Abstract
Hepatitis B virus (HBV) reactivation is a common complication of immunosuppressive treatment in high prevalence countries. Biological disease-modifying antirheumatic drugs (bDMARDs) cause this adverse event more often than conventional immunosuppressants. The incidence of HBV reactivation during treatment for rheumatic diseases in Germany is unclear. Furthermore, it remains open how to treat and monitor patients at risk during immunosuppressive therapy with bDMARDs. We examined 2054 patients from a German tertiary rheumatology center in order to analyze the prevalence of HBc-antibody-positivity and the incidence of HBV reactivation in German rheumatology patients treated with immunosuppressants. Of 1317 patients treated with bDMARDs and 737 conventional synthetic DMARD (csDMARDs) patients between 2008 and 2017, 86 had a history of HBV infection (anti-HBc positive). Only two patients were suffering from chronic infection (HBsAg positive). Three patients were treated pre-emptively with entecavir, and eight patients after HBV DNA reappearance. No liver failure occurred due to HBV reactivation. Compared to anti-HBc-positive patients without reactivation, the reactivation group included more patients exposed to three or more classes of bDMARDs (p = 0.017). The median HBs antibody titer was significantly lower in the reactivation group (15.0 IU/l vs. 293.5 IU/l; p = 0.001). This study shows that bDMARDs and csDMARDs can safely be administered to patients with a history of HBV, provided they are closely monitored. Low titers of anti-HBs antibodies and a history of ≥ 3 classes of immunosuppressants increase the risk of HBV reactivation. These data highlight major differences to high prevalence regions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatitis B virus (HBV) infection represents a major public health issue with more than 400 million people chronically infected worldwide [1]. Roughly 30% of the world’s population show serological evidence of current (HBsAG positivity) or past (anti-HBc positivity) HBV infection [2, 3].
The risk of HBV reactivation is high in HBsAg-positive patients receiving chemotherapy or immunosuppressive therapy including biological disease-modifying antirheumatic drugs (bDMARDs), particularly if rituximab is given alone or in combination with glucocorticoids [4, 5]. In the majority of patients, hepatitis flares are asymptomatic, but icteric flares, hepatic decompensation, and death have been observed [6].
Two consensus statements, Smolen et al. [7] as well as Buch et al. [8], recommend mandatory HBV screening for all patients before starting rituximab therapy. Whilst both screening for prior or chronic HBV infection and close monitoring of affected patients is mandatory, prophylactic antiviral treatment remains controversial for HBs-antigen-negative patients. A main difference from oncologic diseases is that rheumatic diseases are almost always chronic in nature, resulting in lifelong antiviral therapy once the indication is confirmed. Many authors propose different intensities of immunosuppressive regimes and recommend prophylactic antiviral treatment only for intensive regimes (high-risk situation). Rituximab is usually regarded as a high-risk treatment, especially if combined with other immunosuppressants. Tang et al. report a reactivation rate of 9% for hematologic patients treated with chemotherapy including rituximab [9].
The risk posed by tumor necrosis factor α(TNFα) inhibition is less clear, especially since TNFα-inhibitors are usually combined with MTX and low-dose corticosteroids or other conventional synthetic DMARDs (csDMARDs) for the treatment of rheumatoid arthritis [10]. In a meta-analysis including 89 HBs-antigen carriers and 168 anti-HBc-positive patients treated with TNFα-inhibitors, 39% of HBs-antigen carriers and 5% of anti-HBc-positive patients experienced HBV reactivation, leading to six cases of liver failure with fatal outcomes in five of these cases [11]. In contrast, a large, prospective study with 179 HBs-antigen-negative and anti-HBc-positive Caucasian patients with rheumatic diseases from Italy detected no virologic seroreversions during bDMARD therapy (including 14 rituximab patients) [12]. None of the Italian patients were prophylactically treated with antivirals.
It has been recommended that HBsAg-negative, anti-HBc-positive patients with undetectable serum HBV DNA, regardless of anti-HBs status, who receive chemotherapy and/or immunosuppressive therapy should be monitored carefully and treated with nucleos(t)ide analogues (NUC) (pre-emptive therapy) upon confirmation of detectable HBV DNA or HBsAg seroreversion [9]. HBsAg-negative, anti-HBc-positive subjects should receive antiviral prophylaxis if they are at high risk of HBV reactivation. In subjects with moderate or low risk of HBV reactivation, pre-emptive therapy is recommended. Upon HBsAg reappearance (seroreversion), hepatitis flares are inevitable, whereas HBV DNA detection leads to seroreversion and hepatitis in only 50% of cases [13]. Many centers recommend prophylaxis with NUCs in HBsAg-negative, anti-HBc-positive patients who are anti-HBs negative (“anti-HBc-only” status) and receive rituximab and/or combined regimens for hematological malignancies.
Though the prevalence of active hepatitis B in most western countries such as Germany is low, screening for HBV infection is regarded as mandatory before starting immunosuppressive therapies using bDMARDs [9, 10]. Reactivation of HBV infections has been reported during treatment with TNFα-inhibitors and B cell depleting therapies in patients with rheumatic diseases [14,15,16]. However, HBV reactivation risk in combined or sequential immunosuppressive regimes in a rheumatologic setting has not been systematically analyzed. We performed a large-scale study in a German academic rheumatology clinic examining the HBV screening rate, the rate of prophylactic therapy, the prevalence of antibody positivity, and the incidence of HBV reactivation in patients treated with immunosuppression including bDMARDs, targeted synthetic DMARDs (tsDMARDs), and csDMARDs including low-dose methotrexate (MTX).
Patients and methods
Data collection
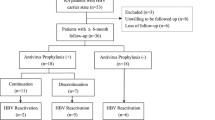
Using an electronic database (EMIL©; version 4.9.7.76; itc-ms.de), we searched our rheumatological out-patient unit for patients treated between April 2008 and April 2017. A total of 2054 patients with information on age, gender, clinical chemistry results, and rheumatologic diagnosis and therapy were included into the analysis. Since the rate of HBV screening in the collective was among the parameters analyzed, we also included patients without known HBV status (see Table 1 for screening rates in the collective). Patients on immunosuppressive therapy were evaluated for HBV screening results at any time during their treatment course. HBs antigen, anti-HBc, and anti-HBs were not regularly available in all patients treated before 2012 but were routinely determined in all patients thereafter. All current and historic screening results since 2008 have been reviewed in the analysis.
Because of the non-interventional nature of the retrospective database investigation, which was primarily based on quality assurance algorithms, ethics approval was dispensable according to German law. This was confirmed by the local ethics committee (file number 20180207/01).
All five EU-approved TNFα-inhibitors (adalimumab, certolizumab, etanercept, golimumab, and infliximab), one IL1-receptor antagonist (anakinra), one IL6-receptor-antagonist (tocilizumab), one T cell co-stimulation modulator (abatacept), an anti-CD20-antibody (rituximab), low-dose methotrexate (5 mg - 25 mg weekly), other conventional synthetic disease-modifying antirheumatic drugs (leflunomide, cyclophosphamide, cyclosporine A, azathioprine, mycophenolate, sulfasalazine), and corticosteroids were included in the analysis. We also included the Janus kinase inhibitors (Jak-inhibitor) baricitinib and tofacitinib. We refer to the Jak-inhibitors as targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs). Jak-inhibitor patients and patients treated with bDMARDs were combined into one group to avoid very small sample sizes resulting from the low number of Jak-inhibitor patients. Patients without immunosuppressive therapy were excluded from the study. csDMARD patients were only included, if the immunosuppressive therapy included MTX during at least one visit in order to exclude patients with very low-risk regimes. We evaluated patients’ age, gender, liver enzymes, migratory status, screening results regarding latent tuberculosis, rheumatologic diagnosis, and immunoglobulin G levels at baseline and at each follow-up visit.
Hepatitis B screening
All 2054 patient files were electronically searched for HBV screening results. Patients were then differentiated by immunosuppressive therapy and by serological HBV status. Records of HBc-antibody-positive patients were searched manually for treatment with polyvalent immunoglobulins to avoid false positive anti-HBc titers. These patients were excluded from the HBV group. All files of patients with a documented exposure to Hepatitis B (HBc-antibody positive) were searched manually for antiviral therapies and signs of HBV reactivation including increase of liver enzymes or HBV DNA positivity.
HBV reactivation
HBV reactivation is defined as an increase in serum HBV DNA levels accompanied by an increase in serum transaminases [17]. For patients with negative baseline HBV DNA, any subsequent positive HBV DNA level was considered as significant in the particular context of immunosuppressive therapy. According to our standard operating procedures, patients with a history of HBV infection and immunosuppressive therapy are screened for elevated HBV DNA levels every 3 to 12 months. In HBV DNA-negative patients, any de novo detection of serum HBV DNA is regarded as a possible sign of early reactivation. Using serological parameters, a formal differentiation of reactivation and de novo infection is not possible. However, all but one of the reactivation patients had negative HBV DNA before the reactivation. The affected patients are closely monitored, and preemptive antiviral therapy with a nucleot(s)ide analogue is initiated upon HBV DNA reappearance.
Statistics
IBM® SPSS® Statistics Version 24 and Microsoft® Excel® 2010 were used for the statistical analysis. Missing values were excluded from the analysis. Fisher’s exact test was used to compare patients with and without reactivation for binary variables. After using the Kolmogorov-Smirnov test to check for a normal distribution, and Levene’s test to compare the variances, Student’s t test was employed to compare the age of patients with and without reactivation, respectively. HBs antibody levels were interpreted as ordinally scaled, as values > 1000 IU/ml were not differentiated. Kendall’s Tau test was used to compare patients with and without reactivation regarding this variable. For all tests, the two-tailed significance level α was set 0.05.
Results
Overall patient characteristics
Of 2054 patients included in our study, 737 (36%) received only csDMARDs including MTX. A total of 1317 (64%) patients were treated with bDMARDs, 487 (37%) of which received rituximab at least once (Table 1). The median observational period of csDMARD patients was 5.98 years (range 0.05–12.22 years) and 7.83 years (range 0.13–14.09 years) among bDMARD patients. About half of the patients in our study suffered from rheumatoid arthritis, and the remaining patients suffered from various other inflammatory rheumatic diseases including psoriatic arthritis, spondyloarthritis, vasculitis, and connective tissue diseases. HBV screening rate was 89% among patients receiving bDMARDs/tsDMARDs and 65% among patients treated only with csDMARDs, resulting in an overall screening rate of 81%. Among tested patients, 86 patients (5%) had serologic evidence of former HBV infection (anti-HBc positive), two of which were HBs-antigen-positive and thus chronically active. Only three patients, including the two HBs-antigen-positive patients mentioned above, received prophylactic antiviral treatment during immunosuppressive therapy. There were no HCV coinfections. We did not specifically look for vaccination titers, but according to our data, the estimated HBV vaccination rate in the cohort is about 2%.
Anti-HBc-positive patients
After exclusion of two patients with chronic HBV infection (HBs-antigen positive), 84 patients with HBc-antibodies were analyzed in further detail (Table 2). Of those 89% who were HBs antibody positive with a median titer of 293.5 IU/l, the remaining patients were HBs antibody negative and thus “anti-HBc only.” Only one out of 84 patients received prophylactic antiviral therapy, no patient developed liver failure for any reason including HBV reactivation. In accordance with other studies of patients with rheumatic diseases, 73% were female. Thirty-one percent had migrated to Germany from a country with a high prevalence of HBV, mainly from the former Soviet Union. About 80% were ever treated with MTX, corticosteroids, and/or other csDMARDs, respectively. Half of the patients ever received TNFα-inhibitors, whereas one fourth received rituximab at least once. Eight percent were treated with multiple immunosuppressive drugs, including three or more different types of bDMARDs sequentially. All patients were monitored by polymerase chain reaction for reappearance of HBV DNA. Eight patients (9.5% of HBs-antigen-negative and anti-HBc-positive patients) showed virological signs of HBV DNA seroreversion during the observational period. Three of these patients also had a concomitant increase in serum transaminases. No cases of HBs-antigen seroreversion occurred.
HBV reactivation
Seven patients developed DNA seroreversion with a de novo detection of HBV DNA during immunosuppressive therapy, all of which received antiviral therapy with entecavir resulting in subsequently negative DNA (Table 3). One patient (patient 4) first presented at our university clinic with a discretely positive HBV DNA titer, which increased under immunosuppressive therapy with MTX. He was then treated with entecavir, upon which hepatitis B DNA was no longer detectable. All eight patients were HBs-antigen-negative throughout the whole observation period and had low or negative anti-HBs antibody titers (ranging from negative to 65 IU/ml). Three patients showed elevated ALT levels, while no increase in bilirubin levels or signs of liver failure was observed. Two patients were treated with infliximab, while one patient received adalimumab, one patient received rituximab, one patient tocilizumab, and one patient tofacitinib, while two patients were treated with csDMARDs only. Patient 1 (Table 3) was treated with tofacitinib, leflunomide, and a low dose of prednisone when HBV DNA reappeared. Tofacitinib was discontinued, while the patient remained on leflunomide. This patient remained HBV DNA-positive until entecavir was started 17 months later, before adalimumab was given for active rheumatoid arthritis. In all other patients, the immunosuppressive therapy was continued without interruptions. The lag time between initiation of a given immunosuppressive therapy and the first sign of HBV reactivation differs widely from 4 to 372 months.
We compared patients with and without HBV DNA reversion to establish risk factors for HBV reactivation under immunosuppression (Table 2). Both groups have a similar age and sex distribution. Migration from a country with high HBV prevalence was slightly more common in the reactivation group without reaching statistical significance (p = 0.111). Both groups had a roughly equal exposure to csDMARDs and bDMARDs, including rituximab. However, the reactivation group included significantly more patients exposed to three or more classes of bDMARDs (p = 0.017). The median HBs antibody titer was significantly lower in the reactivation group than in anti-HBc-positive patients without reactivation (15.0 IU/l vs. 293.5 IU/l; p = 0.001) (Fig. 1). All other HBV seroparameters did not differ significantly. Remarkably, the proportion of anti-HBc-only patients (absence of anti-HBs and HBsAg) was 11% in both groups.
Discussion
International guidelines describe indications for antiviral prophylaxis for HBs-antigen-positive patients [9], but there is no uniform consensus regarding the management of HBs-antigen-negative and anti-HBc-positive patients under immunosuppressive therapies. These patients are treated according to an estimation of their individual risk: immediate (prophylactic) treatment for high risk, pre-emptive therapy, not prophylaxis, for moderate to low risk [9]. In contrast to most publications focusing on oncology patients, we here present retrospective data regarding HBV screening rates, application of prophylactic antiviral therapy, and HBV reactivation from a large German cohort of patients treated with various DMARDs for inflammatory rheumatic diseases. Oncology patients—especially in hematology–are subject to more aggressive immunosuppressive therapies than rheumatology patients. Their reactivation risk is thus much higher. The present study is representing the clinical situation in a country with universal health care and low HBV prevalence. Our cohort has a prevalence of previous HBV infection of 5%, which corresponds to data from Feuchtenberger et al. who report a prevalence of 5.9% [18]. A large analysis of electronic health records of rituximab patients from California shows screening rates between 61 and 90% [19]. Other studies report lower screening rates [20, 21]. Our own cohort of 2054 patients has an overall screening rate of 81%, which rises to 89% if only bDMARD patients are taken into account. In the setting of a developed country, HBV screening should nowadays be regarded as mandatory before initiating immunosuppressive therapy. To increase our own screening rates to 100%, we have implemented checklists in our electronic patient files as one consequence of this quality control measure.
Our data indicates a virologic reactivation rate of 9.5% among HBc-antibody-positive patients with no cases of liver failure or death due to HBV reactivation. Our patients were screened for HBV DNA on a regular basis, and entecavir therapy was initiated once HBV DNA could be detected. In our analysis, rituximab and TNFα-inhibitors did not differ significantly in their potential for HBV reactivation. However, our data show an increased risk of HBV reactivation in patients having received more than three different classes of bDMARDs. This finding is supported by Loras et al., who report treatment with more than two different immunosuppressants as an independent risk factor for HBV reactivation in inflammatory bowel disease patients [22]. It is unclear whether all TNFα inhibiting agents carry the same risk of HBV reactivation. In a review, Carroll and Forgione [23] suggest that infliximab might pose a more severe risk than other TNFα-inhibitors, but this data is not comprehensive. Of 992 patients receiving TNFα-inhibitors in our analysis, three patients showed signs of HBV reactivation: one with adalimumab and two with infliximab.
As the most striking finding from our study, low anti-HBs titers were a strong risk factor for HBV reactivation in our cohort. Among patients with anti-HBs below 100 IU/l in our study, the risk of virological reactivation was 27%. The protective role of anti-HBs in HBsAg-antigen-negative patients is being debated [24], though Koo et al. report an anti-HBc only status as a risk factor for HBV reactivation during poly-chemotherapy containing rituximab in lymphoma patients [25]. Very recent data from Asia in patients with hematological malignancy confirms our finding: Of 1676 patients with negative HBs antigen, 41 (2.4%) experienced HBV reactivation, and a multivariate analysis revealed that low anti-HBs titers (p = 0.016) were an independent risk factor of HBsAg seroconversion [26]. As proposed by our data, there seems to be a cut-off for anti-HBs titers. Out of 77 lymphoma patients with history of HBV infection, ten patients developed HBV reactivation during and following chemotherapy [27]. In this study, univariate and multivariate logistic regression analyses demonstrated that anti-HBc and anti-HBs titers at baseline were significant predictors of HBV reactivation. Furthermore, patients with low anti-HBs titers were significantly more likely to experience HBV reactivation than those with high anti-HBs titers (> 28 mIU/ml). As an important conclusion from this and from our report, patients with an anti-HBs titer below a certain cut-off need to be viewed as high risk with regard to HBV reactivation under immunosuppression. Prophylactic antiviral therapy might be considered in these anti-HBc-positive patients with low anti-HBs titers under intensive immunosuppressive regimes. As another important issue to consider, several authors found decreasing anti-HBs antibody titers in patients treated with TNFα-inhibitors [28] or rituximab [24]. Since we did not longitudinally measure anti-HBs titers in every patient, we are unable to confirm this result. As another consequence from our findings, a serious consideration might be to vaccinate patients with incomplete seroconversion (anti-HBc positive and low or negative anti-HBs titer) in order to decrease the risk of HBV reactivation. This would have to be done before treatment with rituximab, which is known to severely decrease vaccination responses [29].
Our study is limited by the retrospective nature of the data collection. Only 65% of cDMARD patients and 89% of bDMARD patients were screened for HBV infection or vaccination, which further limits the results. Our retrospective findings thus need to be confirmed in future prospective clinical trials before being implemented in clinical guidelines and treatment decisions.
Based on our own and recently published data from Asian hematologic patients, prophylactic antiviral therapy may not be particularly beneficial to anti-HBc-positive patients with negative HBs antigen in a rheumatologic setting. Future clinical decision making may not only be based on the intensity of immunosuppression but also on the level of anti-HBs titers.
References
Lai CL, Ratziu V, Yuen MF, Paynard T (2003) Viral hepatitis B. Lancet 362(9401):2089–2094
Trépo C, Chan HL, Lok A (2014) Hepatitis B virus infection. Lancet 384(9959):2053–2063
MacLachlan JH, Locarnini S, Cowie BC (2015) Estimating the global prevalence of hepatitis B. Lancet 386(10003):17–23 1515–1517
Cornberg M, Protzer U, Petersen J, Wedemeyer H, Berg T, Jilg W (2011) Prophylaxis, diagnosis and therapy of hepatitis B virus infection—the German guideline. Z Gastroenterol 49(7):871–930
European Association for the Study of the Liver (2017) EASL 2017 clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 67:370–398
Lok ASF, McMahon BJ (2009) AASLD practice guideline update: chronic hepatitis B: update 2009. Hepatology 50(3)
Smolen JS, Keystone EC, Emery P, Breedveld FC, Betteridge N, Burmester GR, Dougados M, Ferraccioli G, Jaeger U, Klareskog L, Kvien TK, Martin-Mola E, Pavelka K, Working Group on the Rituximab Consensus Statement (2007) Consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 66:143–150
Buch MH, Smolen JS, Betteridge N, Breedveld FC, Burmester G, Dörner T et al (2011) Updated consensus statement on the use of rituximab in patients with rheumatoid arthritis. Ann Rheum Dis 70:909–920
Tang Z, Li X, Wu S, Liu Y, Qiao Y, Xu D, Li J (2017 Sep) Risk of hepatitis B reactivation in HBsAg-negative/HBcAb-positive patients with undetectable serum HBV DNA after treatment with rituximab for lymphoma: a meta-analysis. Hepatol Int 11(5):429–433
Singh JA, Saag KG, Bridges JRL, Akl EA, Bannuru RR, Sullivan MC et al (2016) 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 68(1):1–26
Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M et al (2011) Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy. Medicine 90(6)
Barone M, Notarnicola A, Lopalco G, Viggiani MT, Sebastiani F, Covelli M, Iannone F, Avolio AW, di Leo A, Cantarini L, Lapadula G (2015) Safety of long-term biologic therapy in rheumatologic patients with a previously resolved hepatitis B viral infection. Hepatology 62:40–46
Mozessohn L, Chan KK, Feld JJ, Hicks LK (2015) Hepatitis B reactivation in HBsAg negative/HBcAb-positive patients receiving rituximab for lymphoma: a meta-analysis. J Viral Hepat 22:842–849
Ostuni P, Botsios C, Punzi L, Sfriso P, Todesco S (2003) Hepatitis B reactivation in a chronic hepatitis B surface antigen carrier with rheumatoid arthritis treated with infliximab and low dose methotrexate. Ann Rheum Dis 62:686–687
Lee YH, Bae SC, Song GG (2013) Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol 31(1):118–121
Tien YC, Yen HH, Chiu YM (2017) Incidence and clinical characteristics of hepatitis B virus reactivation in HBsAg-negative/HBcAb-positive patients receiving rituximab for rheumatoid arthritis. Clin Exp Rheumatol 35(5):831–836
Perrillo RP, Gish R, Falck-Ytter YT (2015) American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 148(1):221–244.e3
Feuchtenberger M, Schaefer A, Nigg AP, Kraus MR (2016) Hepatitis B serology in patients with rheumatic diseases. Open Rheumatol J 10:39–48
Schmajuk G, Tonner C, Trupin L, Li J, Urmimala S, Ludwig D et al (2017) Using health-system-wide data to understand hepatitis B virus prophylaxis and reactivation outcomes in patients receiving rituximab. Medicine 96:13
Paul S, Shuja A, Tam I, Kim EM, Kang S, Kapulsky L, Viveiros K, Lee H (2016) Gastroenterologists have suboptimal hepatitis B virus screening rates in patients receiving immunosuppressive therapy. Dig Dis Sci 61(8):2236–2241
van der Have M, Belderbos TD, Fidder HH, Leenders M, Dijkstra G, Peters CP, Eshuis EJ, Ponsioen CY, Siersema PD, van Oijen M, Oldenburg B, Dutch Initiative on Crohn and Colitis (ICC) (2014) Screening prior to biological therapy in Crohn’s disease: adherence to guidelines and prevalence of infections. Results from a multicentre retrospective study. Dig Liver Dis 46(10):881–886
Loras C, Gisbert JP, Mínguez M, Merino O, Bujanda L, Saro C et al (2010) Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut 59(10):1340–1346
Carroll MB, Forgione MA (2010) Use of tumor necrosis factor α inhibitors in hepatitis B surface antigen-positive patients: a literature review and potential mechanisms of action. Clin Rheumatol 29:1021–1029
Gonzalez S, Perillo RP (2016) Hepatitis B virus reactivation in the setting of cancer chemotherapy and other immunosuppressive drug therapy. Clin Infect Dis 62(S4):S306–S313
Koo YX, Tay M, The YE, Teng D, DSW T, Tan IBH et al (2011) Risk of hepatitis B virus (HBV) reactivation in hepatitis B surface antigen negative/hepatitis core antibody positive patients receiving rituximab-containing combination chemotherapy without routine antiviral prophylaxis. Ann Hematol 90:1219–1223
Chen CY, Tien FM, Cheng A, Huang SY, Chou WC, Yao M et al (2018) Hepatitis B reactivation among 1962 patients with hematological malignancy in Taiwan. BMC Gastroenterol 18(1):6
Matsubara T, Nishida T, Shimoda A, Shimakoshi H, Amano T, Sugimoto A et al (2017) The combination of anti-HBc and anti-HBs levels is a useful predictor of the development of chemotherapy-induced reactivation in lymphoma patients with resolved HBV infection. Oncol Lett 14(6):6543–6552
Vassilopoulos D, Apostolopoulou A, Hadziyannis E, Papatheodoridis GV, Manolakopoulos S, Koskinas J, Manesis EK, Archimandritis AI (2010) Long-term safety of anti-TNF treatment in patients with rheumatic diseases and chronic or resolved hepatitis B virus infection. Ann Rheum Dis 69:1352–1355
Nazi I, Kelton JG, Larché M, Snider DP, Heddle NM, Crowther MA et al (2013) The effect of rituximab on vaccine responses in patients with immune thrombocytopenia. Blood 122:1946–1953
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E.C. Schwaneck, H-P. Tony, O. Gadeholt, and M. Schmalzing have received travel grants, speaker’s fees, research grants, or compensation for board membership and consultancies from Celgene AbbVie, Baxalta (Shire), Chugai, Roche, Janssen, Pfizer, MSD, UCB, Novartis, and Lilly. S. Kreissl-Kemmer received a travel grant from Daiichi Sankyo. M. Krone has no competing interests. J. Weiss has received travel grants, speaker’s fees, or compensation for board membership and consultancies from AbbVie, BMS, and Gilead. A. Geier has received travel grants, speaker’s fees, research grants, or compensation for board membership and consultancies from AbbVie, BMS, Gilead, Janssen, Falk, Sequana, and Novartis. B. Weissbrich has no competing interests.
Additional information
Key messages
• Patients with low anti-HBs antibodies have a higher risk for HBV reactivation.
• Multiple successive, immunosuppressive therapies result in a high risk of HBV reactivation.
• Most patients with a history of HBV can safely receive antirheumatic therapies.
Rights and permissions
About this article
Cite this article
Schwaneck, E.C., Krone, M., Kreissl-Kemmer, S. et al. Management of anti-HBc-positive patients with rheumatic diseases treated with disease-modifying antirheumatic drugs—a single-center analysis of 2054 patients. Clin Rheumatol 37, 2963–2970 (2018). https://doi.org/10.1007/s10067-018-4295-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-018-4295-8