Summary
Since the introduction of tumor necrosis factor (TNF)-α inhibitors, the treatment of rheumatoid arthritis (RA) has been revolutionized. The approach of targeting TNF-α has considerably improved the success in the treatment of RA. Over the last 3 decades five different TNF-α inhibitors have been administered: infliximab, etanercept, adalimumab, golimumab, and certolizumab-pegol. All of them show excellent efficacy with similar rates of clinical response and prevention of radiographic disease progression. With improved therapies, treatment strategies have also changed, with the aims now being to achieve and maintain remission. Most recently, the discussion expands to the issue of treatment reduction in patients who have achieved sustained remission; here, the discontinuation of TNF-α inhibitor therapy has become an area of interest, given obvious economic and risk-benefit evaluations. However, only little is known if “biologic free” remission is possible in patients with sustained remission following intensive TNF-α inhibitor therapy.
Zusammenfassung
Die Entdeckung und Einführung von Tumor-Nekrosis-Faktor Alpha (TNF-α) Blockern hat die Therapie der Rheumatoiden Arthritis (RA) revolutioniert. Der direkte Therapieansatz am Zytokine TNF-α führte zu beachtlichen Erfolgen und Ansteigen von Remissionsraten. Innerhalb der letzten drei Dekaden wurden fünf verschiedene TNF-α Blocker entwickelt und am Markt eingeführt: Infliximab (IFX), Adalimumab (ADA), Etanercept (ETN), Golimumab (GLM) und Certolizumab Pegol (CZP). Alle diese Medikamente zeigen ausgezeichnete Effektivität mit vergleichbaren Erfolgsraten betreffend klinischer Aktivität und radiologischer Progression. Durch Verbesserung der Therapeutika kam es schlussendlich zu Adaptierung und Optimierung von Therapiestrategien, mit Erreichen und Erhalten von Remission als oberstes Therapieziel. Da dauerhafte Remission mittlerweile ein realistisches Szenario darstellt, und in Anbetracht sozio-ökonomischer und Nutzen-Risiko Überlegungen beschäftigen sich neuere Arbeiten mit dem Thema der Therapiereduktion in dieser Patientenpopulation. Bis dato ist allerdings nur wenig darüber bekannt, ob „biologika- freie“ Remission in Patienten mit vorangegangener intensiver TNF-α Therapie eine Möglichkeit darstellt.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease affecting about 1 % of the adult people [1]. Due to its chronicity and destructive disease course RA is associated with major consequences for the individual, causing loss of function and work disability, and poses significant challenges to society given its economic consequences [2, 3].
Treatment of RA has been revolutionized by the discovery of the role of certain cytokines, in particular tumor necrosis factor alpha (TNF-α), in the pathogenesis of the disease. TNF-α is a cytokine that is central to the inflammatory cascade which modulates the immune response, with powerful effects on many aspects of cellular and humoral immunity [4, 5]. Elevation of TNF-α levels have been observed in synovial fluid and the synovium of patients with RA [6]. Due to its influence on various cells in synovial membrane, such as macrophages, synoviocytes, chondrocytes, and osteoclasts, TNF-α induces local inflammation and pannus formation, leading to erosion of cartilage and bone destruction [7]. Higher levels on circulating TNF-α are observed in patients with higher disease activity and destructive disease, as activation of osteoclast is dose dependent [8].
The approach of targeting TNF-α has considerably improved treatment of RA. Since the first developments of TNF-α inhibitors in RA in the 1980s, five different drugs based on blocking TNF-α have entered clinical use: infliximab (IFX), adalimumab (ADA), etanercept (ETC), golimumab (GLM), and certolizumab pepol (CZP) which will be discussed briefly below. Over the last decades various clinical trials have been conducted for these compounds, which have shown excellent and comparable efficacy in improving clinical, functional, and radiological disease outcomes in RA patients.
Overview of the different TNF-α inhibitors
Infliximab
IFX was the first TNF-α inhibitor administered in patients with RA. IFX is a chimeric (mouse/human) monoclonal antibody that hinders the cytokine from triggering the cellular TNF receptor complex. IFX required intravenous application and has a half-life time of 8–10 days, thus is administered every 4–8 weeks. The efficacy of IFX in combination with methotrexat (MTX) was shown in several clinical trials [9–11], with higher improvements of disease activity and prevention of radiographic progression compared with patients treated with MTX plus placebo.
Adalimumab
ADA is a fully human monoclonal antibody of recombinant immunoglobulin (IgG1) against TNF-α, which is also able to prevent the binding of TNF-α to its receptors. Clinical trials like the PREMIER or the OPTIMA study could show that a combination of ADA plus MTX is statistically superior to MTX alone, inhibiting radiographic progression and inducing clinical remission [12–14].
Etanercept
ETN is a genetically engineered protein comprising two molecules of the extracellular domain of TNF receptor II and the Fc-portion of IgG1 [15]. ETN has the shortest half-life of available TNF-inhibitors of 3–5.5 days and is administered subcutaneously, either on a weekly basis (50 mg) or twice a week (25 mg). Increasing the dosage of ETN from 50 mg once a week to 50 mg twice a week in suboptimal responders did improve response rates, but not significantly [16]. In the TEMPO trial a higher clinical response rate and less radiographic progression of patients treated with combination therapy of ETX plus MTX was found compared with those receiving either ETN or MTX monotherapy [17]. The PRESERVE trial looked at the possibility of ETN withdrawal after achieving sustained low disease activity (LDA) and found that patients kept on combination therapy of MTX plus ETN did better in maintaining LDA compared with those where ETN was withdrawn [18].
Golimumab
GLM is also a fully human monoclonal antibody, which is able to bind both, soluble and transmembrane TNF, thereby preventing binding to TNF receptors and inhibiting TNF activity. GLM is administered by subcutaneous injections (50 or 100 mg) every 4 weeks. Several randomized control trials evaluated the efficacy and safety of GLM in different groups of RA patients (MTX-naive: GO-BEFORE [19]; MTX inadequate response: GO-FORWARD [20]; TNF inadequate response: GO-AFTER [21]), showing greater response rates compared with the respective control group.
Certolizumab pegol
CZP is a humanized monovalent Fab antibody fragment linked to polyethylene glycol (PEG). Because of its structure, it has a different mechanism of action and kinetics to other TNF inhibitors. The PEG portion is a bulky hydrophilic inert molecule, which increases the plasma half-life of the drug (estimated to be 2 weeks) [22]. The recommended dose for adults with RA is 400 mg (given as two subcutaneous injections of 200 mg) initially and at weeks 2 and 4, followed by 200 mg every other week. Efficacy of CZP was assessed in combination to MTX in MTX inadequate responders (RAPID1 [23] and RAPID2 [24]) or as monotherapy in disease-modifying antirheumatic drugs (DMARD) inadequate responders (FAST4WARD [25]). Both, combination or monotherapy show better clinical and radiographic outcomes compared with placebo.
Similarities and differences in efficacy and safety
In general, all TNF-α inhibitors in combination with MTX show sustained clinical efficacy and prevention of radiographic progression. Using standard American College of Rheumatology (ACR) response criteria the proportion of patients achieving 20, 50 and 70 % improvement (known as ACR20, ACR50, and ACR70 response rates) are similar for all five drugs: the major determinant of ACR response rates in the population studied, with highest rates in DMARD naïve populations, followed by DMARD insufficient responders and TNF-inhibitor insufficient responders [26]. Even though there seem no striking differences of efficacy and safety between the different agents, they all have distinct pharmacokinetic and—dynamic properties that must be considered when selecting drug therapy for individual patients. For example, there are evident differences in the half-lives of the individual agents, with ETN having the shortest. Furthermore dosing regimens and routes of administration are differently leading to different patient and/or physician preferences, and to different levels of flexibility regarding potential dose optimization.
Only very few head-to-head trials comparing individual TNF-α inhibitors exist; most comparisons to date are based on indirect and retrospective data analyses. In a Danish study conducted in a nationwide biological register comparing IFX, ADA, and ETN, lower rates of treatment response and remission rates were found for IFX, whereas ADA had highest rates of remission and treatment response [27]. Longest drug-free survival was observed for ETN. In a Cochrane review of 2009 comparing safety and efficacy of biological DMARDs, lower risk of tuberculosis was postulated for ETN-treated patients compared with IFX and ADA [28]. Results of a meta-analysis of another systematic literature review show a significant lower risk of discontinuation due to adverse events in patients treated with ETN compared with control [29]. All these results have to be interpreted with caution as heterogeneity in patient populations and clinical trial designs need to be taken into account. Furthermore, studies investigating recent biological DMARDs such as GLM or CZP were not included.
Comparison with non-TNF inhibiting biologics
Recently, head-to-head trials were conducted using ADA as the reference TNF-inhibitor for comparison with other biological agents, such as abatacept or tocilizumab: in the AMPLE study, no differences in response rates or radiographic progression between subcutaneous abatacept and ADA, both in combination with MTX, could be observed [30]. In contrast, ADA as monotherapy was shown to be inferior to tocilizumab monotherapy for reduction of signs and symptoms of RA in the ADACTA trial [31].
Immunogenicity
In some patients, triggered immune response to TNF-α inhibitor therapy might cause the formation of anti-drug antibodies (ADAb). In patients treated with IFX and ADA the development of ADAb has been reported, mainly in the first 6 months of therapy [32, 33]. ADAb can impair clinical response, if they reduce serum levels of the active drug [34]. Many factors such as drug characteristics, treatment dose or duration, genetic background, and co-treatment can influence immunogenicity [35]. Understanding which patients and therapies are at risk for immunogenicity is a hot topic of current research.
The role of TNF inhibitors in the treatment algorithm for RA
With the introduction of biological therapy effective disease control of RA patients was possible. Only with sustained suppression of disease activity, so-called sustained remission, joint damage can be prevented. The ACR and the European League of Rheumatism (EULAR) recommend a treatment approach with the target of remission or LDA as the therapeutic goal [36, 37]. To achieve the goal one need to follow a structured algorithm of add-on and switch of DMARD therapy [38]. It is essential to initiate DMARD therapy as soon as RA is diagnosed—with MTX monotherapy being the recommended initial therapy. If disease activity is not controlled after 3–6 months, TNF-α inhibitors should be added. For patients with early RA (disease duration < 6 months) displaying high disease activity and poor prognostic factors, the ACR recommends the use of a TNF inhibitor as an immediate, first-line course of therapy.
One of the pioneer studies to compare early biological use with more conservative approaches, and thus supporting strategic treatment recommendations, was the BeSt trial, in which patients with recent onset of active RA were randomized into either receiving MTX monotherapy, a step-up combination of MTX plus IFX or a initial combination therapy of MTX plus IFX. An evaluation of the 3-year data from this study reveals that more patients initially receiving MTX plus IFX have been able to taper and stop all anti-rheumatic drugs and still maintain a state of remission (17 %) than in the other groups (10, 5, and 9 %, respectively) [39]. Furthermore, in post hoc analysis of the BeSt study radiographic and functional outcomes patients who started initial MTX plus IFX showed greater improvements in terms of the Health Assessment Questionnaire over time and less radiographic progression compared with those who received the delayed combination therapy (Fig. 1) [40]. These results suggest that the earlier use of combination therapy with IFX resulted in a better function, less radiographic progression and higher rate of IFX discontinuation over time. However, the predetermined sequence of therapies in the groups not receiving TNF-inhibitor from the beginning led to a delay of the biological compound that would be inacceptable by current standards and recommendations (ACR; EULAR; treat to target). In fact, the more recent OPTIMA study [13] has convincingly shown that disease activity, as well as functional and structural effects of the initial combination therapy can be reachieved in patients who started monotherapy of MTX and failed it (i.e., using a step-up approach).
Results of the BeSt trial: significant differences of physical function as measured by the Health Assessment Questionnaire (HAQ) between the initial and delayed infliximab (IFX) group during 3 years of follow-up. (Source: adapted from van der Kooij et al. [40])
According to the EULAR algorithm, if, after 3 months of TNF-α inhibitor therapy, the patient still has no substantive improvement in disease activity he or she should be switched to an alternative TNF-α inhibitor or a non-TNF biologic agent.
Randomized controlled trials (RCTs) could show that in patients with TNF-α failure, marked improvements can be obtained when switched either to another TNF inhibitor [21] or to agents with a distinct mechanism of action (rituximab, abatacept, and tocilizumab) [41–43] with comparable results [44]. Not surprising, response rates decrease with increasing number of previous failed biological agents [45].
Although TNF inhibitor therapies have considerable positive impact on various outcomes of RA, a considerable proportion (approximately 40–44 %) of patients show dissatisfactory improvement of the disease [5]. Although there seems to be no striking differences of efficacy and safety profiles among different agent on the group level, it is important to identify the most appropriate therapy for an individual patient. This poses a highly urgent research agenda, calling for trials on predictors and biomarkers of response to different biological agents.
Discontinuation of TNF-α inhibitors
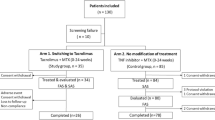
With improving treatment strategies and increasing number of available effective treatments, the proportion of patients reaching sustained remission has grown. Therefore, considering risk-benefit evaluations and economic aspects, discontinuing TNF-α inhibitor therapy after achieving sustained remission has gotten into the focus of interest (Fig. 2). The OPTIMA [13] study indicated that achievement of sustained LDA (two subsequent visits) in early RA is a relatively unstable state, as even one quarter of patients continuing ADA+MTX did not maintain that state over a subsequent year; in those patients withdrawing ADA this group was even 7–9 % higher. In the PRESERVE trial patients staying on ETN 50 plus MTX or reducing ETN to 25 mg + MTX after achieving LDA did similarly well in maintaining LDA and significantly better than those who fully withdrew ETN [18].
Course of rheumatoid arthritis in the twenty-first century: (a) early diagnosis and treatment initiation, (b) following a treat to target approach with frequent assessment of disease activity and adjustment of DMARD therapy, (c) achieving the goal of sustained remission, (d) discontinue biological treatment and (e) achieve biological free sustained remission
It is not clear yet which factors drive biological free remission or lead to an early relapse. Findings of the HONOR study (Humira discontinuation without functional and radiographic damage progressiON fOllowing sustained Remission) indicate deep remission at discontinuation was associated with successful drug-free remission [46]. Animal data suggest that prior TNF-α inhibitor treatment may change the disease course and/or induce immunological remission; however, these findings have not been sufficiently proven among humans [47, 48], where the simple notion remains that biological withdrawal is safest in patients with full remission for a very long period of time [46, 49, 50].
Biosimilars
Biosimilars are defined as “biotherapeutic product which is similar in terms of quality, safety and efficacy to an already licensed reference biotherapeutic product.” RCTs, comparing a biosimilar to an original TNF-α inihibitor (infliximab) have been conducted, demonstrating equivalent efficacy and safety [51]. After patent expiration of the original TNF-α inihibitors, biosimilars will be available on markets, although hopes for considerable price benefits compared with the originator drugs may still be disappointed. Nevertheless, according to a financial analyst biosimilars will hold a market of more than 40 % for therapeutic monoclonal antibodies in Europe by 2018 [52]. This will hopefully also increase the access to TNF-inhibitors for a larger number of patients.
Conclusion
The introduction of TNF-α inhibitors has revolutionized the treatment of RA. The ultimate goal of clinical remission became possible, although treatment reduction in patients who have achieved sustained remission is still afflicted with a considerable risk of relapse.
Conflict of interest
The authors declare that there are no actual or potential conflicts of interest in relation to this article.
References
Spector TD. Rheumatoid arthritis. Rheum Dis Clin North Am. 1990;16(3):513–37.
Kobelt G, Woronoff AS, Richard B, et al. Disease status, costs and quality of life of patients with rheumatoid arthritis in France: the ECO-PR Study. Joint Bone Spine. 2008;75(4):408–15.
Pugner KM, Scott DI, Holmes JW, et al. The costs of rheumatoid arthritis: an international long-term view. Semin Arthritis Rheum. 2000;29(5):305–20.
Bluml S, Scheinecker C, Smolen JS, et al. Targeting TNF receptors in rheumatoid arthritis. Int Immunol. 2012;24(5):275–81.
Feldmann M, Maini RN. Lasker Clinical Medical Research Award. TNF defined as a therapeutic target for rheumatoid arthritis and other autoimmune diseases. Nat Med. 2003;9(10):1245–50.
Di Giovine FS, Nuki G, Duff GW. Tumour necrosis factor in synovial exudates. Ann Rheum Dis. 1988;47(9):768–72.
Tracey D, Klareskog L, Sasso EH, et al. Tumor necrosis factor antagonist mechanisms of action: a comprehensive review. Pharmacol Ther. 2008;117(2):244–79.
Azuma Y, Kaji K, Katogi R, et al. Tumor necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem. 2000;275(7):4858–64.
Smolen JS, Han C, Bala M, et al. Evidence of radiographic benefit of treatment with infliximab plus methotrexate in rheumatoid arthritis patients who had no clinical improvement: a detailed subanalysis of data from the anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study. Arthritis Rheum. 2005;52(4):1020–30.
Maini RN, Breedveld FC, Kalden JR, et al. Sustained improvement over two years in physical function, structural damage, and signs and symptoms among patients with rheumatoid arthritis treated with infliximab and methotrexate. Arthritis Rheum. 2004;50(4):1051–65.
Lipsky PE, van der Heijde DM, St Clair EW, et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N Engl J Med. 2000;343(22):1594–602.
Breedveld FC, Weisman MH, Kavanaugh AF, et al. The PREMIER study: a multicenter, randomized, double-blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum. 2006;54(1):26–37.
Kavanaugh A, Fleischmann RM, Emery P, et al. Clinical, functional and radiographic consequences of achieving stable low disease activity and remission with adalimumab plus methotrexate or methotrexate alone in early rheumatoid arthritis: 26-week results from the randomised, controlled OPTIMA study. Ann Rheum Dis. 2013;72(1):64–71.
Keystone EC, Kavanaugh AF, Sharp JT, et al. Radiographic, clinical, and functional outcomes of treatment with adalimumab (a human anti-tumor necrosis factor monoclonal antibody) in patients with active rheumatoid arthritis receiving concomitant methotrexate therapy: a randomized, placebo-controlled, 52-week trial. Arthritis Rheum. 2004;50(5):1400–11.
Moreland LW, Baumgartner SW, Schiff MH, et al. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337(3):141–7.
Weinblatt ME, Schiff MH, Ruderman EM, et al. Efficacy and safety of etanercept 50 mg twice a week in patients with rheumatoid arthritis who had a suboptimal response to etanercept 50 mg once a week: results of a multicenter, randomized, double-blind, active drug-controlled study. Arthritis Rheum. 2008;58(7):1921–30.
Klareskog L, van der Heijde D, de Jager JP, et al. Therapeutic effect of the combination of etanercept and methotrexate compared with each treatment alone in patients with rheumatoid arthritis: double-blind randomised controlled trial. Lancet. 2004;363(9410):675–81.
Smolen JS, Nash P, Durez P, et al. Maintenance, reduction, or withdrawal of etanercept after treatment with etanercept and methotrexate in patients with moderate rheumatoid arthritis (PRESERVE): a randomised controlled trial. Lancet. 2013;381(9870):918–29.
Emery P, Fleischmann RM, Moreland LW, et al. Golimumab, a human anti-tumor necrosis factor alpha monoclonal antibody, injected subcutaneously every four weeks in methotrexate-naive patients with active rheumatoid arthritis: twenty-four-week results of a phase III, multicenter, randomized, double-blind, placebo-controlled study of golimumab before methotrexate as first-line therapy for early-onset rheumatoid arthritis. Arthritis Rheum. 2009;60(8):2272–83.
Keystone E, Genovese MC, Klareskog L, et al. Golimumab in patients with active rheumatoid arthritis despite methotrexate therapy: 52-week results of the GO-FORWARD study. Ann Rheum Dis. 2010;69(6):1129–35.
Smolen JS, Kay J, Doyle MK, et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor alpha inhibitors (GO-AFTER study): a multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet. 2009;374(9685):210–21.
Pasut G. Pegylation of biological molecules and potential benefits: pharmacological properties of certolizumab pegol. BioDrugs. 2014;28(Suppl 1):15–23.
Keystone E, Heijde D, Mason D, Jr. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319–29.
Smolen J, Landewe RB, Mease P, et al. Efficacy and safety of certolizumab pegol plus methotrexate in active rheumatoid arthritis: the RAPID 2 study. A randomised controlled trial. Ann Rheum Dis. 2009;68(6):797–804.
Fleischmann R, Vencovsky J, van Vollenhoven RF, et al. Efficacy and safety of certolizumab pegol monotherapy every 4 weeks in patients with rheumatoid arthritis failing previous disease-modifying antirheumatic therapy: the FAST4WARD study. Ann Rheum Dis. 2009;68(6):805–11.
Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet. 2007;370(9602):1861–74.
Hetland ML, Christensen IJ, Tarp U, et al. Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum. 2010;62(1):22–32.
Singh JA, Christensen R, Wells GA. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev. 2009(4):CD007848.
Aaltonen KJ, Virkki LM, Malmivaara A, et al. Systematic review and meta-analysis of the efficacy and safety of existing TNF blocking agents in treatment of rheumatoid arthritis. PLoS One. 2012;7(1):e30275.
Schiff M, Weinblatt ME, Valente R, et al. Head-to-head comparison of subcutaneous abatacept versus adalimumab for rheumatoid arthritis: two-year efficacy and safety findings from AMPLE trial. Ann Rheum Dis. 2014;73(1):86–94.
Gabay C, Emery P, van Vollenhoven R, et al. Tocilizumab monotherapy versus adalimumab monotherapy for treatment of rheumatoid arthritis (ADACTA): a randomised, double-blind, controlled phase 4 trial. Lancet. 2013;381(9877):1541–50.
Pascual-Salcedo D, Plasencia C, Ramiro S, et al. Influence of immunogenicity on the efficacy of long-term treatment with infliximab in rheumatoid arthritis. Rheumatology. 2011;50(8):1445–52.
Wolbink GJ, Vis M, Lems W, et al. Development of antiinfliximab antibodies and relationship to clinical response in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54(3):711–5.
Krieckaert C, Rispens T, Wolbink G. Immunogenicity of biological therapeutics: from assay to patient. Curr Opin Rheumatol. 2012;24(3):306–11.
Wolbink GJ, Aarden LA, Dijkmans BA. Dealing with immunogenicity of biologicals: assessment and clinical relevance. Curr Opin Rheumatol. 2009;21(3):211–5.
Smolen JS, Aletaha D, Bijlsma JW, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69(4):631–7.
Felson DT, Smolen JS, Wells G. American College of Rheumatology/European League against Rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann Rheum Dis. 2011;70(3):404–13.
Smolen JS, Landewe R, Breedveld FC, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. 2014;73(3):492–509.
Allaart CF, Breedveld FC, Dijkmans BA. Treatment of recent-onset rheumatoid arthritis: lessons from the BeSt study. J Rheumatol Suppl. 2007;80:25–33.
van der Kooij SM, le Cessie S, Goekoop-Ruiterman YP, et al. Clinical and radiological efficacy of initial vs delayed treatment with infliximab plus methotrexate in patients with early rheumatoid arthritis. Ann Rheum Dis. 2009;68(7):1153–8.
Cohen SB, Emery P, Greenwald MW, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. 2006;54(9):2793–806.
Genovese MC, Becker JC, Schiff M, et al. Abatacept for rheumatoid arthritis refractory to tumor necrosis factor alpha inhibition. N Engl J Med. 2005;353(11):1114–23.
Emery P, Keystone E, Tony HP, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-tumour necrosis factor biologicals: results from a 24-week multicentre randomised placebo-controlled trial. Ann Rheum Dis. 2008;67(11):1516–23.
Nam JL, Winthrop KL, van Vollenhoven RF, et al. Current evidence for the management of rheumatoid arthritis with biological disease-modifying antirheumatic drugs: a systematic literature review informing the EULAR recommendations for the management of RA. Ann Rheum Dis. 2010;69(6):976–86.
Rendas-Baum R, Wallenstein GV, Koncz T, et al. Evaluating the efficacy of sequential biologic therapies for rheumatoid arthritis patients with an inadequate response to tumor necrosis factor-alpha inhibitors. Arthritis Res Ther. 2011;13(1):R25.
Tanaka Y, Hirata S, Kubo S, et al. Discontinuation of adalimumab after achieving remission in patients with established rheumatoid arthritis: 1-year outcome of the HONOR study. Ann Rheum Dis. 2015;74:389–95.
Herrak P, Gortz B, Hayer S, et al. Zoledronic acid protects against local and systemic bone loss in tumor necrosis factor-mediated arthritis. Arthritis Rheum. 2004;50(7):2327–37.
Ji H, Pettit A, Ohmura K, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196(1):77–85.
Tanaka Y, Hirata S, Saleem B, et al. Discontinuation of biologics in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S22–7.
Brocq O, Millasseau E, Albert C, et al. Effect of discontinuing TNFalpha antagonist therapy in patients with remission of rheumatoid arthritis. Joint Bone Spine. 2009;76(4):350–5.
Yoo DH, Hrycaj P, Miranda P, et al. A randomised, double-blind, parallel-group study to demonstrate equivalence in efficacy and safety of CT-P13 compared with innovator infliximab when coadministered with methotrexate in patients with active rheumatoid arthritis: the PLANETRA study. Ann Rheum Dis. 2013;72(10):1613–20.
Todoerti M, De Nard F, Boffini N, et al. Biosimilars: lights and shadows in rheumatology. Rheumatology Reports. 2014;6:5518–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radner, H., Aletaha, D. Anti-TNF in rheumatoid arthritis: an overview. Wien Med Wochenschr 165, 3–9 (2015). https://doi.org/10.1007/s10354-015-0344-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10354-015-0344-y