Abstract
Background and Objective
There are four efficacious subcutaneous anti-tumor necrosis factor alpha (TNF-alpha) agents used for the therapy of ankylosing spondilitis (AS), but apparently little or no differences in their effectiveness was proven. By this study, we aimed to compare Assessment in Ankylosing Spondylitis Response Criteria 20 response patterns (ASAS20) between subcutaneous approved biological agents in patients affected by ankylosing spondylitis by means of a mixed treatment comparison of different randomized controlled trials (RCTs) on the efficacy of biological therapies.
Methods
A search in scientific literature was performed to identify the most complete collection of RCTs available on the selected topic. Similarly designed double-blind, randomized, placebo-controlled trials investigating the efficacy of the subcutaneous and approved TNF-alpha inhibitors such as etanercept, certolizumab pegol, golimumab and adalimumab in the treatment of ankylosing spondylitis patients were identified. The endpoint of interest was ASAS20 response criterium at 12 weeks. Results were analysed simultaneously using Bayesian mixed treatment comparison techniques. Results were expressed as odds ratio (OR) of positive ASAS20 response and associated 95 % credible intervals (CrIs). The probability of being the best treatment was also reported.
Results
Only five RCTs matched the inclusion criteria for consequent data extraction and analysis. Mixed treatment comparison of data from such RCTs demonstrated that all subcutaneous anti-TNF-alpha agents are more effective in inducing an ASAS20 response than placebo. Data from 24 weeks’ follow-up were not taken into account as early escape granted in some of the studies made results at 24 weeks unmatchable. In our analysis, golimumab proved to be the drug that more probably represents the best choice for achieving ASAS20 response at 12 weeks, although no differences were observed when comparing directly every single subcutaneous anti-TNF-alpha agent against another.
Conclusions
Even if the mixed treatment comparisons between adalimumab, golimumab, certolizumab pegol and etanercept did not show a statistically significant difference, this analysis, based on data from only five RCTs, suggests that golimumab, compared to placebo, may be the drug that provides the highest probability of achieving ASAS20 response in AS patients naive to biologic treatments at 12 weeks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
There are no head-to-head studies regarding efficacy of anti-TNF-alpha agents in ankylosing spondylitis. | |
The mixed treatment comparison allows indirect comparison of results obtained by randomized controlled trials regarding efficacy profiles of such treatments. | |
By this analysis, golimumab proved to be the agent that more probably will grant the ASAS20 response at 12 weeks. |
1 Introduction
Ankylosing spondylitis (AS) represents a chronic inflammatory disease predominantly affecting the spine with a reported prevalence in Caucasian populations ranging from 0.05 to 0.23 %. Extra-spinal involvement may be represented by different forms of peripheral arthritis, but often also uveitis, enteritis, and psoriasis are associated. As previously reported, disease modifying antirheumatic drugs (DMARDs) proved to be ineffective for the spinal involvement of the disease [1].
Up to 50 % of patients also experience concomitant peripheral joint arthritis [2]. Genetic characterisation of patients strongly suggested a familial aggregation for AS. Individuals presenting the genetic marker human leukocyte antigen B27 (HLA-B27) have at least a 1 % chance of developing AS, although the actual relationship between the gene and the disease’s development is still not completely understood [3, 4].
Unlike other more common inflammatory joint disorders, the use of biologic treatments is often required to treat AS patients in order to control disease activity [5–11]. Good efficacy and safety profiles have been extensively proven for all anti-tumour necrosis factor (TNF)-based therapies in patients with AS [12, 13], but still a more precise understanding of the therapeutic role of each anti-TNF agent used to treat AS is necessary in order to allow a more accurate choice of treatment options for these patients. Patients presenting an inadequate response to previous NSAID-based therapies are treated with one of the biological agents or TNF blockers adalimumab, certolizumab pegol, etanercept, golimumab and infliximab. Such drugs present biological and clinical differences [9–13] that may result in different efficacy outcomes in different pathologies where they are commonly used.
In order to perform an accurate choice of treatment, it is necessary to compare the efficacy of all therapies available, a comparison that has so far been performed only on a part of clinical endpoints [14]. To date, there are no published randomized controlled trials (RCTs) providing data on a head-to-head comparison of the efficacy of subcutaneous anti-TNF agents, adalimumab, certolizumab pegol, etacercept, golimumab and infliximab in the treatment of AS. This kind of RCT would require very large patient numbers, as the differences in terms of efficacy between the biological treatments would appear to be small, and consequently the cost of conducting this sort of trial would be very high. Mixed treatment comparison (MTC) [15–18], that represents an extension of more conventional and commonly used frequentistic meta-analysis, allows the performing of multiple pair-wise comparisons across a group of different possible treatments for the same disease. The results obtained using this statistical method may provide an objective approach to the difficult choice of treatment, when similarly relevant data are unavailable and when more drugs seem to produce, in the same disease, the same or very similar effects. More specifically, using MTC we aimed to compare the results in terms of efficacy obtained in different RCTs performed on each subcutaneous anti-TNF therapy in patients with AS on clinical characteristics of the disease, as expressed by Assessment in Ankylosing Spondylitis Response Criteria 20 response patterns (ASAS20). Mixed treatment comparison, with respect to common meta-analysis, enables the estimation of data by assembling and analysing data extracted from several studies on the same subject [15–18]. The objective of this study was to compare results as reported by the most common parameter used in clinical practice for evaluating clinical condition of patients affected by AS, ASAS20. For this reason, we concentrated our efforts on determining relative efficacy profiles of currently licensed doses of commonly used subcutaneous biological treatments for AS.
2 Methods
2.1 Identification of Eligible Studies and Data Extraction
An extensive literature search was performed in order to identify all RCTs performed to assess the efficacy of different anti-TNF treatments (etanercept, adalimumab, golimumab, certolizumab pegol) in patients with AS. The MEDLINE and EMBASE databases were both intensively searched and search terms included a combination of free-text and thesaurus terms relevant to AS anti-TNF subcutaneous agents. The primary endpoint for analysis was the ASAS20 response criterium [19] from baseline to 12 weeks.
Only RCTs reporting data on placebo-controlled, double-blind studies with a follow-up of at least 12 weeks on the efficacy, expressed as ASAS20, of subcutaneous anti-TNF agent in patients affected by AS were included. For each selected study, details regarding study design, patients’ demographic and morbidity characteristics, treatment interventions, endpoints and duration of follow-up were analysed. Unless otherwise stated, imputation for non-response was assumed to be through last observation carried forward.
2.2 Data Analysis
An evaluation of the primary trial endpoint was conducted to identify any differences, in terms of ASAS20 response, between the four subcutaneous anti-TNF agents analysed. For subsequent analysis, we used the reported number of patients in each response category in the treatment and placebo groups of each RCT eligible for further analysis. These frequencies were processed by a Bayesian analysis (MTC), by the use of a fixed effect model. In the case of this kind of analysis, it is fundamental to establish whether a fixed-effect model or a random-effect model is the more appropriate for pooling results from different studies [15]. The residual deviance of models obtained using random effects and fixed effects was compared. When the residual deviance obtained by a random-effect model is lower than fixed model residual deviance, a random-effect model may be more appropriate; however, when residual deviance is similar, a fixed-effect model seemed to be the most suitable option. WinBUGS 1.4 statistical software (MRC Biostatistics Unit, Cambridge, UK) was used to perform analysis. WinBUGS provides Bayesian estimates, and in this specific case the analysis was conducted without prior assumptions concerning coefficient sizes. This study reports the results as summary statistics for odds ratio (OR): the mean value that is the most likely value and the correspondent 95 % credible interval (95 % CrIs) that contains the true value of the OR with the 95 % of probability. MTC reports results as an evaluation of OR of response as ASAS20 for each biological agent compared to placebo and also the OR of response, again in terms of ASAS20, between each combination of two biological agents. The probability of best treatment was also reported for each biological agent.
3 Results
3.1 Identified Studies
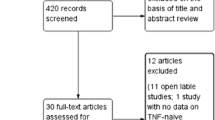
A total of 691 articles were extracted from EMBASE and MEDLINE using the research terms adalimumab or etanercept or golimumab or certolizumab pegol and ankylosing spondylitis and ASAS and randomized controlled trial. Of the selected articles only 81 remained after the research terms “randomized controlled trial” was added. Of the 81 articles selected, only 25 remained after a term search on “ASAS” was included. Only five articles [20–24] met the inclusion criteria established and were consequently included in the study for data extraction. The selected articles and their principal characteristics are shown in Table 1. Length of study, number of patients included, demographic characteristics, different disease duration and eventual concomitant medication, severity of disease and outcome measures were considered as values relevant for a correct statistical comparison, as differences in disease characteristics or demographic characteristics could lead to imprecise results. Consequently, all data were recorded and analysed using a MTC with the analytic methodologies described above. Two studies reported data from RCTs on etanercept, one study on adalimumab, one study on golimumab, one study on certolizumab pegol. The populations of the different studies were similarly represented, in terms of gender and disease duration and characteristics. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) score, which is reported for all studies, is similar in all studies and a statistical examination of any considerable differences did not show any significance. Data such as erythrocyte sedimentation rate, C-reactive protein and presence of HLA-B27 gene polymorphism were not reported in all studies [22] and consequently were not analysed. Data regarding 24 weeks were excluded from analysis as a large part of the studies’ results [23, 24] were composed at 24 weeks by populations who previously took placebo and shifted, at week 12, to active arm.
3.2 ASAS20 Responses
All subcutaneous anti-TNF agents proved to be more efficacious in inducing an ASAS20 response than placebo. In our analysis, golimumab resulted as the anti-TNF agent with the highest probability of being the best treatment in inducing ASAS20 response at 12 weeks with a percentage of 41.28 %. Adalimumab and etanercept had percentages of 29.91 and 28.74, respectively (Table 2). Further analyses, performed in order to make direct comparisons between the four anti-TNF agents included in the study showed a significant difference when comparing etanercept, adalimumab and golimumab versus certolizumab pegol. Etanercept, adalimumab and golimumab proved to be more efficacious than certolizumab pegol in inducing an ASAS20 response (Table 3).
4 Discussion
MTC use, which is growing among scientists dealing with different fields of medicine, allows the simultaneous multiple meta-analysis of different pair-wise comparisons across a range of different interventions. In fact, literature reports an increasing use of this statistical tool in several diseases: it has been used to analyse stroke prevention [25], antidepressants [26], psychological interventions in heart disease [27] and the prevention of vertebral fractures in women with postmenopausal osteoporosis [28], and in the field of rheumatology some papers were produced on rheumatoid arthritis and psoriatic arthritis [29–31]. Previously, we reported results of MTC analysis on psoriatic arthritis and on AS [32]. The study on AS reported data on the three anti-TNF agents available during the time of publication (infliximab, etanercept and adalimumab). Infliximab was shown to be the biologic agent more likely to produce an ASAS20 response with a probability of 72 % of being the best treatment with the above-mentioned efficacy parameter compared to adalimumab and etanercept, which showed a probability of 13 and 15 %, respectively. The decision to undertake this new analysis was made because two new subcutaneous anti-TNF-alpha agents, certolizumab pegol and golimumab, have been approved for therapy in AS and that subcutaneous drugs seem to offer a better adherence to therapy for patients undergoing biologic therapy [33, 34]. The license for the use of certolizumab pegol in AS is still pending in European countries, while golimumab is marketed all over Europe for use in AS.
In addition, intravenous therapies based on the administration of anti-TNF antibodies are often burdened by heavier costs due to hospitalisation and dosage adjustments that are often needed.
The objective of this study was to compare the evidence of efficacy of subcutaneous TNF-alpha blockers in terms of ASAS20 response, also taking into account rapidity of action in symptom relief. Since these drugs are very expensive and may only be used in patients with AS after failure of NSAID treatment, it is extremely important to both physicians and decision makers that the most effective treatment available is chosen, as well as rapidity of onset of action
As no head-to-head studies were available in literature among biologic drugs used in AS, the use of MTC in this field may represent a valid tool for gathering further data.
The five studies included in this MTC were placebo-controlled trials having the same primary endpoint: ASAS20 response. As already reported, these five studies do not differ in terms of disease diagnosis criteria, disease duration and co-medications used or in the demographic/pathologic characteristics of the populations. The fact that the studies had similar disease and demographic characteristics excludes these factors as causing relevant heterogeneity across trials and therefore the indirect estimate was not affected by biases generated by significant differences.
The result of this MTC suggests that golimumab is expected to provide the highest probability of granting ASAS20 response of the TNF-alpha blockers studied in comparison to placebo in the treatment of AS. Golimumab shows a 41.28 % of probability of being the best treatment of all compared with placebo. In short-term therapy, it is essential to know whether a drug may grant a rapid onset of symptom relief. Patients starting a biologic therapy having already failed a previous therapy based on NSAIDs and are at risk of severe impairment due to disease progression. In this sense, golimumab proved to be the most probably efficacious drug to grant an ASAS20 response in the short term. Data from 24 weeks were not taken into account for further examination as part of the studies reached 24 weeks of follow-up after shifting part of the placebo or low dosage arm to different dosages of biologic drugs [23, 24], generating populations who received different treatments during follow-up. Similarly, part of the RCTs taken into account for this analysis reported data on clinical efficacy as ASAS40 and ASAS5/6 [23, 24] and part as ASAS50 and ASAS70 [20–22], and this prevented the comparison between such data.
The lack of a statistically significant difference in the direct comparison between golimumab, adalimumab, certolizumab pegol and etanercept (one compared to another) can be attributed to the small number of trials examined and sample dispersion. The Bayesian approach of MTC allows a probabilistic reading of clinical data and ranking of the interventions. This information may help physicians in choosing the best probable treatment management since the associated uncertainty of each intervention and the estimated size of the treatment effect are translated into one measure: the probability that a certain treatment, amongst compared treatments, might provide the best outcome.
Measuring the probability of choosing the most effective treatment may be helpful to physicians in decision-making settings, in which the compliance, tolerability and safety of each treatment also have to be considered.
Moreover, in the case of spondyloarthritis, we can also consider the differences in stopping radiological disease progression and not merely the improvement in the clinical surrogate measure, i.e. ASAS20.
There are no comparative data regarding the safety or radiologic worsening of treatment using anti-TNF-alpha agents in AS; however, even long-term studies of each of the four agents analysed seem to report good safety profiles for all of them when compared to placebo. Data regarding radiologic worsening are still fragmentary.
As regards patients’ compliance to anti-TNF-alpha therapy for AS, Pavelka et al. reported the follow-up of patients with AS in ATTRA, the Czech National Registry [34], which showed that subcutaneous administration is an efficacious and safe method of treatment.
In this analysis, golimumab demonstrated to be the treatment that at 12 weeks seem to grant the best possibility of reaching ASAS20. This would be essential for a more complete consideration of the therapy allocation for both clinicians and decision makers.
We have to acknowledge for this study several limitations, first of all, the number of trials included in the analysis, based on the RCTs available in scientific literature. Moreover, as mentioned above, there were differences in trial procedures and populations, although they do not seem to invalidate the results obtained; in any case, the MTC technique is able to recognise the possibility of heterogeneity of data and to assess the uncertainty of the estimated relative risks. Another limitation of this study is that it is not possible to perform a randomised effect model MTC, due to the small sample. Indeed, in order to take account of unmeasured or unknown differences in covariates that may act as effects across trials, the use of a random-effect approach would highlight the possibility of the presence of heterogeneity in the compared trials. In this analysis only a fixed-effect model was used.
5 Conclusions
This study provides data for both clinicians and decision makers, contributing to quantify and compare the rate of positive ASAS20 response in patients with AS undergoing subcutaneous biologic therapy, and it may be relevant from a social point of view given the burden of AS affecting the population of working age. This can also improve the ability of clinicians and decision makers to identify the more cost-effective treatment.
Although the MTCs, based on five RCTs available in scientific literature, between golimumab, certolizumab pegol, adalimumab and etanercept did not show a statistically significant difference, this analysis suggests that, of all analysed anti-TNF drugs, golimumab, compared to placebo, seems to provide the highest rate of ASAS20 response in AS patients at 12 weeks. Also, a more complete analysis, including decision factors other than ASAS20, such as compliance, patient preferences, co-morbidities and pharmacoeconomical evaluations are necessary for a more all-encompassing evaluation.
References
Oldroyd J, Schachna L, Buchbinder R, Staples M, Murphy B, Bond M, Briggs A, Lassere M, March L. Ankylosing spondylitis patients commencing biologic therapy have high baseline levels of comorbidity: a report from the Australian rheumatology association database. Int J Rheumatol. 2009;2009:268569. doi: 10.1155/2009/268569. (Epub 2009 Aug 2).
Wittoek R, Mielants H. Clinical assessment in the spondyloarthropathies. Adv Exp Med Biol. 2009;649:1–16.
Sengupta R, Stone MA. The assessment of ankylosing spondylitis in clinical practice. Nat Clin Pract Rheumatol. 2007;3:496–503.
Brandt J, Sieper J, Braun J. Infliximab in the treatment of active and severe ankylosing spondylitis. Clin Exp Rheumatol. 2002;20:S106–10.
Sfikakis PP. The first decade of biologic TNF antagonists in clinical practice: lessons learned, unresolved issues and future directions. Curr Dir Autoimmun. 2010;11:180–210.
McCormack PL, Wellington K. Etanercept: in ankylosing spondylitis. BioDrugs. 2004;18:199–205 (discussion 206).
Breban M, Vignon E, Claudepierre P, et al. Efficacy of infliximab in refractory ankylosing spondylitis: results of asix-month open-label study. Rheumatology (Oxford). 2002;41:1280–5.
Braun J, Davis J, Dougados M, Sieper J, et al. First update of the international ASAS consensus statement for the use of anti-TNF agents in patients with ankylosing spondylitis. Ann Rheum Dis. 2006;65:316–20.
European Medicines Agency. Enbrel 25 mg powder and solvent for solution for injection: summary of product characteristics (online). http:www.emea.eu. Accessed 2006 Sep 5.
Maini RN, Breedveld FC, Kalden JR, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor—monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. 1998;41:1552–63.
Bennett AN, Peterson P, Zain A, et al. Adalimumab in clinical practice. Outcome in 70 rheumatoid arthritis patients, including comparison of patients with and without previous anti-TNF exposure. Rheumatology (Oxford). 2005;44:1026–31.
Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, Reveille JD, Rudwaleit M, van der Heijde D, Stach C, Hoepken B, Fichtner A, Coteur G, de Longueville M, Sieper J. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled phase 3 study. Ann Rheum Dis. 2014;73(1):39–47. doi:10.1136/annrheumdis-2013-204231.
Braun J, Baraliakos X, Hermann KG, van der Heijde D, Inman RD, Deodhar AA, Baratelle A, Xu S, Xu W, Hsu B. Golimumab reduces spinal inflammation in ankylosing spondylitis: MRI results of the randomised, placebo-controlled GO-RAISE study. Ann Rheum Dis. 2012;71(6):878–84. doi: 10.1136/annrheumdis-2011-200308. (Epub 2011 Nov 29. Erratum in: Ann Rheum Dis. 2013 May;72(5):788).
Shu T, Chen GH, Rong L, Feng F, Yang B, Chen R, Wang J. Indirect comparison of anti-TNF-α agents for active ankylosing spondylitis: mixed treatment comparison of randomized controlled trials. Clin Exp Rheumatol. 2013;31(5):717–22. (Epub 2013 Jul 31. Review).
Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics. 2006;24:1–19.
Ades AE, Welton N, Lu G. Introduction to mixed treatment comparisons. Bristol: University of Bristol; 2007.
Caldwell D, Ades A, Higgins J. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900.
Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–24.
van Tubergen A, van der Heijde D, Anderson J, et al. Comparison of statistically derived ASAS improvement criteria for ankylosing spondylitis with clinically relevant improvement according to an expert panel. Ann Rheum Dis. 2003;62:215–21.
van der Heijde D, Kivitz A, Schiff MH, et al. Efficacy and safety of adalimumab in patients with ankylosing spondylitis: results of a multicenter, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2006;54:2136–46.
Davis JC Jr, Van Der Heijde D, Braun J, et al. Recombinant human tumor necrosis factor receptor (etanercept) for treating ankylosing spondylitis: a randomized, controlled trial. Arthritis Rheum. 2003;48:3230–6.
Calin A, Dijkmans BA, Emery P, Hakala M, Kalden J, Leirisalo-Repo M, Mola EM, Salvarani C, Sanmartí R, Sany J, Sibilia J, Sieper J, van der Linden S, Veys E, Appel AM, Fatenejad S. Outcomes of a multicentre randomised clinical trial of etanercept to treat ankylosing spondylitis. Ann Rheum Dis. 2004;63(12):1594–600.
Inman RD, Davis JC Jr, Heijde Dv, Diekman L, Sieper J, Kim SI, Mack M, Han J, Visvanathan S, Xu Z, Hsu B, Beutler A, Braun J. Efficacy and safety of golimumab in patients with ankylosing spondylitis: results of a randomized, double-blind, placebo-controlled, phase III trial. Arthritis Rheum. 2008;58(11):3402–12. doi:10.1002/art.23969.
Landewé R, Braun J, Deodhar A, Dougados M, Maksymowych WP, Mease PJ, Reveille JD, Rudwaleit M, van der Heijde D, Stach C, Hoepken B, Fichtner A, Coteur G, de Longueville M, Sieper J. Efficacy of certolizumab pegol on signs and symptoms of axial spondyloarthritis including ankylosing spondylitis: 24-week results of a double-blind randomised placebo-controlled Phase 3 study. Ann Rheum Dis. 2014;73(1):39–47. doi:10.1136/annrheumdis-2013-204231.
Cooper NJ, Sutton AJ, Lu G, Khunti K. Mixed comparison of stroke prevention treatments in individuals with nonrheumatic atrial fibrillation. Arch Intern Med. 2006;166:1269–75.
Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet. 2009;373:746–58.
Welton NJ, Caldwell DM, Adamopoulous E, et al. Mixed treatment comparison meta-analysis of complex interventions: psychological interventions in coronary heart disease. Am J Epidemiol. 2009;169:1158–65.
Jansen JP, Bergman GJ, Huels J, et al. Prevention of vertebral fractures in osteoporosis: mixed treatment comparison of bisphosphonate therapies. Curr Med Res Opin. 2009;25:1861–8.
Devine EB, Alfonso-Cristancho R, Sullivan SD. Effectiveness of biologic therapies for rheumatoid arthritis: an indirect comparisons approach. Pharmacotherapy. 2011;31(1):39–51.
Bergman GJ, Hochberg MC, Boers M, Wintfeld N, Kielhorn A, Jansen JP. Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum. 2010;39(6):425–41.
Migliore A, Broccoli S, Bizzi E, Laganà B. Indirect comparison of the effects of anti-TNF biological agents in patients with ankylosing spondylitis by means of a mixed treatment comparison performed on efficacy data from published randomised, controlled trials. J Med Econ. 2012;15(3):473–80. doi:10.3111/13696998.2012.660255. (Epub 2012 Feb 16. Review. PubMed PMID: 22335398).
Migliore A, Bizzi E, Broccoli S, Laganà B. Indirect comparison of etanercept, infliximab, and adalumimab for psoriatic arthritis: mixed treatment comparison using placebo as common comparator. Clin Rheumatol. 2012;31(1):193–4. doi:10.1007/s10067-011-1862-7. (Epub 2011 Oct 18. PubMed PMID: 22005889).
Malaviya AP, Ostör AJ. Drug adherence to biologic DMARDS with a special emphasis on the benefits of subcutaneous abatacept. Patient Prefer Adher. 2012;2012(6):589–96. doi:10.2147/PPA.S23786 Epub. PubMed PMID: 22936845; PubMed Central PMCID: PMC3429155.
Pavelka K, Forejtová S, Stolfa J, Chroust K, Buresová L, Mann H, Vencovský J. Anti-TNF therapy of ankylosing spondylitis in clinical practice. Results from the Czech national registry ATTRA. Clin Exp Rheumatol. 2009;27(6):958–63.
Competing interests
This study was conducted with unrestricted funds from ANTIAGE (a non profit National Association for ultrasound-guided intra-articular hip therapy) whose President is Prof. Alberto Migliore. Prof. Alberto Migliore and Prof. Bruno Laganà received grants as consultants from Pfizer, Abbvie and Merck for national and international studies.
Authors’ contribution
Prof. Migliore A. and Prof. Laganà B. both contributed to study concept and realisation, Dr Bernardi M. and Prof. Petrella L. contributed providing statistical analysis and Dr Bizzi E. contributed by analysing results and to the development text.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Migliore, A., Bizzi, E., Bernardi, M. et al. Indirect Comparison Between Subcutaneous Biologic Agents in Ankylosing Spondylitis. Clin Drug Investig 35, 23–29 (2015). https://doi.org/10.1007/s40261-014-0246-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40261-014-0246-6