Abstract
Introduction
Diffusing capacity for carbon monoxide (DLco) reduction is the first detectable pulmonary functional test (PFT) change in systemic sclerosis (SSc)–related pulmonary complications. reduction in patients without cardiopulmonary alterations has also been observed; a good characterisation of these patients is lacking. The objective of this study is to describe the characteristics of SSc patients with isolated DLco reduction and compare these patients to SSc patients with DLco reduction with a known cause.
Methods
SSc patients with DLco < 80% predicted were included and classified into cases (isolated DLco reduction) and controls (DLco reduction in the presence of known pulmonary pathology). SSc clinico-serological data, PFT and echocardiography features were collected and analysed.
Results
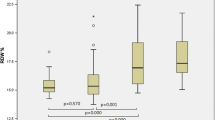
From a total SSc cohort of 115 patients, 75 patients were included: 20 cases (26.7%) and 55 controls (73.3%). Cases were predominantly limited skin subset (90% vs 60%, p < 0.001), were anti-centromere antibody (ACA)-positive (95% vs 40%, p < 0.001) and had an infrequent oesophageal involvement (45% vs 74%; p = 0.016). The mean DLco reduction of cases was mild (65.60% ± 10.56). Only 1 out of 20 patients had normal DLco/VA values, and tricuspid regurgitation was more frequent (85% vs 53.8%, p = 0.014).
Conclusion
There is a subgroup of SSc patients with mild isolated DLco and DLco/VA reduction, predominantly limited SSc with ACA seropositivity, which could identify a particular SSc subset. We hypothesise that isolated DLco/VA reduction could indicate a pulmonary vascular involvement. Nevertheless, a close follow-up is mandatory, as a pre-PAH situation cannot be excluded.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Systemic sclerosis (SSc) is a rare, immune-mediated, multisystem connective tissue disease, characterised by a small vessel inflammatory vasculopathy and activation of fibroblasts with excessive deposition of collagen and extracellular matrix in different organs. It can affect the skin, lung, gastrointestinal tract, heart and kidney. There are two main subsets of SSc, according to the affected skin area: diffuse cutaneous (dSSc) and limited cutaneous (lSSc). These subtypes have different serological profiles [1], with different clinical presentations and different visceral manifestations [2].
Pulmonary involvement, due both to interstitial lung disease (ILD) or pulmonary arterial hypertension (PAH), is one of the main complications related to SSc [3]. Due to the increased mortality of these conditions [4, 5] and given that it could be detected sooner in an asymptomatic phase of disease, physicians are encouraged to carry out active screening for pulmonary SSc–related involvement. There are several pre-existing recommendations for screening. For PAH, most of the guidelines recommend combined screening based on symptoms, pulmonary function test (PFT), echocardiography and biomarkers, with echocardiography being the most cost-effective, but right heart catheterisation (RHC) still remains as gold standard for definite diagnosis [6,7,8,9,10].
In this context, PFT are an important tool in the initial screening of these patients, as alterations often precede the respiratory symptoms. The reduction of diffusing capacity for carbon monoxide (DLco) is the main detectable functional change [11,12,13].
DLco measures the capacity of the lung for gas exchange. It depends on the membrane diffusing capacity and pulmonary capillary volume, as well as the red cell resistance. This may be altered in any pathological condition that causes loss or destruction of the pulmonary capillary membranes (e.g. fibrosis, asbestosis, intrapulmonary shunting, pulmonary hypertension, emphysema, bronchiolitis, anaemia) [14].
It is well-known that patients have a poor outcome in the presence of decreased DLco (values lower to 40% of predicted) [15].
Interestingly, the presence of a group of patients with SSc and isolated reduction of DLco in the absence of cardio-pulmonary complications has been described [16, 17]. In this group, if a mild isolated DLco defect (DLco values between 60 and 79% predicted [18]) is detected, prognosis seems to be similar to the patients with normal PFR values [15]. However, in those patients, when forced vital capacity (FVC)/DLco ratio > 1.4 or isolated DLco < 55% are found, a high risk of PAH development is observed [17].
However, to date, this group of patients with DLco reduction without cardiopulmonary alterations had not yet been well-characterised. There are just a few studies to evaluate the presence and impact of isolated DLco reduction in SSc patients [17, 19].
The aim of this study was to investigate the clinical, immunological, echocardiographic and respiratory functional characteristics of SSc patients with isolated DLco reduction (i.e. DLco values < 80% predicted and absence of known causes that justifies this DLco reduction) and to compare this group with patients with a known cause for DLco reduction.
Methods
Design and subjects
This is a retrospective case-control study that reviews a cohort of 115 patients regularly attending the outpatient clinic of the Rheumatology Unit of the Hospital del Mar in Barcelona with definite SSc diagnosis. All patients were diagnosed by rheumatologists with consolidated expertise in SSc and they fulfilled the 2013 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) Classification Criteria for SSc [20]. All patients had a thorough medical history taken plus physical examination, PFT and echocardiography. Patients who had pulmonary symptoms and/or PFT impairment underwent high-resolution computerised tomography (HRCT) of the thorax.
The inclusion criterion was the presence of DLco values < 80% predicted.
The patients were divided into two groups according case-control definitions (Fig. 1):
- “Cases”: SSc patients with isolated DLco reduction without known causes (i.e. absence of ILD and emphysema by HRCT, asthma, chronic obstructive pulmonary disease, PAH or systolic pulmonary arterial pressure > 36 mmHg estimated by echocardiography) and normal spirometry and static lung volumes.
- “Controls”: SSc patients with a diagnosed cause, as mentioned above, that could justify a DLco reduction.
All participants’ data had been processed in accordance with the Declaration of Helsinki.
Measurements
To characterise the patients, clinical-epidemiological data were collected: age (years), sex (male/female), history of smoking (yes/no), time since disease diagnosis (years), SSc subgroup (dSSc, lSSc, preSSc, sine-SSc) [3], presence of Raynaud’s phenomenon, digital ulcers, telangiectasiae, oesophageal dysfunction and coronary artery disease, and immunological features (ANA, anti-topoisomerase, anti-centromere antibody [ACA], anti-RNA polymerase, anti-RNP, anti-PM-SCL, anti-Ku) [3].
Pulmonary evaluation
All included patients had an HRCT and PFT performed as follows: forced spirometry (Easy-on PC; Easy One ndd Medical Technologies, Andover, MA, EEUU). In addition, static lung volumes, airway resistance, DLco and transfer factor per unit alveolar volume (DLco/VA) (Masterlab; Jaeger, Würzburg, Germany) were determined in SSc patients using standard procedures [21].
Results were expressed as the percentage of predicted values; values < 80% predicted were considered abnormal, according to the American Thoracic Society/European Respiratory Society Standardisation of PFT [22]. Degree of severity of DLco reduction was expressed according to European Respiratory Society/American Thoracic Society guidelines [18]. Predicted values are based on Mediterranean population [23, 24].
The diagnosis of ILD was based on the presence of interstitial lung disease on HRCT, which include several patterns of abnormalities: reticular patterns, nodular patterns, cystic patterns and altered parenchymal attenuation [25].
Cardiological evaluation
All included patients had an echocardiography performed.
Echocardiography measurements were performed by a trained cardiologist. The presence of tricuspid regurgitation (TR), right atrial volume and estimated pulmonary arterial pressure (sPAP) was evaluated. PAP was estimated by velocity of tricuspid regurgitation (VTR). Following clinical practice, right and left ventricles were evaluated both morphologically and functionally (tricuspid annular plane systolic excursion (TAPSE), left ventricle ejection fraction (LVEF), right and left ventricles dimensions, interventricular septum thickness).
According to American College of Cardiology Foundation/American Heart Association (ACCF/AHA) 2009 Expert Consensus Document on Pulmonary Hypertension [26], patients with an estimated PAP > 40 mmHg show a higher PAH risk. In this study, patients with estimated PAP values > 36 mmHg were included as controls and excluded from the case group, in order to diminish the inclusion of misdiagnosed patients at high risk of PAH.
The following echocardiography measurements were collected in all patients: sPAP (mmHg) and presence of TR (yes/no). In the case group, VTR (m/s), right atrial (RA) area (cm2), functional and morphological features were evaluated.
Patients with PAH diagnosis
PAH diagnosis was confirmed with right heart catheterisation if the mean PAP (mPAP) was found to be >25 mmHg at rest, with a pre-capillary pulmonary hypertension (PH), defined by a pulmonary wedge pressure (PAWP) < 15 mmHg and a pulmonary vascular resistance (PVP) > 3 wood units in absence of other causes of pre-capillary PH [27].
Statistical analysis
Data were analysed using SPSS version 18.0 (IBM Inc). A descriptive study was performed. Results are expressed as mean and standard deviation for quantitative variables and as frequencies and percentages for qualitative variables. Cases and controls were compared by means of an unpaired t test for quantitative variables with a parametric distribution, the Mann–Whitney U test for quantitative variables with a non-parametric distribution and chi-squared or Fisher’s exact test for qualitative variables.
Results
From a total SSc cohort of 115 patients, 75 patients with SSc and DLco < 80% predicted were included (65.2%), 20 cases (26.7%) and 55 controls (73.3%). A flow chart and related data are shown in Fig. 1.
Epidemiological, clinical and immunological characteristics
Epidemiological, clinical and immunological characteristics of patients with isolated DLco reduction compared with the control group are shown in Table 1.
The mean age of cases was 62.4 ± 9.2 years; all 20 patients were female and 15% had a history of smoking. There were no significant differences between cases and controls in sociodemographic characteristics and smoking; the time since the diagnosis of SSc was also similar in both groups.
We found significant differences in disease pattern, and subsequently, in their serological profiles, with a greater proportion of cases with lSSc and ACA seropositivity than in the control group (90% vs 38%; p < 0.001, and 95% vs 40%; p > 0.001, respectively).
A lower proportion of cases had oesophageal involvement in comparison to patients from the control group (45% vs 74%; p = 0.016), without significant difference in presence of Raynaud’s phenomenon, digital ulcers, telangiectasiae or coronary artery disease.
Control group disease characteristics are shown in Fig. 1.
Pulmonary function test
For cases, the mean DLco reduction was mild and DLco mean values were higher than the controls (65.60 ± 10.56% vs 55.35 ± 11.3%). Only one out of 20 cases had normal DLco/VA values (Table 2).
Echocardiographic features
We found TR to be more frequent in the cases (85% vs 53.8%, p = 0.014) than in controls. PAP was estimated in 16 out of 20 (80%) from the case group and 23 out of 55 (41.8%) from the control group. The mean values of PAP were 26.29 ± 8.30 mmHg for cases and 39.09 ± 14.18 mmHg for controls, with a significant difference between them (p < 0.001). Cases showed a mean VTR of 2.23 m/s and a mean RA area of 12.10 cm2 (Table 2). None of the patients in cases group showed left ventricular morphology and function alterations.
Discussion
This retrospective study confirms the existence of a group of SSc patients with an isolated DLco reduction in our cohort and characterises this group. From the initial SSc cohort of 115 patients, 20 patients (17.4%), most of them with lSSc, had isolated DLco reduction of mild degree.
Former studies [5, 17] have highlighted the presence of isolated DLco reduction in SSc patients, but these are limited both in number and in patient characterisation, especially lacking HRCT data. Our patients’ prevalence of isolated DLco reduction (17%) is similar to the previously described. Recently, another study evaluates the predictive value of these isolated DLco changes [19].
Interestingly, in our study, all patients with isolated DLco reduction but one also showed low DLco/VA values. DLco/VA is an index of gas exchange efficiency, related to the alveolar–capillary surface to volume ratio. In the absence of known membrane alterations or anaemia and with normal lung volumes, DLco/VA focuses directly on the microvascular compartment [14]. Therefore, this finding may suggest the presence of a SSc subgroup with a hypothetical isolated microvascular lung involvement. To the best of our knowledge, there is only one former metaanalysis which stablishes the association between PAH and decreased DLco/VA [28].
In addition, we found the case group to be predominantly limited skin subset and ACA autoantibodies were more frequent in this group. ACA autoantibodies are well established in their relationship to lSSc, according to LeRoy’s classification [1]. Several studies found lSSc and ACA to be related to the presence of PAH [2, 17, 29]. Although vascular proliferation and occlusion had been demonstrated by autopsy in all skin subsets [29], predominance of primary pulmonary vascular disease in lSSc subset patients has been well described [1,2,3, 30]. We did not find differences in vascular manifestations such as Raynaud’s phenomenon, digital ulcers or telangiectasiae between groups. In contrast, in our study, oesophageal dysfunction was more frequent in the control group. Gastrointestinal manifestations do not usually show any differences in frequency between LeRoy’s skin subsets, with oesophageal involvement around 67–71% [2, 3].
Another point of interest is that the mean DLco and DLco/VA values in the case group were 65.6 ± 10.65% and 62.65 ± 7.35%, respectively, corresponding to a mild reduction [13]. It has been demonstrated that moderate-to-severe DLco reduction, usually < 45–55%, is the best predictor of PAH development [5, 17, 18, 31, 32]. In Colaci et al.’s prospective study, which investigates the clinical relevance of isolated DLco reduction during a follow-up period of 11 years, none of the patients with DLco values > 55% predicted developed PAH [19]. It has also been postulated previously that isolated mild DLco reduction does not indicate a poor prognosis [15].
In the DETECT study, two steps of clinical-biological (telangiectasiae, ACA, NT-proBNP, serum urate, FVC%/DLco% and right axis deviation) and echocardiographic parameters have been proposed to create an algorithm to predict the presence of early PAH [6]. The threshold of DLco values to consider and evaluate the risk of PAH begin < 60% predicted (corresponding to a moderate-to-severe DLco reduction), as it has been demonstrated that prevalence of PAH in SSc patients with a DLco >60% is very low [6, 31, 32]. Thus, the prognosis of SSc patients with isolated DLco reduction > 55–60% remains uncertain.
In contrast, retated to DLco/VA, the Avouac et al. metaanalysis confirms that SSc patients with DLco/VA < 70% are more likely to develop PAH [28]. This association has not been properly described in further studies.
On the other hand, PAH has been generally considered as a late SSc complication. When studies on SSc patients who have developed PAH have been performed, the mean disease duration at PAH onset frequently reaches 10–20 years [19, 33]. In our study, the mean time periods since first non-Raynaud’s symptoms were 9.89 ± 3.56 and 10.56 ± 5.5 years (p = 0.43). These results could suggest that the study has been performed early, so case patients have not had enough time to develop PAH and we are observing a pre-PAH state. However, Hachulla et al. also found that SSc-related PAH can occur at any time during the disease course, as approximately half of SSc patients develop early-onset PAH (less than 5 years after primary diagnosis) [34]. We did not find a statistical difference in disease duration between groups that could suggest a longer disease course to be responsible for the cardiopulmonary disease in the control group. The factor that seems to be important as a predictor of developing PAH is the stability of DLco values over time, when they are mildly decreased. Steen et al. found a progressive decline in the DLco during the years preceding the appearance of objective PAH [5].
In patients with a high risk of developing PAH, echocardiography is recognised as a useful screening tool [7, 9, 31]. To calculate PAP, VTR and RA area are needed. In our patients, we found a measurable VTR in 16 out of 20 (80%) of the case group, similar to the results from Hachulla et al. [32] and also in line with the 86% reached when a highly experienced echocardiographer performs the test [35]. These patients showed a mean VTR of 2.23 ± 0.61 m/s and a mean RA area of 12.12 ± 1.84 cm2. The European Society of Cardiology and European Respiratory Society (ESC–ERS) guidelines establish VRT ≤ 2.8 m/s and RA area ≤ 18 cm2 as values with a low probability of developing PAH [27]. Accordingly, our patients seem to be unlikely to develop PAH, despite the presence of a measurable VTR.
It is important to take into account the predominance of the lSSc subset in the case group (90%), as the relationship between the limited cutaneous subgroup and SSc-associated PAH risk is well known [3, 11, 29]. Additional tests, such as right heart catheterisation, must be carried out in these patients if clinical or echocardiographic risk features are observed, specifically if DLco values show a progressive impairment, in order to achieve earlier detection [5, 6, 27].
Our study has some limitations. This is a cross-sectional retrospective study; therefore, progressive PFT value changes cannot be evaluated. As the main determining factor of PAH development seems to be a progressive decrease of DLco levels [5], a prospective study, similar to Colaci et al.’s [19], could have been helpful to establish SSc PAH risk in these patients.
In addition, following daily clinical practice, not all patients underwent right heart catheterisation, which is considered as the gold standard test to identify PAH. As a consequence, even though we determined a high risk of PAH threshold as estimated PAP > 36 mmHg in order to diminish the underestimation, we cannot firmly declare that none of case patients had SSc-related PAH. Also, not all the echocardiographic variables suggestive for PH were available for all patients’ tests.
Conclusions
In conclusion, in our patients group, we identified a SSc patient homogeneous subgroup with isolated DLco and DLco/VA reduction, who were predominantly lSSc- with ACA-positive. In these patients, DLco reduction is mild and its prognosis remains uncertain. The DLco/VA reduction in these patients could indicate the presence of a pulmonary vascular involvement, which could hypothetically identify a particular SSc subset. It is mandatory to follow-up these patients, as a pre-PAH situation cannot be excluded. Further studies which confirm this finding and long-term prospective studies which evaluate its prognosis should be performed.
References
LeRoy EC, Black C, Fleischmajer R, Jablonska S, Krieg T, Medsger TA Jr et al (1988) Scleroderma (systemic sclerosis): classification, subsets and pathogenesis. J Rheumatol 15:202–205
Walker UA, Tyndall A, Czirják L, Denton C, Farge-Bancel D, Kowal-Bielecka O et al (2007) Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis 66:754–763
Desbois AC, Cacoub P (2016) Systemic sclerosis: an update in 2016. Autoimmun Rev 15:417–426
Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE et al (2010) Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis 69:1809–1815
Steen VD, Medsger TA (2007) Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis 66:940–944
Coghlan JG, Denton CP, Grünig E, Bonderman D, Distler O, Khanna D, Müller-Ladner U, Pope JE, Vonk MC, Doelberg M, Chadha-Boreham H, Heinzl H, Rosenberg DM, McLaughlin VV, Seibold JR, on behalf of the DETECT study group (2014) DETECT study group. Evidence-based detection of pulmonary arterial hypertension in systemic sclerosis: the DETECT study. Ann Rheum Dis 73:1340–1349
Khanna D, Gladue H, Channick R, Chung L, Distler O, Furst DE, Hachulla E, Humbert M, Langleben D, Mathai SC, Saggar R, Visovatti S, Altorok N, Townsend W, FitzGerald J, McLaughlin V, Scleroderma Foundation and Pulmonary Hypertension Association (2013) Recommendations for screening and detection of connective tissue disease-associated pulmonary arterial hypertension. Arthritis Rheum 65:3194–3201
Diot E, Boissinot E, Asquier E, Guilmot JL, Lemarié E, Valat C, Diot P (1998) Relationship between abnormalities on high-resolution CT and pulmonary function in systemic sclerosis. Chest 114:1623–1629
Vandecasteele E, Drieghe B, Melsens K, Thevissen K, De Pauw M, Deschepper E, et al. (2017) Screening for pulmonary arterial hypertension in an unselected prospective systemic sclerosis cohort. Eur Respir J; ; 49
Hao Y, Thakkar V, Stevens W, Morrisroe K, Prior D, Rabusa C et al (2015) A comparison of the predictive accuracy of three screening models for pulmonary arterial hypertension in systemic sclerosis. Arthritis Res Ther 17:7
Hassoun PM (2011) Lung involvement in systemic sclerosis. Presse Med 40:e3–e17
Steen V, Medsger TA Jr (2003) Predictors of isolated pulmonary hypertension in patients with systemic sclerosis and limited cutaneous involvement. Arthritis Rheum 48:516–522
Guarnieri G, Zanatta E, Mason P, Scarpa MC, Pigatto E, Maestrelli P, Cozzi F (2015) Determinants of impairment in lung diffusing capacity in patients with systemic sclerosis. Clin Exp Rheumatol 33(4 Suppl 91):S80–S86
Hughes JM (2003) The single breath transfer factor (TLco) and the transfer coefficient (Kco): a window onto the pulmonary microcirculation. Clin Physiol Funct Imaging 23:63–71
Peters-Golden M, Wise RA, Hochberg MC, Stevens MB, Wigley FM (1984) Carbon monoxide diffusing capacity as predictor of outcome in systemic sclerosis. Am J Med 77:1027–1034
Wilson RJ, Rodnan GP, Robin ED (1964) An early pulmonary physiologic abnormality in progressive systemic sclerosis (diffuse scleroderma). Am J Med 36:361–369
Steen VD, Graham G, Conte C, Owens G, Medsger TA Jr (1992) Isolated diffusing capacity reduction in systemic sclerosis. Arthritis Rheum 35:765–770
Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten C, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J (2005) Interpretative strategies for lung function tests. Eur Respir J 26:948–968
Colaci M, Giuggioli D, Sebastiani M, Manfredi A, Lumetti F, Luppi F et al (2015) Predictive value of isolated DLCO reduction in systemic sclerosis patients without cardio-pulmonary involvement at baseline. Reumatismo 67:149–155
Van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A et al (2013) 2013 classification criteria for systemic sclerosis: an American college of rheumatology/European league against rheumatism collaborative initiative. Ann Rheum Dis 72:1747–1755
García-Río F, Calle M, Burgos F, Casan P, Del Campo F, Galdiz JB et al (2013) Spanish society of pulmonology and thoracic surgery (SEPAR). Arch Bronconeumol 49:388–401
Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A et al (2005) ATS/ERS task force. Standardisation of spirometry. Eur Respir J 26:319–338
Roca J, Rodriguez-Roisin R, Cobo E, Burgos F, Perez J, Clausen JL (1990) Single-breath carbon monoxide diffusing capacity prediction equations from a Mediterranean population. Am Rev Respir Dis 141:1026–1032
Roca J, Burgos F, Barberà JA, Sunyer J, Rodriguez-Roisin R, Castellsagué J, Sanchis J, Antóo JM, Casan P, Clausen JL (1998) Prediction equations for plethysmographic lung volumes. Respir Med 92:454–460
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, McGoon M, Park MH, Rosenson RS, Rubin LJ, Tapson VF, Varga J, American College of Cardiology Foundation Task Force on Expert Consensus Documents, American Heart Association, American College of Chest Physicians, American Thoracic Society, Inc, Pulmonary Hypertension Association (2009) ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J Am Coll Cardiol 53:1573–1619
Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, ESC Scientific Document Group et al (2016) 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation ISHLT. Eur Heart J 37:67–119
Avouac J, Airò P, Meune C, Beretta L, Dieude P, Caramaschi P et al (2010) Prevalence of pulmonary hypertension in systemic sclerosis in European Caucasians and metaanalysis of 5 studies. J Rheumatol 37:2290–2298
Al-Sabbagh MR, Steen VD, Zee BC, Nalesnik M, Trostle DC, Bedetti CD, Medsger TA Jr (1989) Pulmonary arterial histology and morphometry in systemic sclerosis: a case-control autopsy study. J Rheumatol 16:1038–1042
Yousem SA (1990) The pulmonary pathologic manifestations of the CREST syndrome. Hum Pathol 21:467–474
Gladue H, Altorok N, Townsend W, McLaughlin V, Khanna D (2014) Screening and diagnostic modalities for connective tissue disease-associated pulmonary arterial hypertension: a systematic review. Semin Arthritis Rheum 43:536–541
Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, Kahan A, Cabane J, Francès C, Launay D, Mouthon L, Allanore Y, Tiev KP, Clerson P, Groote P, Humbert M (2005) Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum 52:3792–3800
Hsu VM, Chung L, Hummers LK, Wigley F, Simms R, Bolster M, Silver R, Fischer A, Hinchcliff ME, Varga J, Goldberg AZ, Derk CT, Schiopu E, Khanna D, Shapiro LS, Domsic RT, Medsger T, Mayes MD, Furst D, Csuka ME, Molitor JA, Alkassab F, Steen VD (2014) Development of pulmonary hypertension in a high-risk population with systemic sclerosis in the Pulmonary Hypertension Assessment and Recognition of Outcomes in Scleroderma (PHAROS) cohort study. Semin Arthritis Rheum 44:55–62
Hachulla E, Launay D, Mouthon L, Sitbon O, Berezne A, Guillevin L, Hatron PY, Simonneau G, Clerson P, Humbert M, French PAH-SSc Network (2009) Is pulmonary arterial hypertension really a late complication of systemic sclerosis? Chest 136:1211–1219
Borgeson DD, Seward JB, Miller FA Jr, Oh JK, Tajik AJ (1996) Frequency of Doppler measurable pulmonary artery pressures. J Am Soc Echocardiogr 9:832–837
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Informed consent
The study does not contain recognisable patient data. For this type of study format, consent is not required.
Additional information
Key Messages
• There is a SSc patient subgroup with isolated DLco and DLco/VA reduction, predominantly lSSc with ACA-positive.
• This DLco/VA reduction could indicate the presence of a pulmonary microvascular involvement
• The prognosis of this patients has not beet yet established; therefore, a close pulmonary follow-up is mandatory
Rights and permissions
About this article
Cite this article
Corzo, P., Pros, A., Martinez-Llorens, J. et al. Isolated DLco/VA reduction in systemic sclerosis patients: a new patient subset?. Clin Rheumatol 37, 3365–3371 (2018). https://doi.org/10.1007/s10067-018-4342-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4342-5