Abstract
Imbalance of natural killer (NK) cells is associated with the development of systemic lupus erythematosus (SLE). However, little is known about the dynamic changes on NK cells following therapy. This study aimed at examining the impact of classic therapies on the numbers of different subsets of NK cells in new-onset SLE patients. The numbers of different subsets of peripheral blood NK cells in 24 new-onset SLE patients before, 4 and 12 weeks post the classic therapies, and 7 healthy controls were determined by flow cytometry. The potential correlation between the numbers of NK cells and the values of clinical measures was analyzed. In comparison with that before treatment, the numbers of NK, NKG2C+, and KIR2DL3+ NK cells were significantly increased while the numbers of NKp46+ and NKG2A + NK cells significantly decreased at 4 and/or 12 weeks post the treatment only in the drug well-responding patients, but not in those poor responders (P < 0.05 for all). The numbers of NKG2C + NK cells were correlated positively with the levels of serum C3 while the numbers of KIR2DL3+ NK cells were correlated negatively with the scores of SLEDAI in these patients at 4 weeks post the treatment. The classic therapies modulated the numbers of some subsets of NK cells in drug well-responding SLE patients. The changes in the numbers of some subsets of NK cells may serve as biomarkers for evaluating the therapeutic responses of SLE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Systemic lupus erythematosus (SLE) is an inflammatory autoimmune disease. SLE patients produce several types of autoantibodies against nuclear antigens, leading to formation of immune complexes, inflammation, and progressive injury of many organs and systems [1]. It is well known that genetic and environment factor, sex hormone, infection, and some drugs are associated with the development of SLE and autoimmunity is crucial for the pathogenesis of SLE. Self-reactive T and B cells spontaneously activate and play important roles in tissue damage. Other immunocomptent cells also regulate autoimmunity and the progression of SLE.
Natural killer (NK) cells are innate immune lymphocytes and play an important role in both innate and adaptive immunity. The function of NK cells is regulated by a balance between activating and inhibitory signals originating from a diverse array of surface receptors [2]. NK cells express predominantly activation and cytotoxicity receptors, including NKG2C, NKG2D, NKp30, NKp46, and others [3–5]. Furthermore, NK cells also express inhibitory receptors, such as the lectin-like CD94-NKG2A and the killer cell immunoglobulin-like receptors (KIRs) [6–8]. In addition, NK cells participate in immune regulation by producing a wide range of inflammatory cytokines and other cytotoxic factors mediating cytotoxicity against target cells. Previous studies have shown that dysfunction and deficiency of NK cells are associated with the development of SLE [9] and SLE patients displayed significantly lower ratio of NKG2A + to NKG2D NK cells [10]. Our previous study has shown lower frequency of NK cells expressing inhibitory receptors but higher frequency of NK cells expressing activation receptors in SLE patients[11]. Therefore, the imbalance of activating and inhibitory NK cells contributes to the development and progression of SLE.
Currently, SLE patients are treated with the classic therapies of glucocorticoid (GC) and immunosuppressants of cyclophosphamide (CTX) and hydroxychloroquine (HCQ). However, there is no information on how the dynamic changes in the frequency and numbers of different subsets of peripheral blood NK cells following the classic therapies in newly diagnosed SLE patients. It is unclear whether there is any difference in the frequency and numbers of different subsets of peripheral blood NK cells between drug responders and nonresponders.
In this study, we aimed to characterize the dynamic changes in the numbers of different subsets of peripheral blood NK cells following classic therapies with GC, HCQ, and CTX. We found that the classic treatment increased the numbers of NK, NKG2C+, and KIR2DL3+ but decreased the frequency and numbers of NKp46+ and NKG2A + NK cells in newly diagnosed SLE patients. We discussed the implications of our findings.
Materials and methods
Patients and controls
A total of 24 newly diagnosed SLE patients were recruited from the inpatient service of the First Hospital of Jilin University, Changchun, China, from September, 2011 to August, 2012. All SLE patients met the diagnostic criteria of the American College of Rheumatology (ACR). The degree of disease activity was assessed using SLE disease activity index (SLEDAI) by experienced physicians, and a score ≥6 was considered active SLE. Patients were excluded if she/he had other autoimmune disease, recent infection, or had received immunosuppressant therapies or GC therapies within the past 6 months. Additional 7 age and gender-matched healthy controls were recruited from the Physical Examination Center of our hospital. Written informed consent was obtained from individual participants. The study was designed in accordance with the guidelines of the Declaration of Helsinki and was approved by the Human Ethics Committee of Jilin University.
All patients with SLE were treated orally with GC (1 mg/kg/day) and HCQ (0.4 g/day) and intravenously with CTX (0.4 g/week) for more than 12 weeks.
Data collection and clinical evaluation
The demographic and clinical data of individual patients were collected from the hospital records. The data included age, gender, a history of diseases and current medications. Their venous blood samples were collected before treatment and 4 and 12 weeks after treatment. Their blood white blood cell (WBC) count and blood platelet count were routinely examined and the concentrations of serum, anti-dsDNA, anti-Sm, C-reactive protein (CRP), and complement C3 and C4 were determined using scatter turbidimetry on a Siemens special protein analysis instrument (Siemens Healthcare Diagnostics Products, GmbH, Germany). The demographic and clinical characteristics of patients are summarized in Table 1.
Flow cytometry analysis
Fasting venous blood samples were collected from individual patients and were subjected to flow cytometry analysis. Briefly, individual blood samples (100 μl) were stained a mixture of fluorescein isothiocyanate (FITC)-conjugated anti-CD3, allophycocyanin (APC)-conjugated anti-CD56, peridinin chlorophyll protein (PerCP)-conjugated anti-CD16, and phycoerythrin (PE)-conjugated anti-NKG2D, anti-NKG2C, anti-NKp30, anti-NKp46, anti-NKG2A, anti-KIR2DL3, anti-KIR3DL1, anti-CD158a, and anti-CD158b (BD Biosciences, San Diego, USA) for 30 min at room temperature, respectively. The same isotype fluorescent mouse IgG1 and IgG2a were used as negative controls. The remaining erythrocytes were lysed using BD FACS Lysing Solution 2 (BD Biosciences), and the frequency of different subsets of NK cells was determined by flow cytometry using a FACS Calibur instrument (BD Bioscience) and FlowJo software (v7.6.2) (TreeStar, Ashland, OR, USA). The numbers of different subsets of NK cells per ml peripheral blood were calculated, according to the numbers of lymphocytes.
IFN-γ production
Peripheral blood mononuclear cells (PBMCs) were isolated from individual blood samples by Ficoll density gradient centrifugation using Ficoll-Paque Plus (Amersham Biosciences, Little Chalfont, UK). The PBMCs (106 cells/well) were stimulated in duplicate with 50 ng/ml of phorbol myristate acetate (PMA; Sigma-Aldrich St. Louis, MO, USA) and 1.0 μg/ml of ionomycin (Sigma-Aldrich) in complete RPMI-1640 medium (Invitrogen, Carlsbad, CA, USA) at 37 °C for 2 h in a humidified incubator of 5 % CO2 and then exposed to 1.0 μg/ml of Brefeldin A (GolgiPlug, Becton Dickinson) for an additional 4 h. After being washed, the cells were stained with FITC-anti-CD56 and APC-anti-CD3 (BD Biosciences), and fixed with 4 % paraformaldehyde at room temperature for 30 min. The cells were permeabilized and stained with PE-anti-IFN-γ, followed by flow cytometry analysis. The same cells were cultured in medium alone and used as negative controls. The frequency of IFN-γ + CD3 − CD56+ NK cells was determined by flow cytometry analysis.
The degranulation of NK cells
The PBMCs (106 cells/well) were cocultured with K562 cells at a ratio of 10:1 in RPMI-1640 medium for 1 h in the presence of anti-CD107a (H4A3; Becton Dickinson) or control IgG2a and exposed to 2-μl monensin (GolgiStop; BD Sciences) for 5 h. The PBMCs alone served as negative controls. After being washed, the cells were stained with PE-anti-mouse IgG2a and were continually stained with FITC-anti-CD3 and APC-anti-CD56. The frequency of CD107a + CD3 − CD56+ NK cells was determined by flow cytometry.
Statistical analysis
All data are expressed as median unless specified. The difference in category data among different time points was analyzed by Fisher exact test. The difference in other values among groups was analyzed by the Kruskal-Wallis test. the difference between two groups was analyzed by the Wilcoxon signed-rank test, a nonparametric test using the SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA). A two-side P value of <0.05 was considered statistically significant.
Results
Classic treatment mitigates disease severity in newly diagnosed SLE patients
To determine the impact of the classic therapies on the dynamic changes in the numbers of peripheral blood NK cells, 24 patients with new-onset SLE and 7 healthy controls (HC) were recruited. As shown in Table 1, there was no significant difference in the distribution of age and gender between the patients and HC in this population. Following the classic therapies with GC, CTX, and HCQ, the values of SLEDAI were significantly reduced and the numbers of peripheral blood lymphocytes significantly increased in those patients at 4 and 12 weeks posttreatment (P < 0.05 vs. baseline). Similarly, the concentrations of serum C3 and C4 significantly increased and CRP decreased as well as the positivity of anti-DNA in those patients at 12 weeks posttreatment (P < 0.05. vs. baseline). Stratification analysis indicated that 16 and 20 patients (well responders, WR) responded to the classic therapies and achieved a value of SLEDAI <6.0 at 4 and 12 weeks posttreatment, respectively (Table 2). The well responders displayed significantly reduced values of SLEDAI and higher concentrations of serum C3 and C4. In contrast, there was no significant change in the values of these measures in the drug poor responders (PR). These data clearly indicated that the classic therapies mitigated the severity of SLE in those patients, particularly in the WR.
Classic therapies modulate the numbers of different subsets of peripheral blood NK cells in newly diagnosed SLE patients
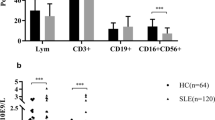
Next, we examined the numbers of different subsets of peripheral blood NK cells by flow cytometry. We found that while there was significantly less numbers of CD3 − CD56+ NK cells in patients before treatment, as compared with that in the HC, the numbers of NK cells significantly increased in the patients at 12 weeks posttreatment (P = 0.001) and were similar to that in the HC (Fig. 1). Further analyses indicated that in comparison with that before treatment, the numbers of NKG2C + NK cells also significantly increased in those patients at 4 and 12 weeks posttreatment (P = 0.049 and P < 0.001, respectively) (Fig. 2(A and a)) and were insignificantly different from that in the HC. Similarly, the numbers of KIR2DL3+ NK cells significantly increased in those patients at 12 weeks posttreatment (P = 0.027) and were not significantly different from that in HC (Fig. 2(B and b)). In contrast, the numbers of NKp46+ NK cells were significantly reduced at 12 weeks posttreatment (P = 0.039) and NKG2A + NK cells significantly increased at 4 and 12 weeks posttreatment in those patients (P = 0.043 and P = 0.037, respectively) (Fig. 2(C and c), (D and d)). However, there was no significant change in the numbers of NKG2D+, NKp30+, CD158a+, and CD158b + NK cells at 4 and 12 weeks posttreatment in those patients (data not shown). In addition, there was no significant change in the numbers of IFN-γ + and CD107a + NK cells in those patients following classic therapies for 12 weeks in this population (data not shown). Apparently, classic therapies modulated the balance of different subsets of NK cells in SLE patients.
Characterization of different subsets of CD3−CD56+ NK cells in SLE patients after drug treatment. Peripheral blood cells were stained with different fluorescent antibodies, and after lysis of red blood cells, the remaining cells were gated on living lymphocytes and further gated on CD3 − CD56+ NK cells. Data shown are representative charts of flow cytometry and expressed as the mean values of individual subjects at the indicated time points. (A) Flow cytometry analysis of CD3−CD56+ NK cells; (a) Quantitative analysis of the numbers of CD3−CD56+ NK cells. The horizontal lines indicate the median values
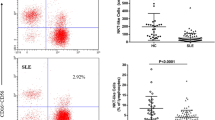
Characterization of different subsets of CD3−CD56+ NK cells in SLE patients after drug treatment. Peripheral blood cells were stained with different fluorescent antibodies, and after lysis of red blood cells, the remaining cells were gated on living lymphocytes and further gated on CD3 − CD56+ NK cells. The frequency of different subsets of NK cells were analyzed by flow cytometry. Data shown are representative charts of flow cytometry and expressed as the mean values of individual subjects at the indicated time points. (A) Flow cytometry analysis of NKG2C+ NK cells; (a) Quantitative analysis of the numbers of NKG2C+ NK cells; (B) Flow cytometry analysis of KIR2DL3+ NK cells; (b) Quantitative analysis of the numbers of KIR2DL3+ NK cells. (C) Flow cytometry analysis of NKp46+ NK cells; (c) Quantitative analysis of the numbers of NKp46+ NK cells; (D) Flow cytometry analysis of NKG2A+ NK cells; (d) Quantitative analysis of the numbers of NKG2A+ NK cells. There is no significant difference in the numbers of other subsets of NK cells in the patients among these time points (data not shown). The horizontal lines indicate the median values in individual groups
Further analyses revealed that in comparison with that before treatment, the numbers of NKG2C + NK cells significantly increased at 4 and 12 weeks posttreatment only in the WR group of patients (P = 0.023 and P < 0.001, respectively, Fig. 3a). Similarly, the numbers of KIR2DL3+ NK cells also significantly increased at 12 weeks posttreatment (P = 0.022, Fig. 3b). In contrast, the numbers of NKp46+ NK cells significantly decreased at 12 weeks posttreatment in the WR group of patients, but not in the PR group (P = 0.043, Fig. 3c). In addition, the numbers of NKG2A + NK cells were significantly reduced at 4 and 12 weeks in the WR group, but not in the PR group (P = 0.042 and P = 0.027, respectively, Fig. 3d). Therefore, classic therapies modulated the balance of different subsets of NK cells in the patients who well responded, but not in those poor responding.
Characterization of different subsets of CD3 − CD56+ NK cells in SLE patients after drug treatment. Patients were stratified according the values of SLEDAI, and the numbers of NKG2C+, KIR2DL3+, NKp46+ NKG2A + NK cells in individual patients were analyzed before and 4 and 12 weeks after the treatment. Data shown are the mean values of different subsets of NK cells in individual subjects. a Stratification analysis of the numbers of NKG2C+ NK cells between the well responders (WR) and poor responders (PR); b Stratification analysis of the numbers of KIR2DL3+ NK cells between the WR and PR; c Stratification analysis of the numbers of NKp46+ NK cells between the WR and PR; d Stratification analysis of the numbers of NKG2A+ NK cells between the WR and PR. The horizontal lines indicate the median values in individual groups
Positive correlation between the NKG2C + NK cells and C3 while negative correlation between KIR2DL3+ NK cell subsets and SLEDAI score in the patients at 4 weeks posttreatment
We analyzed the potential relationship between the numbers of different subsets of NK cells and clinical indicators. We found that the numbers of NKG2C + NK cells were correlated positively with the levels of serum C3, while the numbers of KIR2DL3+ NK cells were correlated negatively with the values of SLEDAI scores at 4 weeks posttreatment (P < 0.05, Fig. 4a, b). There was no other significant correlation between the numbers of any other subsets of NK cells tested with the values of clinical measures in this population (data not shown). Clearly, the increased numbers of NKG2C + and KIR2DL3+ NK cells were clinically relevant.
Discussion
NK cells are heterogeneous lymphocytes and their functions are regulated by the balance of activating and inhibitory signals via receptors on their cell surface [2]. In this study, we examined the dynamic changes in the numbers of different subsets of NK cells following the classic therapies. We found that treatment with the classic therapies for 4 and 12 weeks significantly reduced the disease severity, positivity of anti-DNA, and the levels of serum CRP but increased the numbers of peripheral blood lymphocytes and the levels of serum C3 and C4, accompanied by increased numbers of NK cells in the patients. Given that dysfunction and deficient in the lymphocytes and lower levels of C3 and C4 have been detected in new-onset SLE patients, the increased numbers of lymphocytes and the levels of serum C3 and C4, together with significant reduced scores of SLEDAI and levels of serum CRP, clearly demonstrated that classic therapies mitigated the disease severity in SLE patients.
Previous studies have shown abnormally lower numbers of peripheral blood NK cells in the patients with new-onset SLE [12, 13, 10], which may be attributed to immune complexes-related NK cell exhaustion [14]. In addition, imbalance of different subsets of NK cells has been associated with the development of SLE [9]. However, there is little information on the dynamic changes in the numbers of different subsets of NK cells following the classic therapies in SLE patients. Our previous study has shown that significantly less numbers of NKG2C + and KIR2DL3+, but greater numbers of NKp46+ NK cells in Chinese patients with new-onset SLE [11]. In this study, we found that the numbers of NKG2C + and KIR2DL3+ NK cells significantly increased, but the numbers of NKp46+ and NKG2A + NK cells significantly decreased at 4 and/or 12 weeks post the classic therapies in this population. Interestingly, the dynamic changes in the numbers of these subsets of NK cells only occurred in the patients who responded well to the classic therapies, but not those poorly responding to the therapies. Because NKp46+ and NKG2A + NK cells are associated with active SLE [13], the significantly reduced numbers of these subsets of NK cells were consistent with decreased disease activity in this population. KIR2DL3+ NK cells are inhibitory NK cells, which can downregulate autoimmunity. The significantly increased numbers of KIR2DL3+ NK cells suggest that the classic therapies may mitigate autoimmunity-related inhibitory NK cell exhaustion and help in control of disease progression. Indeed, the numbers of KIR2DL3+ NK cells were correlated negatively with the scores of SLEDAI in these patients at 4 weeks posttreatment. Deficient numbers of NKG2C + NK cells have been found in new-onset SLE patients [11]. The significantly increased numbers of NKG2C + NK cells following the classic therapies may reflect the redistribution of NKG2C + NK cells from inflammatory sites to peripheral blood. Alternatively, the increased numbers of NKG2C + NK cells may stem from upregulated expression of NKG2C receptor expression in NK cells. More importantly, we found that the numbers of NKG2C + NK cells were correlated positively with the levels of serum C3 in the patients. It is possible that the classic therapies inhibit immune complex formation and inflammation, leading to increase in the numbers of NKG2C + NK cells. The significant changes in the numbers of some subsets of NK cells following the classic therapies in the drug well responders, but not in the poor responders, suggest that these changes may serve as biomarkers to evaluate the therapeutic responses of SLE patients to the classic therapies. We are interested in further investigating the function of these subsets of NK cells and why the classic therapies modulate the numbers of different subsets of NK cells in a varying efficient manner.
Active NK cells can also secret abundant IFN-γ and other cytokines and have potent cytotoxicity against target cells [15–17]. Previous studies have shown that IFN-γ + and CD107a + NK cells are crucial for the pathogenesis of SLE [18–20]. It is well known that IFN-γ can upregulate MHC I and II expressions and enhance antigen-presenting cell’s activity [21, 22]. Furthermore, IFN-γ can also promote immunoglobulin gene switch and enhance antibody production[23, 24]. However, we did not find that the classic therapies significantly alter the numbers of IFN-γ + and CD107a + NK cells in these patients, although the classic therapies did reduce significantly disease severity in these patients. We are interested further investigating the potential mechanisms by which the classic therapies modulate different subsets of NK cells in SLE patients.
We recognized that this study had limitations, including small sample size, a short period of observation, and the lack of functional tests of different subsets of NK cells as well as one therapeutic protocol in these patients. Therefore, further studies in a bigger population to address these questions are warranted.
In summary, our data indicated that the classic therapies for 12 weeks significantly reduced disease severity and inflammation but elevated the levels of serum C3 and C4 in patients with new-onset SLE. Furthermore, the classic therapies for 12 weeks significantly modulated some subsets of NK cells by decreasing activating NK cells but increasing inhibitory and regulatory NK cells in new-onset SLE patients who responded well to the therapies. More importantly, the numbers of NKG2C + NK cells were correlated positively with the levels of serum C3, while the numbers of KIR2DL3+ NK cells were correlated negatively with the scores of SLEDAI in these patients at 4 weeks post the treatment. Therefore, the changes in the numbers of these subsets of NK cells may serve as biomarkers for evaluating the therapeutic responses in new-onset SLE patients.
References
Sekigawa I, Naito T, Hira K, Mitsuishi K, Ogasawara H, Hashimoto H, Ogawa H (2004) Possible mechanisms of gender bias in SLE: a new hypothesis involving a comparison of SLE with atopy. Lupus 13(4):217–222
Raulet DH, Vance RE, McMahon CW (2001) Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol 19:291–330. doi:10.1146/annurev.immunol.19.1.291
Pende D, Parolini S, Pessino A, Sivori S, Augugliaro R, Morelli L, Marcenaro E, Accame L, Malaspina A, Biassoni R, Bottino C, Moretta L, Moretta A (1999) Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J Exp Med 190(10):1505–1516
Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A (1997) p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med 186(7):1129–1136
Vitale M, Bottino C, Sivori S, Sanseverino L, Castriconi R, Marcenaro E, Augugliaro R, Moretta L, Moretta A (1998) NKp44, a novel triggering surface molecule specifically expressed by activated natural killer cells, is involved in non-major histocompatibility complex-restricted tumor cell lysis. J Exp Med 187(12):2065–2072
Bryceson YT, March ME, Ljunggren HG, Long EO (2006) Activation, coactivation, and costimulation of resting human natural killer cells. Immunol Rev 214:73–91. doi:10.1111/j.1600-065X.2006.00457.x
Moretta L, Moretta A (2004) Unravelling natural killer cell function: triggering and inhibitory human NK receptors. EMBO J 23(2):255–259. doi:10.1038/sj.emboj.7600019
Vivier E, Nunes JA, Vely F (2004) Natural killer cell signaling pathways. Science 306(5701):1517–1519. doi:10.1126/science.1103478
Baxter AG, Smyth MJ (2002) The role of NK cells in autoimmune disease. Autoimmunity 35(1):1–14
Li WX, Pan HF, Hu JL, Wang CZ, Zhang N, Li J, Li XP, Xu JH, Ye DQ (2010) Assay of T- and NK-cell subsets and the expression of NKG2A and NKG2D in patients with new-onset systemic lupus erythematosus. Clin Rheumatol 29(3):315–323. doi:10.1007/s10067-009-1322-9
Ye Z, Ma N, Zhao L, Jiang ZY, Jiang YF (2014) Differential expression of natural killer activating and inhibitory receptors in patients with newly diagnosed systemic lupus erythematosus. Int J Rheum Dis. doi:10.1111/1756-185X.12289
Green MR, Kennell AS, Larche MJ, Seifert MH, Isenberg DA, Salaman MR (2005) Natural killer cell activity in families of patients with systemic lupus erythematosus: demonstration of a killing defect in patients. Clin Exp Immunol 141(1):165–173. doi:10.1111/j.1365-2249.2005.02822.x
Hervier B, Beziat V, Haroche J, Mathian A, Lebon P, Ghillani-Dalbin P, Musset L, Debre P, Amoura Z, Vieillard V (2011) Phenotype and function of natural killer cells in systemic lupus erythematosus: excess interferon-gamma production in patients with active disease. Arthritis Rheum 63(6):1698–1706. doi:10.1002/art.30313
Ortaldo JR, Mason AT, O'Shea JJ (1995) Receptor-induced death in human natural killer cells: involvement of CD16. J Exp Med 181(1):339–344
Lauzurica P, Sancho D, Torres M, Albella B, Marazuela M, Merino T, Bueren JA, Martinez AC, Sanchez-Madrid F (2000) Phenotypic and functional characteristics of hematopoietic cell lineages in CD69-deficient mice. Blood 95(7):2312–2320
Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M (2006) CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 440(7083):540–544. doi:10.1038/nature04606
Testi R, D'Ambrosio D, De Maria R, Santoni A (1994) The CD69 receptor: a multipurpose cell-surface trigger for hematopoietic cells. Immunol Today 15(10):479–483. doi:10.1016/0167-5699(94)90193-7
Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N (2008) Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol 181(3):2211–2219
Viallard JF, Pellegrin JL, Ranchin V, Schaeverbeke T, Dehais J, Longy-Boursier M, Ragnaud JM, Leng B, Moreau JF (1999) Th1 (IL-2, interferon-gamma (IFN-gamma)) and Th2 (IL-10, IL-4) cytokine production by peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE). Clin Exp Immunol 115(1):189–195
Zhuang H, Kosboth M, Lee P, Rice A, Driscoll DJ, Zori R, Narain S, Lyons R, Satoh M, Sobel E, Reeves WH (2006) Lupus-like disease and high interferon levels corresponding to trisomy of the type I interferon cluster on chromosome 9p. Arthritis Rheum 54(5):1573–1579. doi:10.1002/art.21800
Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F (2004) Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat Immunol 5(12):1260–1265. doi:10.1038/ni1138
Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B (1994) Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science 265(5168):106–109
Sijts A, Sun Y, Janek K, Kral S, Paschen A, Schadendorf D, Kloetzel PM (2002) The role of the proteasome activator PA28 in MHC class I antigen processing. Mol Immunol 39(3–4):165–169
Snapper CM, Paul WE (1987) Interferon-gamma and B cell stimulatory factor-1 reciprocally regulate Ig isotype production. Science 236(4804):944–947
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Conflict of interest
All authors declared no conflict of interests involved.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ma, H., Zhao, L., Jiang, Z. et al. Dynamic changes in the numbers of different subsets of peripheral blood NK cells in patients with systemic lupus erythematosus following classic therapy. Clin Rheumatol 33, 1603–1610 (2014). https://doi.org/10.1007/s10067-014-2712-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-014-2712-1