) Marketing online food images via color saturation: a sensory imagery perspective. J Bus Res 151:366–378. https://doi.org/10.1016/j.jbusres.2022.06.061
Schifferstein HNJ, Wehrle T, Carbon CC (2019) Consumer expectations for vegetables with typical and atypical colors: the case of carrots. Food Qual Prefer 72:98–108. https://doi.org/10.1016/j.foodqual.2018.10.002
Iijima Y, Joh A (2014) Pigment composition responsible for the pale yellow color of ginger (Zingiber officinale) rhizomes. Food Sci Technol Res 20(5):971–978. https://doi.org/10.3136/fstr.20.971
Ren Z, Yu X, Yagoub AEA et al (2021) Combinative effect of cutting orientation and drying techniques (hot air, vacuum, freeze and catalytic infrared drying) on the physicochemical properties of ginger (Zingiber officinale Roscoe). LWT-Food Sci Technol 144:111238. https://doi.org/10.1016/j.lwt.2021.111238
Ghafoor K, Al Juhaimi F, Özcan MM et al (2020) Total phenolics, total carotenoids, individual phenolics and antioxidant activity of ginger (Zingiber officinale) rhizome as affected by drying methods. LWT-Food Sci Technol 126:109354. https://doi.org/10.1016/j.lwt.2020.109354
Amiri ZN, Najafpour GD, Moghadamnia AA (2018) Subcritical water extraction of bioactive compounds from ginger (Zingiber officinale Roscoe). Int J Eng 31(12):1991–2000. https://doi.org/10.5829/ije.2018.31.12c.01
Osae R, Apaliya MT, Kwaw E et al (2021) Drying techniques affect the quality and essential oil composition of Ghanaian ginger (Zingiber officinale Roscoe). Ind Crops Prod 172:114048. https://doi.org/10.1016/j.indcrop.2021.114048
Chen CC, Ho CT (1987) Gas chromatographic analysis of thermal degradation products of gingerol compounds in steam-distilled oil from ginger (Zingiber officinale Roscoe). J Chromatogr A 387:499–504. https://doi.org/10.1016/S0021-9673(01)94559-5
Shaw PE, Tatum JH, Berry RE (1971) 2,3-Dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one, a degradation product of a hexose. Carbohydr Res 16(1):207–211. https://doi.org/10.1016/S0008-6215(00)86115-7
Acknowledgements
We are grateful to the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, CAPES, especially to the CAPES-PRINT Program. G. A. Valencia thanks the Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), and A. R. Monteiro thanks the National Council for Scientific and Technological Development (CNPq) for financial support. The authors gratefully acknowledge the Federal University of Santa Catarina (UFSC) for the support.
Funding
This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível superior (CAPES) [Finance Code 001]; Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC) [grants 2021TR000418 and 2021TR001887]; Programa CAPES PRINT [project number 88887.310560/2018–00 – UFSC]; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) [grant 302434/2022–4 and Projeto Universal, grant 202304/2021–7].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study's conception, design, and methodology definition. Raul Remor Dalsasso conducted the formal analysis, curated and investigated the data, wrote the original draft, and created the visualizations. Germán Ayala Valencia supervised the study, provided critical review, and obtained funding and resources. Alcilene Rodrigues Monteiro administered the work, validated and critically reviewed the manuscript, and obtained funding and resources.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors have agreed to publish this paper without any reservations whatsoever.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dalsasso, R.R., Valencia, G.A. & Monteiro, A.R. Improving Ginger’s Bioactive Composition by Combining Innovative Drying and Extraction Technologies. Plant Foods Hum Nutr 78, 755–761 (2023). https://doi.org/10.1007/s11130-023-01109-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01109-y





 ), 80 °C (
), 80 °C (
 ), or 120 °C (
), or 120 °C (
 ) and vacuum microwave drying (
) and vacuum microwave drying (
 ). The dotted line represents the polynomial adjustment of the vacuum microwave drying
). The dotted line represents the polynomial adjustment of the vacuum microwave drying