Abstract
The purpose of the present review was to provide a comprehensive picture of the efficacy of the different tumor necrosis factor (TNF)-α inhibiting agents in the treatment of acute anterior uveitis (AAU), the most common extra-articular manifestation of ankylosing spondylitis (AS). AS related, AAU may lead to severe visual impairment, due to frequent flare recurrences, anterior, and posterior segment complications and traditional treatment side effects. Considerably higher levels of tumor necrosis factor (TNF) have been assessed in the aqueous humor and inflamed joints of patients with AS. Anti-TNF drugs have shown efficacy in preventing relapses of rheumatological manifestations of spondyloarthropathies. Several studies have underlined the sustained efficacy of the monoclonal anti-TNF antibodies also in reducing the recurrence of anterior chamber flares in patients with AS-related AAU. On the other hand, retrospective studies and observational reports have indicated lower effectiveness and some paradoxical occurrence of uveitis following treatment with the soluble receptor agent etanercept. Growing evidence suggests that a prophylactic strategy could be advocated in subjects with frequent and recalcitrant attacks of AS-AAU. In this regard, the administration of monoclonal anti-TNF antibodies such as adalimumab (ADA) has been shown to significantly reduce the rate of AAU recurrences. Indeed, during ADA treatment about 90 % of patients have shown to remain completely free of attacks for the entire follow-up period, in most studies. Further studies are needed to confirm the long-term efficacy of TNF inhibitors in AS related AAU and also their role in preventing ocular complications and visual impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acute anterior uveitis (AAU) is the most common form of uveitis. Half of patients with anterior uveitis are human leucocyte antigen (HLA) B27 positive [1]. Several epidemiological studies have confirmed the high prevalence of systemic disease in patients with AAU, most commonly seronegative spondyloarthropathies (SpA) [2–5]. Among this group of patients, AAU occurs in up to 50 % of patients with AS during the course of their disease, and in contrast, it affects roughly 2 to 5 % of patients with inflammatory bowel disease (IBD)-associated arthritis and approximately 7 % of patients with psoriatic arthritis (PsA) [6].
AS is a chronic inflammatory systemic disease of unknown cause, characterized primarily by radiographic changes of sacroiliac joints and the spine, affecting about 1 % of the general population [7]. According to the Modified New York Criteria, AS is the most severe form of axial spondyloarthropathies (axial-SpA). It was traditionally considered more common in males, but it is now recognized that it may be equally common in females, although less severe. HLA-B27 is found in nearly 90 % of patients with AS. AS is characterized also by a variety of extra-articular manifestations, such as uveitis, inflammatory bowel disease, and psoriasis. Besides HLA-B27 positivity, genetic predisposition to uveitis has been widely investigated. In particular, genetic analyses have recognized a locus at chromosome 9p21–9p24 possibly predisposing to ocular involvement in these patients [8]. In addition, because of the increasing use of TNF inhibitors for the treatment of AS and AS-related UAA, TNF gene and other related regions functionally associated with TNF expression have been widely studied in the last years with diverse results. In this regard, some TNF polymorphism has been associated with a reduced risk of uveitis as for the TNFα –308 GA/AA genotype [9]; conversely, other gene alleles have been related to an increased risk of UAA susceptibility, as described for the TNF Receptor-Associated Factor 5 (TRAF5) [10]. As a whole, these findings indicate that TNF plays out its main role not only systemically but also at local sites in AS patients. Although a modulating effect of polymorphisms on TNF expression has been suggested, the exact mechanisms by which TNF induces inflammation in AS are mostly unknown and why these polymorphisms do influence eye inflammation is not clear, thus deserving further studies.
Between 20 and 40 % of patients with AS will experience a sudden onset of a unilateral AAU sometime during the course of their disease and more usually before the diagnosis of systemic disease [11]. Hence, underlying AS is diagnosed in up to 40 % of patients with AAU, particularly in the presence of the HLA-B27. Presence of AAU in AS patients may be a prognostic marker for worse outcome, as it has been shown to associate with higher disease activity, poorer functional ability, and physical mobility [12, 13]. However, a delayed diagnosis is common accounting for several years. AAU may be the first interaction with medical care and the best opportunity to identify an associated rheumatologic disease. Patients with AAU and positive HLA-B27 should be questioned about inflammatory low back pain and also evaluated for other clinical features of AS. A novel algorithm has been recently proposed—the Dublin uveitis evaluation tool (DUET)—with the aim of recognizing coexisting SpA in these patients and of improving early referral and diagnosis. In a large two-phase study, DUET algorithm was noted to have excellent sensitivity (96 %) and specificity (97 %) [14].
In axial SpA, studies of magnetic resonance imaging as well as tumor necrosis factor (TNF) inhibitor treatment and withdrawal studies all suggest that early effective suppression of inflammation has the potential to reduce radiographic damage. This potential would suggest that the concept of a window of opportunity is relevant to axial SpA. The opportunity to detect early high-risk patients, such as those carrying HLA-B27 AAU, allows commencing treatment without delay preventing ocular and systemic structural damage and sequelae [15, 16].
Clinical features of AS-related AAU uveitis
AAU associated with AS is most commonly HLA-B27-positive and has some distinct characteristics, including male preponderance, earlier age of onset, higher incidence of fibrinous reaction or hypopyon formation, unilateral or alternating between eyes, anterior rather than posterior uveitis, and a greater number of ocular complications [17–23]. Uveitis associated with the HLA-B27 antigen is a distinct clinical entity with characteristic clinical features that are usually distinguishable from HLA-B27-negative AAU [24]. Additionally, the pattern of uveitis seen in association with AS tends to differ from the pattern often seen with either PsA or IBD which can present with an insidious onset, being anterior and intermediate, bilateral, chronic, and more frequent in females [6].
The main symptoms of AS-AAU are sudden onset of ocular pain, photophobia, and blurred vision. The main signs are limbal hyperemia, fine whitish gray keratic precipitates, and prominent cellular reaction with fibrinous exudation in the anterior chamber that contributes to the formation of posterior synechiae. The cellular response can be severe enough to cause hypopyon. HLA-B27-associated AAU tends to be recurrent and to induce posterior complication and consequently visual impairment, and therefore, a systemic prophylactic strategy could be useful in subjects with frequent and recalcitrant attacks, especially if associated with systemic disease such as AS.
Complications include extensive and persistent synechiae, cataract, ocular hypertension, and glaucoma secondary to the inflammation itself or to the use of topical steroids [25]. Severe vitreous inflammation, papillitis, and retinal vasculopathy may occasionally occur. Cystoid macular edema (CME) may be associated with prolonged or severe cases of anterior uveitis [26]. Posterior segment involvement in HLA-B27 associated uveitis is an under recognized phenomenon. It has been emphasized in European data: Dodds and associates reported that among HLA-B27-positive AAU patients, posterior segment manifestations were found in 21.05 % and included diffuse vitritis (75.0 %), CME (29.1 %), and papillitis (25.0 %) [21]. In this regard, Rodriguez et al. found consistent results with vitritis, papillitis, and CME involving 93.1, 82.7, and 37.9 % of patients, respectively [27]. Similarly, Power et al. have reported a posterior involvement in 17 % of patients [28]. Although this may be an overestimation due to the concentration of difficult cases in subspecialty referral centers, it nevertheless emphasizes that HLA-B27-associated disease is not necessarily restricted to the anterior segment of the eye. The HLA-B27 patients are also more likely than the idiopathic patients to require systemic therapy for the ocular condition, and this is even more so in patients with systemic disease associated with the HLA-B27-positive uveitis.
Treatment strategies for uveitis associated with ankylosing spondylitis
TNF is present at high concentrations in the aqueous humor and serum of patients with uveitis and in the joints of patients with AS, and it actively participates in the pathogenesis of both uveitis and AS [29]. In the eye, TNF receptors I and II are expressed by the pigment epithelial cells of the iris, ciliary body, and retina [30]. Moreover, these cells are able to produce TNF-α that plays an essential role in the intraocular immune response called “anterior-chamber associated immune deviation” and in the auto-regulation of the physiologic apoptosis of resident ocular cells [31]. TNF-α levels are elevated both in the serum and aqueous humor of patients with uveitis, and these increased levels correlate with disease status [32]. T cells extracted from the aqueous humor of noninfectious uveitis patients spontaneously produce significant levels of TNF-α [33]. As previously seen in experimental models, persistent production of TNF-α is associated with tissue damage via reactive oxygen species, promotion of angiogenesis, and breakdown of the blood ocular barrier [30]. TNF-α has also been associated with sight-threatening uveitis complications, such as CME and choroidal neovascularization (CNV). This could be related to the TNF-α interaction with vascular endothelial growth factor (VEGF) because TNF-α is known to upregulate VEGF production in choroidal endothelial cells [34, 35].
In recent years, trials of anti-TNF therapy in AS have yielded impressive results and a recent systematic review, and meta-analysis has described the benefits of anti-TNF therapy in patients with AS [36–44]. Additionally, some of these studies explored the efficacy of anti-TNF therapy in controlling intraocular inflammation related to AS. The aim of this review is to provide an up-to-date on the clinical efficacy of anti-TNF therapy for AS-related AAU.
Traditionally, treatment of acute anterior uveitis is with topical steroids and cycloplegics. Periocular and systemic steroids are indicated in severe inflammation. The intraocular inflammation associated with the B27 gene shows higher recurrence and complication rate. Systemic short-term corticosteroids may help to reach quiescence but are not indicated long-termly due to the well-known systemic and ocular adverse events (AEs). Therefore, according to a long-term stepladder approach, corticosteroid-sparing drugs titrated to the severity of intraocular inflammation are the treatment of choice to prevent visual impairment and AEs. Some evidence indicates that immunosuppressive drugs used by the ophthalmologists to treat refractory uveitis, such as azathioprine and methotrexate, do not have much efficacy on the axial-SpA disease activity [45]. Furthermore, there is some evidence on the use of sulfasalazine to reduce the recurrence rate of uveitis in SpA patients [46, 47]. However, the efficacy of disease-modifying antirheumatic drugs (DMARDs) in axial SpA is rather disappointing. Moreover, careful monitoring for side effects and complications of steroids and immunosuppressive therapy is required. Non-steroidal anti-inflammatory drugs (NSAIDs), used as first line treatment in SpA to control joint inflammation, can only relieve uveitis symptoms for a short period in AS patients but cannot change the course of their ocular and systemic disease or prevent structural damage. In the presence of history of gastrointestinal complaints or a high cardiovascular risk, NSAIDs should be used with caution.
According to the Expert Panel Recommendations, infliximab and adalimumab can be considered as potential second-line immunomodulatory agents for the treatment of severe ocular inflammatory conditions including posterior uveitis, panuveitis, and severe uveitis associated with seronegative spondylarthropathy [48]. In particular, adalimumab has been recently approved for the treatment of noninfectious intermediate and posterior uveitis and panuveitis when an inadequate response to corticosteroids has been proved. This makes adalimumab the first and only Food and Drug Administration-approved steroid-sparing drug for uveitis therapy. As widely accepted, a diagnosis of non-radiographic axial SpA is a condition itself to start, following failure of NSAIDS treatment, TNF inhibitors protocol, as this form can progress toward AS within a couple of year if not adequately treated. Additionally, in the presence of AS, TNF inhibitors become first-line treatment choice in patients with persistently high disease activity despite conventional treatments [48]. Up to now, large placebo-controlled trials have explored the efficacy of TNF inhibitors in axial-SpA patients also in terms of controlling extra spinal disease manifestation, including uveitis recurrence. As reported in several retrospective studies, a first or at least early line anti-TNF-α treatment targeting spinal lesions of AS may produce concomitant beneficial effects on related intraocular inflammation and on topical and systemic steroid tapering. Moreover, a different efficacy of the various TNF inhibitors has been highlighted for extra-articular manifestations, especially IBD. In particular, monoclonal antibodies are more effective than the fusion protein, while differences regarding acute anterior uveitis are less evident [48–50].
The TNF-inhibiting agents currently available in clinical practice are as follows: infliximab (Remicade, Schering-Plough Pharma Inc, Kenilworth, New Jersey, USA), a chimeric mouse–human monoclonal immunoglobulin G (IgG) antibody; adalimumab (Humira, Abbott Pharmaceuticals Inc, Abbott Park, Illinois, USA); golimumab (Simponi Aria, Janssen, Beerse, Belgium) and certolizumab pegol (Cimzia, UCB Pharma, Bruxelles, Belgium) which are humanized monoclonal anti-TNF-α antibodies; and etanercept (Enbrel, Wyeth Pharmaceuticals Inc, Madison, New Jersey, USA), a chimeric fusion protein of the TNF receptor linked to the Fc portion of human IgG1. Infliximab is the only anti-TNF-α agent administered intravenously, whereas the remaining four are administered subcutaneously. Table 1 briefly reports the main characteristics of TNF-α inhibitors available for the treatment of AS.
In order to summarize the actual evidence reported on the role of TNF inhibitors in the AS-related AAU, we captured all relevant studies published before July 2016 on MEDLINE, EMBASE, and the Cochrane Library using the following search terms: ankylosing spondylitis, acute anterior uveitis, intraocular flare, infliximab, etanercept, adalimumab, golimumab, certolizumab, TNF inhibitor/blocker/antagonists, or anti-TNF.
TNF-α inhibitors
Infliximab and adalimumab
Studies on infliximab (IFX) and adalimumab (ADA) therapy in AS related uveitis have yielded interesting results in terms of significant positive benefits on intraocular inflammation. Beyond AS-related UAA, both agents have proved to be highly effective in refractory inflammatory with significant corticosteroid-sparing effect and good safety profile [43].
INF has shown to reduce the recurrence rate of uveitis in SpA patients and, in refractory anterior uveitis, constitutes an efficient alternative to corticosteroid treatment. Braun et al. compared seven placebo-controlled studies in order to analyze the efficacy of IFX and ETN in decreasing the number of uveitis flares in 717 patients [50]. The difference between the incidence of anterior uveitis flares during placebo treatment and the incidence during treatment with anti-TNF-α agents was significant. This trend was somewhat stronger for IFX than for ETN, but the difference between the two drugs did not reach statistical significance.
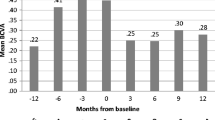
Reports on the efficacy of ADA on uveitis are mainly based on retrospective analysis of placebo-controlled trials showing beneficial results. A large prospective multinational study, known as the Rhapsody study, on 1250 AAU patients with active AS, reported a significant decrease of the recurrence rate of uveitis during ADA treatment. All AAU flares were documented throughout the treatment period plus 70 days: 40 mg every other week of ADA treatment resulted in a substantial preventive effect on AAU flares in patients with active AS, including patients with a recent history of AAU flares. Treatment with ADA decreased the rate of AAU flares by 51 % for all enrolled patients to an AAU flare rate of 7.4/100 patients-years [51]. By comparison, Braun et al. [50] reported a pooled AAU flare rate of 3.4/100 patients-years for all INF-treated patients and a pooled AAU flare rate of 7.9/100 patients for ETN-treated patients. In another prospective study, AS patients were treated with ADA because of their high disease activity and screened by an ophthalmologist on uveitis. Confirming previous findings, this study demonstrated a significant decrease (up to 80 %) of the recurrence rate of uveitis during ADA treatment. Among patients with uveitis in the year before treatment, ADA therapy significantly reduced the number of patients with uveitis attacks by 62 % [52]. These data on ADA have been confirmed by a recent Korean study exploring the efficacy of anti-TNF α therapy in patients affected by AS HLA-B27-positive AAU [53]. A further retrospective study by Durrany et al. on 32 patients with different ocular inflammatory conditions highlighted a moderate effectiveness in controlling inflammation and corticosteroid-sparing even in patients with recalcitrant inflammation resistant to multiple other previously administered therapies [44]. In conclusion, in subjects with frequent and recalcitrant attacks, ADA has been observed to significantly and substantially reduce the rate of recurrences with about 90 % of patients remaining completely free of attacks during ADA treatment for the entire follow-up period in most studies. Finally, IFX and ADA are associated with significantly fewer flares and new onset of uveitis when compared with placebo.
Etanercept
There is much debate about the efficacy of etanercept (ETN) on uveitis [50, 53–60]. Braun et al. [50] reported that the reduction in AS related uveitis flares was greater in patients treated with IFX compared with those treated with ETN. These findings were consistent with those identified in another single center study conducted on 46 AS patients [53]. Accordingly, a retrospective observational analysis from a large claims database on 2115 anti-TNF-naïve patients with a diagnosis of AS and no history of uveitis confirmed a higher risk of developing new-onset uveitis in patients treated with ETN than those treated with ADA and IFX. The authors concluded that ETN has lower efficacy than monoclonal anti-TNF-α either because of a possible paradoxical paradigm or because it is less effective in preventing de novo intraocular inflammation in such patients [54].
The number of studies suggesting a differentiated therapeutic response of AS-related uveitis to ETN with respect to monoclonal antibodies is increasing. However, besides TNF-α, ETN also inhibits TNF-β: According to findings from animal models of uveitis, higher TNF-β levels are associated with intraocular inflammation. Consequently, ETN would therefore be expected to be even more effective. In support of this theory, a recent meta-analysis comparing anti-TNF therapy with placebo in patients with AS indicated significant positive benefits of anti-TNF agents, including ETN, to treat uveitis [55]. Similarly, in spite of their differences in mechanism of action, Kim et al. [56] observed no differences on uveitis relapse and improvement rates among the three anti-TNF-α agents IFX, ADA, and ETN, as all agents successfully induced uveitis improvement in about 3 weeks and reduced anterior uveitis flare-ups.
As a whole, there is ongoing debate whether uveitis during anti-TNF-α treatment is a paradoxical effect or an inadequate response to therapy [57]. Reasons for the reported lower rates of therapeutic response with ETN are unknown but may be related to genetic predisposition [58] as well as to a possible class effect, a hypothesis that should be confirmed in future studies comparing ETN with certolizumab pegol and golimumab. Most authors agree that patients with ETN-related paradoxical uveitis may benefit from switching to another anti-TNF-α agent. Therefore, current evidence suggests the preferential use of monoclonal anti-TNF-α rather than fusion proteins in patients with AS with an active or previous history of uveitis; however, given the weak power of evidence and some controversial report, further study is warranted on this issue.
Regarding other extra-articular manifestations, unlike ADA and IFX, ETN is not effective in IBD further supporting the importance of taking into account the extra-articular involvement when choosing the TNF inhibitors in any specific case [59].
Certolizumab pegol and golimumab
Certolizumab pegol (CZP) is a PEGylated FC-free anti-TNF agent shown to improve spinal and extra-articular manifestations, such as enthesitis, in patients with both AS and non-radiographic axial SpA [61, 62]. Effectiveness in refractive uveitis has also been reported after systemic administration [63]. Emerging data from a retrospective case series including two patients with AS and a prospective and controlled trial enrolling 218 axial SpA patients posit a treatment effect of CZP in active refractory uveitis. In particular, CZP showed an impact on reducing uveitis flares which was comparable to rates observed for other anti-TNF antibodies [63, 64]. Nevertheless, sufficient evidence in AS-AAU is still advocated to draw firm conclusions on this issue.
Golimumab (GOL) has been approved for the treatment of rheumatoid arthritis, PsA, AS, and ulcerative colitis. The few published studies on the efficacy of GOL in refractory uveitis are based on small case series or heterogeneous subgroups of patients with this disorder [65–69]. A very recent study by a Spanish research group reported eight cases of refractory uveitis related to AS and one related to non-radiographic axial SpA showing a rapid and maintained improvement of intraocular inflammation parameters, macular thickness, and best corrected visual acuity [70].
Safety issues
Although the use of TNF inhibitors has led to an efficient control of signs and symptoms of AS, allowing a significant improvement in quality of life, most of these patients will need a long-term treatment, in many cases for indeterminate period of time, especially in AS. Despite the high safety profile of biologic agents, ophthalmologists and rheumatologists must be aware of their potential side effects. According to the information reported from the large national registries published in the last 5 years, anti-TNF α therapy may expose patients with AS to an increased incidence/risk of developing infection, especially tuberculosis [53, 71]. This has to be taken into account especially in countries where the incidence of tuberculosis is high; therefore, tuberculosis screening procedures are mandatory before starting TNF inhibitors, as the risk of reactivation of latent infection has been found slightly higher with monoclonal antibodies [72]. On the other hand, actual available published data have shown that there is no increased risk of malignancies associated with the use of ADA and ETN in AS. Similarly, also the risk of demyelinating disorders, interstitial lung disease, and mortality is not significantly increased in patients using TNF inhibitors for AS compared with patients not using biological drugs [71]. Table 2 shows the advantages and disadvantages of the current available TNF inhibitors to consider when treating AS-related AAU.
Conclusions
There is mounting evidence that early effective treatment of inflammation in axial-SpA can change disease outcome. Therefore, the identification of high-risk individuals and prompt institution of therapy is going to have great importance in clinical practice. Patients with AAU and HLA-B27 haplotype should be informed about the recurrent nature of their disease, the probable association with systemic manifestations, and the potential for developing sight-threatening ocular complications beyond articular disability. In this context, ophthalmologists and rheumatologists should keep a low threshold for the initiation of systemic therapy in patients with this condition.
According to the present evidences, systemic administration of TNF blockers induces quick and sustained efficacy in reducing and/or preventing inflammatory intraocular activity of AS-related uveitis. Data reports mainly support the use of IFX and ADA as first-line anti-TNF-α agents for treating uveitis in preference to ETN because of possible paradoxical intraocular inflammation or less efficacy on uveitis of the soluble receptor agent [35, 51, 73, 74]. This and other evidences reported in the present review highlight the importance of taking into account extra-articular manifestations related to AS when choosing TNF inhibitors in such patients.
Finally, regarding the novel monoclonal antibodies commercialized (CZP and GOL), they have shown some efficacy, but strong evidence from larger studies is still lacking.
In any case, data currently available need to be confirmed by further studies, as high-quality, large prospective RCTs with longer follow-up are required to confirm current knowledge about long-term efficacy of biologic agents in controlling intraocular inflammation related to AS.
References
Jones NP (2015) The Manchester uveitis clinic: the first 3000 patients—epidemiology and casemix. Ocul Immunol Inflamm 23:118–126
Karaconji T, Maconochie Z, McCluskey P (2013) Acute anterior uveitis in Sydney. Ocul Immunol Inflamm 21:108–114
Pato E, Bañares A, Jover JA, Fernández-Gutiérrez B, Godoy F, Morado C et al (2000) Undiagnosed spondyloarthropathy in patients presenting with anterior uveitis. J Rheumatol 27:2198–2202
Canouï-Poitrine F, Kemta Lekpa F, Farrenq V, Boissinot V, Hacquardo-Bouder C, Comet D et al (2012) Prevalence and factors associated with uveitis in spondyloarthropathies patients in France: results from the EXTRA observational survey. Arthritis Care Res (Hoboken) 64:919–924
Zeboulon N, Dougados M, Gossec L (2015) Prevalence and characteristics of uveitis in the spondyloarthropathies: a systematic literature review. Ann Rheum Dis 67:955–959
Rosenbaum JT (2015) Uveitis in spondyloarthritis including psoriatic arthritis, ankylosing spondylitis, and inflammatory bowel disease. Clin Rheumatol 34:999–1002
Sieper J, Rudwaleit M, Khan MA, Braun J (2006) Concepts and epidemiology of spondyloarthritis. Best Pract Res Clin Rheumatol 20:401–417
Martin TM, Zhang G, Luo J, Jin L, Doyle TM, Rajska BM et al (2005) A locus on chromosome 9p predisposes to a specific disease manifestation, acute anterior uveitis, in ankylosing spondylitis, a genetically complex, multisystem, inflammatory disease. Arthritis Rheum 52:269–274
Nossent JC, Sagen-Johnsen S, Bakland G (2014) Tumor necrosis factor-α promoter −308/238 polymorphism association with less severe disease in ankylosing spondylitis is unrelated to serum TNF-α and does not predict TNF inhibitor response. J Rheumatol 41:1675–1682
Xiang Q, Chen L, Fang J, Hou S, Wei L, Bai L et al (2013) TNF receptor-associated factor 5 gene confers genetic predisposition to acute anterior uveitis and pediatric uveitis. Arthritis Res Ther 15:R113
Stolwijk C, van Tubergen A, Castillo-Ortiz JD, Boonen A (2015) Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: a systematic review and meta-analysis. Ann Rheum Dis 74:65–73
Chen CH, Lin KC, Chen HA, Liao HT, Liang TH, Wang HP et al (2007) Association of acute anterior uveitis with disease activity, functional ability and physical mobility in patients with ankylosing spondylitis: a cross-sectional study of Chinese patients in Taiwan. Clin Rheumatol 26:953–957
Essers I, Ramiro S, Stolwijk C, Blaauw M, Landewé R, van der Heijde D et al (2015) Characteristics associated with the presence and development of extra-articular manifestations in ankylosing spondylitis: 12-year results from OASIS. Rheumatology (Oxford) 54:633–640
Haroon M, O’Rourke M, Ramasamy P, Murphy CC, FitzGerald O (2015) A novel evidence-based detection of undiagnosed spondyloarthritis in patients presenting with acute anterior uveitis: the DUET (Dublin Uveitis Evaluation Tool). Ann Rheum Dis 74:1990–1995
Robinson PC, Brown MA (2014) The window of opportunity: a relevant concept for axial spondyloarthritis. Arthritis Res Ther 16:109
Cantarini L, Fabbroni M, Talarico R, Costa L, Caso F, Cuneo GL et al (2015) Effectiveness of adalimumab in non-radiographic axial spondyloarthritis: evaluation of clinical and magnetic resonance imaging outcomes in a monocentric cohort. Medicine (Baltimore) 94, e1170
D’Ambrosio EM, La Cava M, Tortorella P, Gharbyia M, Campanella M, Iannetti L (2016) Clinical features and complications of the HLA-B27-associated acute anterior uveitis: a meta-analysis. Semin Ophthalmol. doi:10.3109/08820538.2016.1170158
Rothova A, Buitenhuis HJ, Christiaans BJ, Linssen A, van der Gaag R, Kijlstra A et al (1983) Acute anterior uveitis (AAU) and HLA-B27. Br J Rheumatol 22(4 Suppl 2):144–145
D’Alessandro LP, Forster DJ, Rao NA (1991) Anterior uveitis and hypopyon. Am J Ophthalmol 112:317–321
Tuncer S, Adam YS, Urgancioglu M, Tugal-Tutkun I (2005) Clinical features and outcomes of HLA-b27-positive and HLA-B27-negative acute anterior uveitis in a Turkish patient population. Ocul Immunol Inflamm 13:367–373
Huhtinen M, Karma A (2000) HLA-B27 typing in the categorisation of uveitis in a HLA-B27 rich population. Br J Ophthalmol 84:413–416
Sampaio-Barros PD, Conde RA, Bonfiglioli R, Bertolo MB, Samara AM (2006) Characterization and outcome of uveitis in 350 patients with spondyloarthropathies. Rheumatol Int 26:1143–1146
Power WJ, Rodriguez A, Pedroza-Seres M, Foster CS (1998) Outcomes in anterior uveitis associated with the HLA-B27 haplotype. Ophthalmology 105:1646–1651
Chang JH, McCluskey PJ, Wakefield D (2005) Acute anterior uveitis and HLA-B27. Surv Ophthalmol 50:364–388
Neri P, Azuara-Blanco A, Forrester JV (2004) Incidence of glaucoma in patients with uveitis. J Glaucoma 13:461–465
Loh AR, Acharya NR (2010) Incidence rates and risk factors for ocular complications and vision loss in HLA-B27-associated uveitis. Am J Ophthalmol 150:534–542
Dodds EM, Lowder CY, Meisler DM (1999) Posterior segment inflammation in HLA-B27. acute anterior uveitis: clinical characteristics. Ocul Immunol Inflamm 7:85–92
Rodriguez A, Akova YA, Pedroza-Seres M, Foster CS (1994) Posterior segment ocular manifestations in patients with HLA-B27-associated uveitis. Ophthalmology 101:1267–1274
Pérez-Guijo V, Santos-Lacomba M, Sánchez-Hernández M, Castro-Villegas Mdel C, Gallardo-Galera JM, Collantes-Estévez E (2004) Tumour necrosis factor-alpha levels in aqueous humour and serum from patients with uveitis: the involvement of HLA-B27. Curr Med Res Opin 20:155–157
Khera TK, Dick AD, Nicholson LB (2010) Mechanisms of TNF alpha regulation in uveitis: focus on RNA-binding proteins. Prog Retin Eye Res 29:610e21
Ferguson TA, Griffith TS (2007) The role of Fas ligand and TNF related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem Immunol Allergy 92:140e54
Santos Lacomba M, Marcos Martin C, Gallardo Galera JM, Gómez Vidal MA, Collantes Estévez E, Ramírez Chamond R et al (2001) Aqueous humor and serum tumor necrosis factor alpha in clinical uveitis. Ophthalmic Res 33:251e5
Sakaguchi M, Sugita S, Sagawa K, Itoh K, Mochizuki M (1998) Cytokine production by T cells infiltrating in the eye of uveitis patients. Jpn J Ophthalmol 42:262e8
Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR (2006) Sequential induction of angiogenic growth factors by TNFalpha in choroidal endothelial cells. J Neuroimmunol 171:45e56
Giraudo E, Primo L, Audero E, Gerber HP, Koolwijk P, Soker S et al (1998) Tumor necrosis factor alpha regulates expression of vascular endothelial growth factor receptor-2 and of its co-receptor neuropilin-1 in human vascular endothelial cells. J Biol Chem 273:22128e35
Rudwaleit M, Claudepierre P, Wordsworth P, Cortina EL, Sieper J, Kron M et al (2009) Effectiveness, safety, and predictors of good clinical response in 1250 patients treated with adalimumab for active ankylosing spondylitis. J Rheumatol 36:801–808
Sieper J, van der Heijde D, Dougados M, Brown LS, Lavie F, Pangan AL (2012) Early response to adalimumab predicts long-term remission through 5 years of treatment in patients with ankylosing spondylitis. Ann Rheum Dis 71:700–706
Machado MA, Barbosa MM, Almeida AM, de Araújo VE, Kakehasi AM, Andrade EI et al (2013) Treatment of ankylosing spondylitis with TNF blockers: a meta-analysis. Rheumatol Int 33:2199–2213
Braun J, van der Horst-Bruinsma IE, Huang F, Burgos-Vargas R, Vlahos B, Koenig AS et al (2011) Clinical efficacy and safety of etanercept versus sulfasalazine in patients with ankylosing spondylitis: a randomized, double-blind trial. Arthritis Rheum 63:1543–1551
Giardina AR, Ferrante A, Ciccia F, Impastato R, Miceli MC, Principato A et al (2010) A 2-year comparative open label randomized study of efficacy and safety of etanercept and infliximab in patients with ankylosing spondylitis. Rheumatol Int 30:1437–1440
Braun J, Baraliakos X, Hermann KG, van der Heijde D, Inman RD, Deodhar AA et al (2012) Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo- controlled GO-RAISE study. Ann Rheum Dis 71:878–884
Baraliakos X, Listing J, Fritz C, Haibel H, Alten R, Burmester GR et al (2011) Persistent clinical efficacy and safety of infliximab in ankylosing spondylitis after 8 years–early clinical response predicts long-term outcome. Rheumatology (Oxford) 50:1690–1699
Vallet H, Seve P, Biard L, Baptiste Fraison J, Bielefeld P et al (2016) Infliximab versus adalimumab in the treatment of refractory inflammatory uveitis: a multicenter study from the French uveitis network. Arthritis Rheumatol 68:1522–1530
Durrani K, Kempen JH, Ying GS, Kacmaz RO, Artornsombudh P, Rosenbaum JT et al (2016) Adalimumab for Ocular Inflammation. Ocul Immunol Inflamm 22:1–8
Haibel H, Brandt HC, Song IH, Brandt A, Listing J, Rudwaleit M et al (2007) No efficacy of subcutaneous methotrexate in active ankylosing spondylitis: a 16-week open-label trial. Ann Rheum Dis 66:419–421
Muñoz-Fernández S, Hidalgo V, Fernández-Melón J, Schlincker A, Bonilla G, Ruiz-Sancho D et al (2003) Sulfasalazine reduces the number of flares of acute anterior uveitis over a one-year period. J Rheumatol 30:1277–1279
Benitez-del-Castillo JM, Garia-Sanchez J, Iradier T, Banares A (2000) Sulfasalazine in the prevention of anterior uveitis associated with ankylosing spondylitis. Eye 14:340–343
Levy-Clarke G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN et al (2014) Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology 121:785–796
Braun J, van den Berg R, Baraliakos X, Boehm H, Burgos-Vargas R, Collantes-Estevez E et al (2011) 2010 update of the ASAS/EULAR recommendations for the management of ankylosing spondylitis. Ann Rheum Dis 70:896–904
Braun J, Baraliakos X, Listing J, Sieper J (2005) Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum 52:2447–2451
Rudwaleit M, Rødevand E, Holck P, Vanhoof J, Kron M, Kary S et al (2009) Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: results of a prospective open-label study. Ann Rheum Dis 68:696–701
van Denderen JC, Visman IM, Nurmohamed MT, Suttorp-Schulten MS, van der Horst-Bruinsma IE (2014) Adalimumab significantly reduces the recurrence rate of anterior uveitis in patients with ankylosing spondylitis. J Rheumatol 41:1843–1848
Guignard S, Gossec L, Salliot C, Ruyssen-Witrand A, Luc M, Duclos M et al (2006) Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondyloarthropathy: a retrospective study. Ann Rheum Dis 65:1631–1634
Wendling D, Joshi A, Reilly P, Mittal M, Bao Y (2014) Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: an analysis of a large US claims database. Curr Med Res & Opin 30:2515–2521
Migliore A, Bizzi E, Bernardi M, Picchianti Diamanti A, Laganà B (2015) Indirect comparison between subcutaneous biologic agents in ankylosing spondylitis. Colin Drug Invesst 35:23–29
Kim M, Won JY, Choi SY, Ju JH, Park YH (2016) Anti-TNFα treatment for HLA-B27 positive ankylosing spondylitis-related uveitis. Am J Ophthalmol. doi:10.1016/j.ajo.2016.07.016
Raffeiner B, Ometto F, Bernardi L, Botsios C, Punzi L (2014) Inefficacy or paradoxical effect? Uveitis in ankylosing spondylitis treated with etanercept. Case Rep Med 2014:2014471319
Killian M, Touraine R, Amouzougan A, Thomas T, Marotte H (2015) Impact of genetic predisposition of de novo uveitis with etanercept in ankylosing spondylitis. Ann Rheum Dis 74, e22
Mozaffari S, Nikfar S, Abdolghaffari AH, Abdollahi M (2014) New biologic therapeutics for ulcerative colitis and Crohn’s disease. Expert Opin Biol Ther 14:583–600
Lian F, Zhou J, Wei C, Wang Y, Xu H, Liang L (2015) Anti-TNFα agents and methotrexate in spondyloarthritis related uveitis in a Chinese population. Clin Rheumatol 34:1913–1920
Sieper J, Landed R, Rudwaleit M, van der Heijde D, Dougados M, Mease PJ et al (2015) Effect if certolizumab peg over ninety-six weeks in patients with axial spindyloarthritis: results from a phase III randomized trial. Arthritis Rheumatol 67:688–77
Sieper J, Kivitz A, van Tubergen A, Deodhar A, Coteur G, Woltering F et al (2015) Impact of certolizumab peg on patient-reported outcomes in patients with axial spondyloarthritis. Arthritis Care Res (Hoboken) 67:1475–1480
Llorenc V, Mesquida M, Sainz de La Maza M, Blanco R, Calvo V, Maiz O et al (2016) Certolizumab pegol, a new anti-TNF-alpha in the armamentarium against ocular inflammation. Ocul Immunol Inflamm 24:167–172
Rudwaleit M, Rosenbaum JT, Landed R, Marzo-ortega H, Sieper J, van der Heijde D (2016) Observed Incidence of uveitis following certolizumab peg treatment in patients with axial spondyloarthritis. Arthritis Care Res (Hoboken) 68:838–844
Miserocchi E, Modorati G, Pontikaki I, Meroni P, Gerloni V (2014) Long-term treatment with golimumab for severe uveitis. Ocul Immunol Inflamm 22:90–95
Miserocchi E, Modorati G, Pontikaki I, Meroni P, Gerloni V (2013) Golimumab treatment for complicated uveitis. Clinical Exp Rheumatol 31:320–321
Cordero-Coma M, Calvo-Rio V, Adan A, Blanco R, Alvarex-Castro C, Mesquida M et al (2014) Golimumab as rescue therapy for refractory immune-mediated uveitis: a three-center experience. Mediators of Inflamm 2014:717598
Faez S, Lobo AM, Sobrin L, Papaliodis GN (2014) Treatment of seronegative spondyloarthropathy-associated uveitis with golimumab: retrospective case series. Clin Experiment Opthalmolol 42:392–395
Calvo-Rio V, de la Hera D, Blanco R, Beltran-Catalan E, Loricera J, Canal J et al (2014) Golimumab in uveitis previously treated with other anti-TNF-alpha drugs: a retrospective study of three cases from a single centre and literature review. Clin Exp Rheumatol 32:864–868
Calvo-Río V, Blanco R, Santos-Gómez M, Rubio-Romero E, Cordero-Coma M, Gallego-Flores A et al (2016) Golimumab in refractory uveitis related to spondyloarthritis. Multicenter study of 15 patients. Semin Arthritis Rheum 46:95–101
Sampaio-Barros PD, van der Horst-Bruinsma IE (2014) Adverse effects of TNF inhibitors in SPA: are they different from RA? Best Pract Res Clin Rheumatol 28:747–763
Ai JW, Zhang S, Ruan QL, Yu YQ, Zhang BY, Liu QH et al (2015) The risk of tuberculosis in patients with rheumatoid arthritis treated with tumor necrosis factor-alpha antagonist: a meta-analysis of both randomized controlled trials and registry/cohort studies. J Rheumatol 42:2229–2237
Martin-Mola E, Sieper J, LEirisalo-Repo M, Dijkmans BA, Vlahos B, Pedersen R et al (2010) Sustained efficacy and safety, including patient-reported outcomes, with etanercept treatment over 5 years in patients with ankylosing spondylitis. Clin Exp Rheumatol 28:238–245
Moisseiev E, Shulman S (2014) Certolizumab-induced uveitis: a case report and review of the literature. Case Rep Ophthalmic 5:54–59
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
None.
Rights and permissions
About this article
Cite this article
Fabiani, C., Vitale, A., Lopalco, G. et al. Different roles of TNF inhibitors in acute anterior uveitis associated with ankylosing spondylitis: state of the art. Clin Rheumatol 35, 2631–2638 (2016). https://doi.org/10.1007/s10067-016-3426-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-016-3426-3