Abstract
Our study aimed to determine whether proatherogenic lipid profiles exist in patients with active Takayasu arteritis (TA) and assess the relationship between different lipid profiles and disease activity in TA. A total of 132 premenopausal female patients with TA and 100 sex-, age-, and body mass index-matched healthy controls were included in our study. The clinical data were collected in detail from all participants. Patients with active TA had significantly lower levels of apolipoprotein A1 (apoA1) (1.47 ± 0.30 vs. 1.99 ± 0.33 mmol/L, p < 0.001) and lower levels of high-density lipoprotein cholesterol (HDL-C) (1.23 ± 0.33 vs. 1.68 ± 0.38 mmol/L, p < 0.001) than patients with inactive TA. However, they had higher ratios of apolipoprotein B (apoB)/apoA1 (0.74 ± 0.27 vs. 0.48 ± 0.14, p < 0.001) compared with patients with inactive TA. Multiple linear regression analysis demonstrated that the apoB/apoA1 ratio was independently associated with TA activity (β = 0.38, p = 0.04). In addition, multivariate stepwise forward regression analysis showed that the apoB/apoA1 ratio was the major determinant for high-sensitivity C-reactive protein (β = 0.58, p = 0.002). Our findings indicate that patients with active TA had proatherogenic lipid profiles. In addition, the ratio of apoB to apoA1 could be used as a marker for monitoring and targeting patients with TA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Takayasu arteritis (TA) is a systemic inflammatory disease that involves the aorta and its main branches, resulting in luminal stenosis and aneurysmal changes in the large vessels [1]. The view that inflammation can promote the incidence of atherosclerotic diseases has been widely accepted. Currently, the leading cause of cardiovascular mortality in TA is congestive heart failure, which is mainly due to uncontrolled hypertension and aortic valve insufficiency in TA. Nevertheless, accelerated atherosclerosis may be associated with stroke and transient ischemic attack, which occur in up to 20 % of this disease [2–4]. In addition, autopsy and ultrasonic and image examinations have revealed atherosclerotic lesions in TA patients [5, 6]. However, whether increased atherosclerosis in TA patients has an effect on vessel walls, leads to augmented and sustained inflammatory activities, or is associated with traditional atherosclerotic risk factors (such as hypertension and dyslipidemia) have not yet been clarified [2]. Previous studies on the characterization of 25 TA patients reported that TA has a proatherogenic lipid profile, and this profile is characterized by low levels of high-density lipoprotein cholesterol (HDL-C) [7]. Our study aimed to characterize the spectrum of lipid profiles in TA patients to investigate whether proatherogenic lipid profiles exist in patients with active TA and assess the relationship between inflammatory activity of TA and lipid profiles.
Materials and methods
Inclusion criteria and exclusion criteria
A total of 132 premenopausal female TA patients and 100 sex-, age-, and body mass index (BMI)-matched healthy controls were consecutively screened in Fuwai hospital from 2011 to 2013. Patients with diabetes mellitus, acute infection, cardiovascular disease, liver or kidney disorder, nephritic syndrome, alcoholism, and thyroid abnormalities were excluded.
Diagnostic and classification criteria
All the patients satisfied the 1990 American College of Rheumatology criteria [8] and fulfilled at least three of the following: age at disease onset ≤40 years, claudication of the extremities, decreased brachial artery pressure, blood pressure difference between both arms ≥10 mmHg, bruit over the subclavian arteries or aorta, and abnormalities on arteriography. All 132 patients underwent aortic angiography at the time of diagnosis. They were classified into six types (types I, IIa, IIb, III, IV, and V), according to the International TA Conference in Tokyo 1994 angiographic classification [9] as follows: type I, involvement of the main branches from the aortic arch; type IIa, involvement of the ascending aorta, aortic arch, and its branches; type IIb, involvement of the ascending aorta, aortic arch and its branches, and thoracic descending aorta; type III, involvement of the thoracic descending aorta, abdominal aorta, and/or renal arteries; type IV, involvement of the abdominal aorta and/or renal arteries; and type V, the combined features of types IIb and IV. The active group with disease activity satisfied the NIH criteria [10] if at least two of the following criteria were satisfied: systemic features (e.g., fever or musculoskeletal features) with no other cause identified; elevated erythrocyte sedimentation rate; features of vascular ischemia or inflammation, such as claudication, diminished or absent pulses, bruit, vascular pain (carotidynia), and asymmetric blood pressure in either the upper or lower limbs (or both); and typical angiographic features.
Laboratory measurement
Blood samples were collected early in the morning to measure fasting lipid profiles. Serum lipids were measured using biochemical assays. Serum high-sensitivity C-reactive protein (hsCRP) and erythrocyte sedimentation rate were determined using immunoturbidimetry (Beckmann Assay 360, Bera, CA, USA).
Statistical analysis
The mean levels of variables were compared using ANOVA, and the χ 2 test was used for categorical variables. Comparisons between each combination of two groups were conducted using the Student–Newman–Keuls test. Multiple linear regression analysis was used to determine the association between different lipid profiles and disease activity after adjusting the dose of glucocorticoids and other confounding factors. The association between hsCRP and other variables were examined by Pearson’s correlation analysis. Multivariable stepwise forward analysis was used to examine the independent correlated factor of hsCRP. A p value of <0.05 in the two-sided test was considered statistically significant. All statistical analyses were conducted with the SPSS 19.0 statistical package.
Results
Clinical characteristics
Table 1 shows the characteristics of the patients. The mean disease duration was 10.5 ± 8.0 and 15.0 ± 8.6 years in patients with active and inactive TA, respectively. A total of 49 (37.1 %) of the 132 patients had type I, and 11 patients (8.3 %) had type II, which comprised five (3.7 %) type IIa and six (4.5 %) type IIb. Moreover, 10 patients (7.6 %) had type III, 22 (16.7 %) had type IV, and 40 (30.3 %) had type V.
Lipid profiles in TA patients and healthy controls
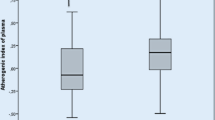
TA patients had significantly higher levels of apolipoprotein B (apoB) (0.97 ± 0.30 vs. 0.84 ± 0.19 mmol/L, p = 0.01), as well as lower levels of HDL-C (1.40 ± 0.41 vs. 1.55 ± 0.37 mmol/L, p = 0.05) and apolipoprotein A1 (apoA1) (1.71 ± 0.38 vs. 1.79 ± 0.27 mmol/L, p = 0.04), compared with the healthy controls (Table 2). The serum levels of total cholesterol (TC) (4.35 ± 0.96 vs. 4.80 ± 1.13 mmol/L, p = 0.008), HDL-C (1.23 ± 0.33 vs. 1.68 ± 0.38 mmol/L, p < 0.001), and apoA1 (1.47 ± 0.30 vs. 1.99 ± 0.33 mmol/L, p < 0.001) were significantly lower in patients with active TA compared with those in patients with inactive TA. By contrast, the serum ratios of apoB/apoA1 (0.74 ± 0.27 vs. 0.48 ± 0.14 mmol/L, p < 0.001), non-HDL-C/HDL-C (2.73 ± 1.10 vs. 2.01 ± 0.75 mmol/L, p < 0.001), and apoB/low-density lipoprotein cholesterol (LDL-C) (0.41 ± 0.09 vs. 0.35 ± 0.07 mmol/L, p < 0.001) were significantly higher in patients with active TA compared with those in patients with inactive TA. Multiple linear regression analyses indicated that the apoB/apoA1 ratio had a significant positive correlation with TA activity after adjusting for age, BMI, glucocorticoid dose, dose of statins, classification, hypertension, microalbuminuria, serum creatinine, aspartate aminotransferase, alanine aminotransferase, TC, and triglyceride (TG) (β = 0.38, p = 0.04) (Tables 3 and 4). No significant association was found between different doses of glucocorticoids and the alteration in lipid profiles, except serum triglyceridemia.
Relationship between lipid profiles of TA and inflammation markers
Pearson’s correlation analysis indicated that serum hsCRP correlated positively with the ratios of apoB/apoA1 (r = 0.45, p < 0.01), non-HDL-C/HDL-C (r = 0.29, p = 0.01), apoB/LDL-C (r = 0.33, p < 0.01), and levels of apoB (r = 0.23, p < 0.05), but correlated negatively with the levels of HDL-C (r = −0.36, p = 0.001) and apoA1 (r = −0.41, p < 0.001). The hsCRP level was not significantly correlated with the LDL-C level (r = − 0.03, p = 0.80) (Table 5). Multivariate stepwise forward regression analysis was performed to estimate the independent predictor of hsCRP. In this regression formula, age, BMI, hypertension, MAU, Scr level, TG level, TC level, HDL-C level, apoA1 level, apoB level, apoB/apoA1 ratio, and non-HDL-C/HDL-C ratio were used as independent variables. The hsCRP level was used as the dependent variable. The apoB/apoA1 and non-HDL-C/HDL-C ratios and apoA1 entered the regression model, and the apoB/apoA1 ratio was the major determinant for hsCRP (β = 0.58, p = 0.002).
Discussion
Our study demonstrated that patients with active TA had altered atherogenic lipoprotein and apolipoprotein abnormalities. The ratio of apoB to apoA1 was independently associated with disease activity. In addition, the apoB/apoA1 ratio was the major determinant for hsCRP.
Traditional indicators, such as higher LDL-C and lower HDL-C, are known risk factors for atherosclerosis [11]. In recent years, studies found that apoB-containing particles are the main triggers in the atherogenic process. Moreover, the higher the ratios of apoB/apoA1 and non-HDL-C/HDL-C, the more cholesterol is likely to be deposited in the arterial wall, thereby provoking atherogenesis and increasing cardiovascular disease (CVD) risk [12–15]. TA patients exhibited serious atherogenic lipoprotein and apolipoprotein abnormalities, such as higher levels of LDL-C and apoB and lower levels of HDL-C and apoA1, compared with the healthy controls in our data. All these combined risk factors contribute to increasing the CVD risk in TA patients.
Experimental studies on animals and humans suggested that inflammation alters HDL composition [16, 17]. Consistent with previous reports, our data show that patients with active TA displayed lower levels of HDL-C and apoA1 compared with patients with inactive TA. These particles were negatively correlated with hsCRP level. Although the exact mechanisms remain unclear, studies demonstrated that displacement of HDL-apoA1 by serum amyloid A is thought to be a major factor in inflammation-induced HDL dysfunctional transformation. Loss of apoA1 functionality and impaired lecithin–cholesterol acyltransferase and lipoprotein-associated phospholipase A2 activities result in abnormal anti-inflammatory and antioxidative activities of HDL, as well as a reduction in circulating apoA1 levels in inflammation [18, 19].
Increases in markers of inflammation or infection are commonly associated with a reduction in TC and LDL-C in primates and humans [18]. In our study, no significant changes were observed in the levels of LDL-C between patients with active and inactive TA. This finding may be due to the fact that the effect of glucocorticoids on hyperlipidemia may partially neutralize the circulating levels of TC and LDL-C during inflammation [20]. Another explanation for this finding is that LDL-C measurements assess the mass of cholesterol in the LDL particle but not its number and size. LDL-C with small particle size is called small dense LDL, which has a potent atherosclerosis-inducing effect because of its low affinity for LDL receptors and susceptibility to oxidative modification [12]. Therefore, the concentration of LDL-C might not reflect inflammatory activity in patients with TA.
In our study, the active TA group showed higher levels of apoB and higher ratio of apoB to apoA1 than the inactive TA group. As of this writing, the mechanism for the increase in the apoB level during inflammation has not been clarified. The total level of apoB is believed to be closely correlated with the levels of small dense LDL particles [12] and represents the total atherogenic particle number and pro-inflammatory lipoproteins [21]. ApoB is the primary predictor of inflammatory markers in nondiabetic postmenopausal women among various risk parameters, including adiposity and insulin resistance [22]. Onat et al. [23] found that serum apoB is independently predictive of metabolic syndrome and diabetes in women, and the predictive ability of elevated apoB levels might be ascribed to the increase in small LDL particles and potential role of apoB as a subclinical inflammatory agent.
Our study found that the apoB/apoA1 ratio was the primary determinant for hsCRP, possibly because it could reflect the balance between concentrations of proatherogenic versus antiatherogenic lipoprotein particles. Thus, the improvement in apoB/apoA1 ratio might be associated with the remission of inflammation and should be monitored as a potential indicator of inflammatory status in TA patients. In addition, both inflammatory and dyslipidemia processes in TA could contribute to increased CVD risk. Inflammation also adversely affected the lipid profiles in patients with active TA in our study. Thus, the effective control of inflammatory status may suppress the atherosclerotic process in patients with active TA.
Glucocorticoids are the gold standard for TA treatment, and long-term use of these drugs in high dosage is known to cause dyslipidemia [24]. However, no significant association between the use of glucocorticoids and alteration in lipoprotein and apolipoprotein profiles were found in this study. A high ratio of apoB/apoA1 was independently associated with disease activity. This ratio might be used to estimate the effectiveness of glucocorticoid treatment. The apoB/apoA1 ratio was useful in identifying the potential higher risk for CVD, especially in the stratum of patients with normal LDL-C levels [25]. Lipid-modifying therapy on the apolipoprotein profiles might be an effective approach for patients with high apoB/apoA1 ratios. Studies have confirmed that statins can be used to modulate endothelial function and reduce inflammatory processes [26]. Therefore, statins might be suggested to TA patients for their beneficial effects on inflammation and lipid levels.
The major limitation of this study is that its design was cross-sectional. Longitudinal studies and intervention studies are necessary to confirm the association between lipid profiles and disease activity in TA patients. Patients with some factors that affect lipid profiles were excluded. Therefore, the exclusion criteria in TA patients could limit the description of real lipid profiles in the general TA population.
In conclusion, our findings suggest that patients with active TA had an aggregation of proatherogenic lipoprotein and apolipoprotein profiles. Effective anti-inflammatory treatment should be recommended in the early stage of TA. In addition, the ratio of apoB to apoA1 could be monitored and controlled in patients with active TA to limit the long-term risk of CVD.
References
Subramanyan R, Joy J, Balakrishnan KG (1989) Natural history of aortoarteritis (Takayasu’s disease). Circulation 80:429–437
Park KC, Kim JH, Yoon SS, Heo SH (2008) Takayasu’s disease presenting with atherothrombotic ischaemic stroke. Neurol Sci 29:363–366
Seyahi E, Ugurlu S, Cumali R, Balci H, Seyahi N, Yurdakul S et al (2006) Atherosclerosis in Takayasu arteritis. Ann Rheum Dis 65:1202–1207
Numano F, Okawara M, Inomata H, Kobayashi Y (2000) Takayasu’s arteritis. Lancet 356:1023–1025
Raninen RO, Kupari MM, Hekali PE (2002) Carotid and femoral artery stiffness in Takayasu’s arteritis. An ultrasound study. Sacnd J Rheumatol 31:85–88
Park SH, Chung JW, Lee JW, Han MH, Park JH (2001) Carotid artery involvement in Takayasu’s arteritis: evaluation of the activity by ultrasonography. J Ultrasound Med 20:371–378
de Carvalho JF, Bonfá E, Bezerra MC, Pereira RM (2009) High frequency of lipoprotein risk levels for cardiovascular disease in Takayasu arteritis. Clin Rheumatol 28:801–805
Arend WP, Michel BA, Bloch DA, Hunder GG, Calabrese LH, Edworthy SM et al (1990) The American College of Rheumatology 1990 criteria classification of Takayasu arteritis. Arthritis Rheum 33:1129–1134
Hata A, Noda M, Moriwaki R, Numano F (1996) Angiographic findings of Takayasu arteritis: new classification. Int J Cardiol 54(Suppl):S155–S163
Kerr GS, Hallahan CW, Giordano J, Leavitt RY, Fauci AS, Rottem M et al (1994) Takayasu arteritis. Ann Intern Med 120:919–929
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (2001) JAMA 285:2486–2497
Tani S, Saito Y, Anazawa T, Kawamata H, Furuya S, Takahashi H et al (2011) Low-density lipoprotein cholesterol/apolipoprotein B ratio may be a useful index that differs in statin-treated patients with and without coronary artery disease: a case control study. Int Heart J 52:343–347
Kim SW, Jee JH, Kim HJ, Jin SM, Suh S, Bae JC et al (2013) Non-HDL-cholesterol/HDL-cholesterol is a better predictor of metabolic syndrome and insulin resistance than apolipoprotein B/apolipoprotein A1. Int J Cardiol 3:2678–2683
Sierra-Johnson J, Fisher RM, Romero-Corral A, Somers VK, Lopez-Jimenez F, Ohrvik J et al (2009) Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J 30:710–717
Mattsson N, Magnussen CG, Rönnemaa T, Mallat Z, Benessiano J, Jula A et al (2010) Metabolic syndrome and carotid intima-media thickness in young adults: roles of apolipoprotein B, apolipoprotein A-I, C-reactive protein, and secretory phospholipase A2: the cardiovascular risk in young Finns study. Arterioscler Thromb Vasc Biol 30:1861–1866
Paradis ME, Badellino KO, Rader DJ, Deshaies Y, Couture P, Archer WR et al (2006) Endothelial lipase is associated with inflammation in humans. J Lipid Res 47:2808–2813
de Carvalho JF, Borba EF, Viana VS, Bueno C, Leon EP, Bonfá E (2004) Anti-lipoprotein lipase antibodies: a new player in the complex atherosclerotic process in systemic lupus erythematosus? Arthritis Rheum 50:3610–3615
Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR et al (2004) Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res 45:1169–1196
Dullaart RP, Perton F, Kappelle PJ, de Vries R, Sluiter WJ, van Tol A (2010) Plasma lecithin: cholesterol acyltransferase activity modifies the inverse relationship of C-reactive protein with HDL cholesterol in nondiabetic men. Biochim Biophys Acta 1801:84–88
Faggiano A, Pivonello R, Spiezia S, De Martino MC, Filippella M, Di Somma C et al (2003) Cardiovascular risk factors and common carotid artery caliber and stiffness in patients with Cushing’s disease during active disease and 1 year after disease remission. J Clin Endocrinol Metab 88:2527–2533
Sniderman AD (2005) Apolipoprotein B versus non-high-density lipoprotein cholesterol—and the winner is…. Circulation 112:3366–3367
Faraj M, Messier L, Bastard JP, Tardif A, Godbout A, Prud’homme D et al (2006) Apolipoprotein B: a predictor of inflammatory status in postmenopausal overweight and obese women. Diabetologia 49:1637–1646
Onat A, Can G, Hergenç G, Yazici M, Karabulut A, Albayrak S (2007) Serum apolipoprotein B predicts dyslipidemia, metabolic syndrome and, in women, hypertension and diabetes, independent of markers of central obesity and inflammation. Int J Obes 31:1119–1125
Seko Y (2007) Giant cell and Takayasu arteritis. Curr Opin Rheumatol 19:39–43
Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD (2004) The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med 42:1355–1363
Blum A, Shamburek R (2009) The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis 203:325–330
Acknowledgments
This study is upported by the National Natural Science Foundation of China (grant no. 81170285) and the Research Fund for the Doctoral Program of Higher Education of China (grant no. 20101106110012).
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, X., Chen, B., Lv, N. et al. Association of abnormal lipid spectrum with the disease activity of Takayasu arteritis. Clin Rheumatol 34, 1243–1248 (2015). https://doi.org/10.1007/s10067-014-2819-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2819-4