Abstract
We evaluated inflammation at the small joints of feet in psoriasis patients without clinical arthritis (PsO) as against clinically overt psoriatic arthritis (PsA) patients, using a low field magnet extremity MRI (eMRI). Patients with psoriasis recruited from dermatology and rheumatology clinics of a tertiary care institution in southern India were divided into PsO and PsA groups. Demographic and physical examination details were recorded. Consenting patients underwent non-contrast eMRI of the right foot. Two trained readers scored the MRI parameters of inflammation (synovitis, tenosynovitis, osteitis) using a modification of the PsA magnetic resonance imaging score (PsAMRIS). Proportion of patients with any sign of MRI inflammation was noted. Clinical variables were compared with inflammation scores for any association. A total of 83 patients (30 PsA and 53 PsO), with 75% males and mean age of 42.2 ± 11.6 years were included. There was no statistical difference between the median eMRI inflammatory scores in PsA and PsO patients (p = 0.493). Evidence of inflammation was present in 33.9% and 50% patients in the PsO and PsA groups, respectively. Early arthritis for psoriatic patients screening questionnaire (EARP) score of ≥ 3 was significantly associated with imaging features of inflammation in PsO group (p = 0.044). This study corroborates a high proportion of subclinical inflammation in small joints of foot in PsO patients, which needs to be reproduced in larger, longitudinal cohorts to predict risk factors for progression to future PsA development.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Psoriatic arthritis (PsA) is a chronic, multi-system, potentially rapidly progressive disease, associated with cutaneous and nail psoriasis in 7–42% of patients, and manifesting as peripheral or axial arthritis, along with enthesitis and dactylitis [1,2,3]. Reported incidence and prevalence rates of PsA show wide variability across populations due to heterogeneity of the disease and lack of standardized classification criteria. With the development and validation of classification criteria for psoriatic arthritis (CASPAR) [4], PsA has been well characterized, and shown to be occurring in 0.3–1% of the population, similar to rheumatoid arthritis [5]. PsA is known to cause significant psychosocial and functional impairment in patients [6, 7]. Added to this, the substantial burden of comorbidities namely, type 2 diabetes mellitus, metabolic syndrome, insulin resistance, fatty liver, and increased cardiovascular risk associated with PsA increases the global burden of this disease [8].
Early diagnosis and treatment of PsA is important for better long-term outcomes, both in terms of structural joint damage, as well as quality of life and long-term physical function [9, 10]. Transposing the “treat-to-target” paradigm from rheumatoid arthritis to PsA has been proven to benefit joint outcomes significantly in early disease [11]. Identification of risk factors for progression from skin to joint disease in patients with psoriasis is an evolving process. Advanced imaging techniques have enabled detection of PsA before clinical presentation, thus providing a therapeutic window of opportunity.
Subclinical enthesitis detected by ultrasound has been shown to have aggressive underlying inflammatory or vascular process in PsA as compared to those with cutaneous psoriasis alone [12]. Magnetic resonance imaging (MRI) is efficacious in early diagnosis and monitoring disease activity in PsA [13]. Subclinical inflammatory lesions in hand joints of patients with psoriasis, detected by MRI is associated with development of future PsA [14, 15]. MRI has demonstrated characteristic nail changes in psoriasis patients even in the absence of clinically evident onychopathy [16]. MRI STIR sequence is sensitive in demonstrating bone edema, soft tissue edema, and tenosynovitis [17]. Prevalence of inflammatory MRI lesions in the feet has been noted to be high in patients with pure cutaneous psoriasis [18]; but comprehensive MRI studies are lacking.
The present study sought to evaluate inflammation at the small joints of feet in a subset of patients with psoriasis without clinical arthritis, using an office-based extremity MRI (eMRI), as compared to findings in overt PsA patients.
Methods
Patients
Patients (> 16 years) with a diagnosis of psoriasis, attending dermatology clinics of a tertiary care teaching institution in southern India were referred to a combined PsA clinic for detailed musculoskeletal evaluation. After physical examination, patients were divided into PsA (fulfilling CASPAR criteria) and cutaneous psoriasis (PsO) without any evidence of clinical arthritis. Institutional review board and ethics committee approval was sought prior to commencement of this study.
Clinical examination
Clinical and demographic details of these patients were noted after obtaining informed consent. Demographic characteristics included age, sex, body mass index, duration of skin/nail disease, duration of arthritis, type of arthritis, and family history. Physical examination on all domains of psoriatic disease, including peripheral joint activity (68 tender and 66 swollen joint counts), skin activity (psoriasis area and severity index i.e. PASI score), dactylitis assessment (active dactylitis score), enthesitis (Leeds enthesitis index), nail assessment (NAPSI score), spine assessment (modified Schober test), and quality of life assessment (SF-36 form and Indian health assessment questionnaire) were performed by a rheumatologist. PsO patients completed the early arthritis for psoriatic patients screening questionnaire (EARP) before consulting the rheumatologist. C-reactive protein (CRP) was measured at the time of recruitment.
MRI sequences and protocols
Non-contrast MRI scans of the right foot were performed on consenting patients with a 0.2T Esaote C-scan (Genova, Italy) dedicated extremity scanner. Standard protocols were applied for all the scans. T2-weighted axial image (turbo spine echo -TSE) sequences [field of view (FOV)-160 mm, echo time (TE) -80 ms, repetition time (TR)-2880 ms, and slice thickness-3 mm], and gradient echo (GE) short-tau inversion recovery (STIR) axial images [FOV-160 mm, TE-20 ms, TR-2700 ms, and slice thickness -3.5 mm] were used to assess synovitis and tenosynovitis. GE-STIR coronal images [FOV160 mm, TE20 ms, TR900 mm, and slice thickness 4 mm] were used to define bone edema (osteitis).
MRI scoring
Two blinded and independent readers (AJM, PB) interpreted and scored the MRI scans. Both had expertise in assessing the modified outcome measures in rheumatology clinical trials (OMERACT) PsAMRI score (PsAMRIS) and scored for MRI inflammation (synovitis, tenosynovitis, and osteitis) [19].
Metatarsophalangeal joints [MTPs (1-5)] and interphalangeal joint [IP (1)] were assessed for tenosynovitis and synovitis and the proximal and distal ends of each phalanx were assessed for osteitis. As no contrast was used for any of the scans, thickness of synovitis and tenosynovitis were measured and graded on a 0–3 scale, which included absent, mild, moderate, and severe thickening of synovial membrane by thirds of the maximum synovial thickness and fluid around the tendons. Similar grades were applied for osteitis (0–3), corresponding to thirds of bone assessed and showing increased water content. In the absence of contrast enhancement, synovitis and tenosynovitis scores can have high false positivity. To mitigate against this problem we included only the PsAMRI synovitis and tenosynovitis scores > 1 in each region (MTP and IP) for analysing both groups. Proportion of patients with any sign of MRI inflammation was noted.
Statistical analysis
Sample size for this study was calculated based on a study by Erdem et al. examining MRI of feet in patients with PsO without any symptoms of clinical arthritis, which noted 92% of 26 patients having inflammatory changes identified by MRI, and no abnormalities in the healthy control group. [18] To reproduce a similar result in PsO without clinical arthritis, with 80% power and 7.5% level of significance, the required sample size was calculated to be 53.
Demographic, clinical and MRI data were entered and analyzed using SPSS software for statistics (IBM SPSS v.21.0, IBM Corporation, Armonk, New York, USA). Continuous variables are depicted as median (IQR) or mean ± SD, and categorical variables as numbers and percentages. Mann-Whitney U test was used to compare inflammation scores of PsO with PsA patients. Inter-class coefficient was calculated to assess inter-reader reliability in detecting inflammatory MRI pathologies. Clinical variables were compared with inflammation scores for any association. p values of < 0.05 were considered as statistically significant.
Results
A total of 83 patients (30 PsA and 53 PsO) were included; 75% were males and mean age was 42.2 ± 11.6 years. Baseline demographic and clinical characteristics of patients are depicted in Table 1. Median duration of skin and joint involvement in the PsO and PsA groups were 7 years and 1 year, respectively. There was a positive family history in 26% of PsO group. Patient global assessment, PASI and NAPSI in the PsO group reflected mild disease activity. Maximum score in the EARP questionnaire was 4. Enthesitis was present in half the patients with PsA. High sensitive CRP (hsCRP) was normal in all the PsO patients.
Inter-reader agreement
For intra-class correlation between the scores, both readers scored 42 scans randomly. The remaining scans were scored by a single reader. ICC between the readers is represented in Table 2 . There was very good correlation between the readers for all the inflammatory variables. Osteitis scores ICC (.881) was marginally lower as compared to the other two scores.
Inflammatory lesions in MRI
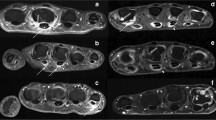
MRI inflammation was present in 33.9% of PsO patients and 50% of PsA patients (Figs. 1 and 2 ). The median inflammatory score in PsA patients was 1.5 (IQR 1.5–3.0) (Table 3 ). This corresponded well with the low patient global assessment scores. Univariate analysis using Mann-Whitney U test (Table 4 ) showed significant association between EARP questionnaire score of ≥ 3 and imaging features of inflammation (p = 0.044) in the PsO arm.
Discussion
This study demonstrated imaging signs of inflammation in a third of PsO patients without arthritis, dactylitis, or enthesitis, as per a low field, dedicated eMRI without contrast enhancement. This finding is greater than the findings expected in a control group. A recent meta-analysis evaluating the prevalence of MRI features in symptom-free persons using the rheumatoid arthritis MRI score (RAMRIS) at MCPs has described presence of osteitis in 9.5% and synovitis in up to 4.8% of individuals [20]. The concept of subclinical inflammation in psoriatic disease is still in the evolutionary state and the findings from present study needs to be interpreted in the light of scarcity of data in this field. Presence of MRI inflammatory lesions in these patients cannot be considered as markers for development of arthritis at this stage, neither do they warrant therapeutic intervention. However, the MRI findings are interesting in terms of evolution of pathogenesis of the disease. Additionally, these patients are being followed up longitudinally to study MRI-based risk factors that could predict development of future arthritis in this group.
Erdem et al. imaged the feet of 26 PsO patients without clinical arthritis using a 1.5T magnet and observed joint effusion/synovitis in 46%, tenosynovitis in 19%, and plantar fascia enthesopathy in 15% patients using non-contrast STIR and T2W images [18]. Ultrasound studies have addressed this concept in a more robust way. Freeston et al. have reported knee (21.4%) and MTP (26.5–33.7%) synovitis in very early PsA patients [21]. Naredo et al. compared 162 patients with plaque PsO with 60 age-matched controls using power Doppler ultrasound (PDUS). US enthesopathy was noted in 7.4% psoriatic patients, while none in the control group had similar finding [22].
Clinical correlations with the MRI-based inflammation findings have not been studied extensively. In the present study, inflammatory lesions in MRI were associated only with an EARP score of ≥ 3 in the PsO group. Inflammation in MRI was not associated with duration or intensity of skin lesions (PASI scores). In a recent study, Mishra et al. compared four validated PsA screening questionnaires to diagnose arthritis in patients with PsO and EARP to have the best sensitivity [23]. The findings of present study are in line with this clinical observation. A larger sample size may be needed to address the issue of clinical associations with imaging characteristics in PsO. Also, surprisingly there was no difference in the inflammatory features in both the groups (PsO vs. PsA). This may again be due to the small sample size studied, and also because of mild disease activity in PsA patients. The upcoming entity of whole-body MRI (WBMRI) may evolve as a comprehensive method to demonstrate inflammatory changes throughout the body and correlate with clinical characteristics. Preliminary studies have come up with global inflammation scores using WBMRI in PsA patients [24].
In their longitudinal analysis, Faustini et al. observed that subclinical MRI based inflammation can considerably predict risk of patients with PsO progressing to PsA [14]. However, the mean follow-up period in this study was 426 ± 88 days. Event rates of patients with psoriasis evolving into PsA are very low. Hence, more studies are needed to address the predictive ability of these inflammatory lesions for future development of clinical arthritis.
Findings of the present study need to be interpreted cautiously, keeping in mind its limitations. The sample size was small to answer association of imaging variables with clinical features. Although inflammation is not well discriminated by low field magnets, overall this user-friendly scanner produces clinically appreciable results [25]. Absence of contrast enhancement in this study may have further affected reporting of synovitis and tenosynovitis. This has, however, been addressed by eliminating low scores of synovitis and tenosynovitis during analysis. Future studies should define threshold for clinically significant inflammatory findings.
The major strength of this study is perhaps addressing a research area with scarcity of data, as well as adding new data to the existing evidence of subclinical inflammatory findings in PsO patients without arthritis. A composite index to predict onset of PsA encompassing genetics, imaging variables, inflammatory markers, and cytokine profile, along with clinical characteristics at baseline is a future goal.
Conclusion
In conclusion, this study has provided valuable information into the concept of subclinical arthritis in psoriatic patients without clinical arthritis, demonstrating high prevalence of MRI-based inflammation in this subgroup.
References
Gladman DD (2005) Psoriatic arthritis: epidemiology, clinical features, course and outcome. Ann Rheum Dis 64(Suppl2):ii14–ii17
Zachariae H (2003) Prevalence of joint disease in patients with psoriasis: implications for therapy. Am J Clin Dermatol 4:441–447
Scarpa R, Oriente P, Pucino A, Torella M, Vignone L, Riccio A et al (1984) Psoriatic arthritis in psoriatic patients. Br J Rheumatol 23:246–250
Taylor W, Gladman D, Helliwell P, Marchesoni A, Mease P, Mielants H, CASPAR Study Group (2006) Classification criteria for psoriatic arthritis: development of new criteria from a large international study. Arhtritis Rheum 54:2665–2673
Catanoso M, Pipitone N, Salvarani C (2012) Epidemiology of psoriatic arthritis. Reumatismo 64:66–70
Lewinson RT, Vellerand IA, Lowerison MW, Parsons LM, Frolkis AD, Kaplan GG et al (2017) Depression is associated with an increased risk of psoriatic arthritis among patients with psoriasis: a population-based study. J Invest Dermatol 137:828–835
Kavanaugh A, Helliwell P, Ritchlin CT (2016) Psoriatic arthritis and burden of disease: patient perspectives from the population-based Multinational Assessment of Psoriasis and Psoriatic Arthritis (MAPP) Survey. Rheumatol Ther 3:91–102
Ogdie A, Schwartzman S, Husni ME (2015) Recognizing and managing comorbidities in psoriatic arthritis. Curr Opin Rheumatol 27:118–126
Haroon M, Gallagher P, FitzGerald O (2015) Diagnostic delay of more than 6 months contributes to poor radiographic and functional outcome in psoriatic arthritis. Ann Rheum Dis 74:1045–1050
Kirkham B, de Vlam K, Li W, Boggs R, Mallbris L, Nab HW et al (2015) Early treatment of psoriatic arthritis is associated with improved patient-reported outcomes: findings from the etanercept PRESTA trial. Clin Exp Rheumatol 33:11–19
Coates LC, Moverley AR, McParland L, Brown S, Navarro-Coy N, O’Dwyer JL et al (2015) Effect of tight control of inflammation in early psoriatic arthritis (TICOPA): a UK multicentre, open label, randomized controlled trial. Lancet 386:2489–2498
Aydin SZ, Ash ZR, Tinazzi I, Castillo-Gallego C, Kwok C, Wilson C et al (2013) The link between enthesitis and arthritis in psoriatic arthritis: a switch to a vascular phenotype at insertions may play a role in arthritis development. Ann Rheum Dis 72:992–995
Mathew AJ, Coates LC, Danda D, Conaghan PC (2017) Psoriatic arthritis: lessons from imaging studies and implications for therapy. Exp Rev Clin Immunol 13:133–142
Faustini F, Simon D, Oliveira I, Kleyer A, Haschka J, Englbretcht M et al (2016) Subclinical joint inflammation in patients with psoriasis without concomitant psoriatic arthritis: a cross-sectional and longitudinal analysis. Ann Rheum Dis 75:2068–2074
Mathew AJ, Panwar J, Bird P, George R, Danda D (2016) Utility of extremity magnetic resonance imaging (eMRI) without contrast enhancement in detecting preclinical psoriatic arthritis (abstract). Arthritis Rheumatol 68 (suppl 10)
Soscia E, Sirignano C, Catalano O, Atteno M, Costa L, Caso F et al (2012) New developments in magnetic resonance imaging of the nail unit. J Rheumatol 89:49–53
Ghanem N, Uhl M, Pache G, Bley T, Walker UA, Langer MMRI (2007) In psoriatic arthritis with hand and foot involvement. Rheumatol Int 27:387–393
Erdem CZ, Tekin NS, Sarikaya S, Erdem LO, Gulec SMR (2008) Imaging features of foot involvement in patients with psoriasis. Eur J Radiol 67:521–525
Glinatsi D, Bird P, Gandjbakhch F, Mease PJ, Boyesen P, Peterfy CG et al (2015) Validation of the OMERACT psoriatic arthritis magnetic resonance imaging score (PsAMRIS) for the hand and foot in a randomized placebo-controlled trial. J Rheumatol 42:2473–2479
Mangnus L, Schoones JW, van der Helm-van Mil AH (2015) What is the prevalence of MRI-detected inflammation and erosions in small joints in the general population? A collation and analysis of published data. RMD Open 1:e000005
Freeston JE, Coates LC, Nam JL, Moverley AR, Hensor EM, Wakefield RJ et al (2014) Is there subclinical synovitis in early psoriatic arthritis? A clinical comparison with gray-scale and power Doppler ultrasound. Arthritis Care Res (Hoboken) 66:432–439
Naredo E, Moller I, de Miguel E, Batlle-Gualda E, Acebes C, Brito E et al (2011) High prevalence of ultrasonographic synovitis and enthesopathy in patients with psoriasis without psoriatic arthritis: a prospective case-control study. Rheumatology (Oxford) 50:1838–1848
Mishra S, Kancharla H, Dogra S, Sharma A (2017) Comparison of four validated psoriatic arthritis screening tools in diagnosing psoriatic arthritis in patients with psoriasis (COMPAQ study). Br J Dermatol 176:765–770
Poggenborg RP, Pedersen SJ, Eshed I, Sorensen IJ, Moller JM, Madsen OR et al (2015) Head-to-toe whole-body MRI in psoriatic arthritis, axial spondyloarthritis and healthy subjects: first steps towards global inflammation and damage scores of peripheral and axial joints. Rheumatology (Oxford) 54:1039–1049
Mathew AJ, Bird P (2015) Utility of in-office extremity magnetic resonance imaging in rheumatology. Indian J Rheumatol 10:140–146
Acknowledgements
We are grateful to the patients who participated in this research study. We thank Rasu E for his role in performing the MRI scans. This work was presented as a poster at the EULAR 2017 in Madrid, Spain.
Funding
This study is supported by the Asia Pacific League of Associations for Rheumatology (APLAR) Research Grant 2015 and Christian Medical College, Vellore Institutional fluid grant.
Author information
Authors and Affiliations
Contributions
AM was responsible for planning, execution, patient examination, MRI scoring, preparing the initial manuscript draft and completing manuscript revision. PB was the second scorer for MRI and critically revised the manuscript. AG participated by recruiting patients for this study and critically revising the manuscript. RG helped in design of the study and critically revised the manuscript. DD acted as the supervisor for this study, helping in designing, interpreting data and critically revising the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Disclosures
None.
Ethics approval and registration
Christian Medical College Institution Review Board and Ethics Committee (IRB Min. No 9543 dt 22.07.15, CMC Vellore).
Rights and permissions
About this article
Cite this article
Mathew, A.J., Bird, P., Gupta, A. et al. Magnetic resonance imaging (MRI) of feet demonstrates subclinical inflammatory joint disease in cutaneous psoriasis patients without clinical arthritis. Clin Rheumatol 37, 383–388 (2018). https://doi.org/10.1007/s10067-017-3895-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-017-3895-z