Abstract
Poncet’s disease (PD) is an entity described as a reactive arthritis due to tuberculous infection elsewhere from the joints. PD existence has been questioned; however, more cases have been reported over the years. Due to its rare nature, little is known about the clinical picture of this disease and no prospective studies had been made to address this issue. We performed a systematic review of the written literature on PD in different databases using the key words “Poncet’s disease,” “tuberculous rheumatism,” and “tuberculous reactive arthritis.” Out of 78 articles, 198 patients were included in the analysis, plus our patient. Several characteristic patterns were found. Also, a review of the pathogenesis and some hypotheses are made. PD is a well-defined entity, which should be taken as a reactive arthritis for future studies given the increase in TB incidence and prevalence around the world, especially in high-burden countries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Throughout the years, the incidence of tuberculosis (TB) has increased exponentially. According to the World Health Organization, in 2007, the incidence of new tuberculosis cases was 9.27 million (139 new cases per 100,000 populations) [1].
This worldwide resurgence of TB raised interest on the disease including extrapulmonary TB. Approximately 10–11 % of extrapulmonary TB involves joints and bones (about 1–3 % of all cases of TB) [2]. Almost half of these cases are spinal TB, followed by TB arthritis, TB osteomyelitis, TB dactylitis, and reactive arthritis [2]. The latter reactive arthritis can be divided in arthritis associated to adjuvant mycobacterial treatment for bladder carcinoma and Poncet’s disease (PD).
Antonin Poncet first introduced PD in 1897 when he described a polyarthritis in an acute stage of TB, which resolved without joint damage [3]. His broad concept was based on the association of polyarthritis with active or inactive visceral TB or a family history of TB or the presence of true TB arthritis in a patient before, coincident with, or following a polyarthritis of any type [4]. This definition lacked diagnostic precision and was rapidly rejected by fellow colleges [5]. Continuous reports on patients with similar characteristics led authors to improve the definition and, in 1978, Bloxham and Addy defined PD as a para-infective arthritis [6]. To the authors, PD was similar to a reactive arthritis, an aseptic arthritis triggered by an infection outside the joint, in this case TB, but with complete resolution of the arthritis and no joint damage or chronicity once the infection was treated.
Nevertheless, PD existence continued to be questioned by some authors. Summers and Jayson found no evidence of PD in 50 cases of TB. Later on, Holoshitz et al. proposed that PD should be considered only in the presence of synovial biopsy, after finding a patient with clinical features of PD and true TB joint infection, which was confirmed by biopsy [7, 8]. To this date, the disease is not completely accepted.
We took interest in the disease after treating a patient with, what we believe is PD. After reviewing the literature, we came upon the contradictory statements mentioned before and found no complete definition, classification, and characteristics of the disease. We decided to gather an important amount of cases, by reviewing different databases around the world, in order to characterize, define, explain, and implement PD as a real well-defined disease.
Case report
A case of PD was identified together with the Rheumatology and Infectious Diseases departments at the Clínica Universitaria Colombia in Bogotá. A 36-year-old male without relevant medical history except as smoker of 10 cigarettes per day since youth, was admitted with a 34-day history of chills, fever, bilateral flank pain, cervical pain, and widespread myalgias. Physical examination revealed tachycardia, temperature of 38 °C, cervical spasm, and several ender points. Initial laboratory testing showed increased C-reactive protein (40 mg/dL), increased erythrocyte sedimentation rate (80 mm/h), and leucocytes of 11.5 × 109 E/l.
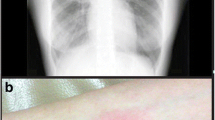
The patient was hospitalized, and additional laboratory testing was performed, which resulted negative for mononucleosis, toxoplasmosis, cytomegalovirus, HIV, and syphilis. Later on, the patient complained of pain and swelling of elbows, right knee, and ankles. A synovial fluid analysis was made of the right knee revealing no crystals. Standard cultures and cultures for TB of synovial fluid, blood, and sputum were negative. X-rays of the knee, elbows, and ankles showed no abnormalities. Autoimmune laboratory tests including anti-cyclic citrullinated peptide and antinuclear antibodies were negative. A chest CT scan was performed showing a dense nodule of 12 mm of diameter in the lower lobe of the left lung. Fine needle aspiration cytology resulted in an insufficient specimen and an open lung biopsy with partial pleurectomy was carried out. Results from the biopsy revealed caseating necrosis. A diagnosis of pulmonary TB and PD was made and isoniazid, rifampicin, pyrazinamide, and ethambutol were started with complete resolution of all symptoms including joint pain and swelling within the following 7 days.
Literature review
The electronic databases (MEDLINE, PUBMED, PUBMED CENTRAL, SCIELO, BIREME, LILACS, WPRIM, AIM, IMSEAR, and IMEMR) were systematically searched up to March 2013 for all case reports and studies concerning PD. For the search strategy, the following mesh terms and text words were used: “Poncet’s disease,” “tuberculous rheumatism,” and “tuberculous reactive arthritis.” No other limits were employed. Articles in all languages were included. The cases that met the following requirements were included: patients with active or latent TB infection in any organ, presence of arthritis (swelling or pain) in any joint, resolution of joint symptoms with anti-TB therapy, and exclusion of other causes of arthritis (autoimmune, infectious, degenerative, deposit, trauma). The following information was abstracted for the analysis: characteristics of the clinical presentation of the disease (number of type of arthritis, site of arthritis, time of resolution of arthritis), demographics (sex, age, origin), site of TB infection, adenopathy and site of adenopathy, and presence of other tuberculous manifestations. After an extensive and complete search of the literature, 78 reports were detected [4, 6, 9–83], from which 198 patients were extracted and 199 patients were used for the analysis (including our own patient).
Clinical characteristics
Of 199 patients, 42.2 % (n: 84) were male and 39.2 % (n: 78) were female. The remaining patients (18.6 %; n: 37) had no gender specification. Of these, 25 were reported by Duggal and Khosla [9], 8 by Garg et al. [79], 3 by Greenwood [10], and one by Kowalzki and Seitz [11]. The mean age was 33.7 years (SD ± 12.5 years) with a range of 2–78 years.
Of the patients, 35 % were from India (n: 70), 13.1 % (n: 26) were from Brazil, 10.1 % (n: 20) were from Mexico, 6.0 % (n: 12) were from England, and 3.0 % (n: 6) were from Nigeria. The remaining patients were from: Russia, Spain, USA, and Pakistan with five cases each (2.5 %), Iran with four cases (2.0 %), Japan, Korea, and France with three cases each (1.5 %), Syria, Zambia, China, and Turkey with two cases each (1.0 %), and Ethiopia, Italy, Peru, Hong Kong, Germany, Tanzania, South Africa, Suriname, Switzerland, Sri Lanka, Serbia, Zaire, Chile, Colombia, Finland, and Argentine with one case each (0.5 %). Only two patients (1.0 %) did not have information on origin which corresponded to the paper by Wilkinson and Roy [12].
A site of TB infection was specified in 96.5 % (n: 192), being 56.8 % (n: 113) extrapulmonary TB and 42 % of patients (n: 84) had lymph node involvement. Only in 8.5 % of patients (n: 17) had the presence of erythema nodosum reported by the authors.
The most common affected joints were the ankles (n: 126, 63.3 %) followed by knees (n: 117, 58.8 %), wrists (n: 58, 29.1 %), and elbows (n: 46, 23.1 %). Forty percent (n: 81) of the patients presented with oligoarthritis, 27.6 % (n: 55) with polyarthritis, and 24.6 % (n: 49) with monoarthritis. Joint arthritis resolved in an average of 51.6 days (SD ± 37.5 days) after antituberculous therapy. The clinical features of the study population are summarized in Table 1.
PD is characterized by oligoarthritis, mainly affecting large joints like ankles, knees, wrists, and elbows, with no axial involvement. The arthritis tends to resolve weeks after antituberculous therapy with no tendency to chronicity. Also, no extra-articular manifestations were found and only a few patients presented erythema nodosum. Although extrapulmonary TB was the most frequent site of infection, our study showed that the lung is the most affected organ associated to PD, and lymph nodes are frequently affected.
Our findings add even more strength to the diagnostic criteria proposed by Novaes et al. (Table 2) [70]. As expected, Asian countries reported most of the cases of PD; however, one should expect more cases from Africa. This could be explained by the lack of research publication from African countries.
Pathogenesis
Although little is known about PD, some hypotheses have risen throughout the years. It is well known that tuberculosis is arthritogenic and several findings have proved this statement. Holoshitz et al. demonstrated antigenic similarity between a fraction of Mycobacterium tuberculosis and human cartilage [8]. They also induced arthritis in rats, using a T lymphocyte clone that recognized both M. tuberculosis antigens and antigens in human synovial fluid cartilage [84]. Bhattacharya et al. demonstrated circulating immune complexes that could be trapped in the synovium of patients with active tuberculosis [85]. Southwood et al. found in a patient with PD exaggerated reaction of synovial fluid lymphocytes to purified protein derivative compared to peripheral blood lymphocytes [86]. The use of immunotherapy with bacillus Calmette–Guérin as an adjuvant for the treatment of bladder carcinoma may be followed by mono or polyarthritis, which has proven to enhance Th1 cell-mediated responses and suppression of Th2-cell activity [87]. Finally, the animal model of adjuvant arthritis in which injection of heat-killed desiccated M. tuberculosis (complete Freund′s adjuvant) results in chronic arthritis resembling rheumatoid arthritis, due to a T cell-mediated cross-reactivity between mycobacterial antigens and human cartilage [88].
Molecular mimicry is thought to be the explanation to all these events. Some epitopes of microbe heat-shock proteins (HSP) have shown a high degree of sequence homology with certain host’s normal proteins or with the host’s HSP, which are produced at the site of inflammation [89]. In TB infection, a cross-reactivity between the epitope of mycobacterial 65 kDa HSP and the human cartilage proteoglycans is the main cause for molecular mimicry [90].
It is believed that all these findings are the key to the pathogenesis of PD, if they are present in a genetically predisposed patient. Those who are HLA-DR3 and/or HLA-DR4, show T cell hyper responsiveness to mycobacterial antigens [89]. Also, several authors have demonstrated the presence of these HLA alleles in patients with PD [26, 30, 72, 77]. In a recent study, however, HLA typing was performed to 16 Mexican mestizo patients with PD, finding a significantly increased frequency of HLA-B27 and DQB1*0301 alleles when compared with healthy controls [77]. In fact, our study shows that of the 15 cases of PD found in the literature that were tested for HLA-B27, 4 (26.6 %) were positive.
The sum of the hypothesis explained before and others not yet discovered, could be the basis of the pathogenesis of PD. Therefore, in a genetically predisposed patient with a TB infection, bacterial antigens migrate to the joints where cross-reactivity between mycobacterial antigens and host cartilage occurs, inducing arthritis conducted by T cells. Therefore, by definition, PD should be considered a reactive arthritis (ReA).
Reactive arthritis and Poncet’s disease
ReA was originally defined as joint inflammation triggered by an extra-articular bacterial infection, and it was distinguished from post-infectious arthritis by absence of bacterial components in the joint tissue, hence sterile arthritis [91]. Classically, ReA has been related to arthritides triggered by Chlamydia, Yersinia, Salmonella, Shigella, Campylobacter, and Clostridium, which are HLA-B27 related and display similar symptoms to spondyloarthropathies, like axial involvement, tendency to chronicity, and extra-articular manifestations (conjunctivitis and uveitis) [92]. However, other infections have shown to trigger ReA without a clear association to HLA-B27. These include Borrelia, Brucella, Haemohilus, Hafnia, Leptospira, Neisseria, Staphylococcus, Streptococcus, Ureaplasma, Vibrio, and Mycobacterium [93]. Due to the lack of association to HLA-B27, and maybe because of that, the clinical picture of the “non-classical” ReA has less tendency to chronicity, no axial involvement, and less extra-articular manifestations [93]. Because of this issue, controversy has arisen on the classification of ReA, which has led to several categorizations [94, 95]. Toivanen and Toivanen state that depending on the triggering agent, the arthritides fulfilling the original definition of ReA could be considered to occur in two forms, one, HLA-B27 associated, and another, HLA-B27 non-associated [93]. According to our analysis, PD shares both characteristics of HLA-B27 associated and HLA-B27 non-associated ReA, as shown in Table 3. Early hospital-based studies have reported a frequency between 60 and 90 % of HLA-B27 in ReA patients [96]. However, in more recent studies based on outbreaks and surveys at population level, only a slight or no increased frequency of HLA-B27 has been reported [97, 98]. That is why some authors question the utility of HLA-B27 testing in ReA [98].
Once more, controversy arises on this disease. On one hand, the clinical characteristics of PD are more similar to that of an HLA-B27 non-associated ReA, no axial involvement, no tendency to chronicity, and no extra-articular manifestations and improvement with antibiotics. On the other hand, there is an association between PD and HLA-B27 as demonstrated on the study by Lugo-Zamudio et al. [77].
Conclusion
In summary, PD is a ReA, caused by molecular mimicry between TB antigens and host cartilage in a genetically predisposed patient, which is characterized by an oligoarthritis predominantly affecting knees and ankles, with no axial involvement, associated to HLA-B27, which tend to improve weeks after antituberculous therapy with no sequelae. It is a clear entity that should be taken as such for future studies, clinical classification and diagnosis.
Even though PD diagnosis still raises a challenge for the clinician, PD should be suspected in any patient with oligoarthritis and TB infection, especially in countries with high prevalence of TB. We suggest that more studies aiming HLA typing should be undertaken to clarify the involvement of HLA alleles in the pathogenesis of PD.
References
Global Tuberculosis Control 2009: Surveillance, Planning, Financing; WHO Report (2009) Geneva, Switzerland, WHO/HTM/TB/2009.411
Malaviya AN, Kotwal PP (2003) Arthritis associated with tuberculosis. Best Pract Res Clin Rheumatol 17:319–343
Poncet A. (1897) De la polyarthrite tuberculeuse deformante ou pseudo-rheumatisme chronique tuberculeux. Congr Fr Chir. 732
Isaacs AJ, Sturrock RD (1974) Poncet’s disease; fact or fiction? Tubercle 55:135–142
Brav EA, Hench PS (1934) Tuberculous rheumatism: a résumé. J Bone Joint Surg Am 16:839–866
Bloxham CA, Addy DP (1978) Poncet’s disease: para-infective tuberculous polyarthropathy. Br Med J 1:1590
Summers GD, Jayson MIV (1980) Does Poncet’s disease exist? Rheumatol Rehabil 19:149–150
Holoshitz J, Drucker I, Yaretzky A et al (1986) T lymphocytes of rheumatoid arthritis patients sow augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet ii:305–309
Duggal L, Khosla P (2006) Bilateral ankle arthritis with mediastinal lymphadenopathy: a clinician’s perspective. APLAR J Rheumatol 9:264–268
Greenwood BM (1970) Polyarthritis in western Nigeria. 3. Other forms of polyarthritis. Ann Rheum Dis 29:56–63
Kowalski M, Seitz M (1999) Tuberculosis manifestations in the musculoskeletal system exemplified by Poncet’s disease. Schweiz Med Wocheschr 129:1839–1842
Wilkinson AG, Roy S (1984) Two cases of Poncet’s disease. Tubercle 65:301–303
Khoury MI (1989) Does reactive arthritis to tuberculosis (Poncet’s disease) exist? J Rheumatol 16:1162–1164
Pasternak MS, Mark EJ (1993) Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 3–1993. A 51-year-old Ethiopian woman with myalgia, weight loss, and mediastinal lymphadenopathy. N Eng J Med 1(328):195–202
Allen SC (1981) A case in favor of Poncet’s disease. Br Med J (Clin Res Ed) 283:952
Ideguchi H, Ohno S, Takase K et al (2009) A case of Poncet's disease (tuberculous rheumatism). Rheumatol Int 29:1097–1099
Lee HL, Lee IH, Son BK et al (2001) A case of Poncet’s disease with tuberculosis of the intestine and lymph nodes. Korean J Med 60:211–214
Miki Y, Fujita Y, Kawai R et al (2003) A case of Poncet's disease (tuberculous rheumatism) in a patient with chronic renal failure undergoing hemodialysis therapy. Ryumachi 43:678–682
Ames PR, Capasso G, Testa V et al (1990) Chronic tuberculous rheumatism (Poncet's disease) in a gymnast. Br J Rheumatol 29:72–74
Enriquez J, Vial J, Flores H (2006) Enfermedad de Poncet (reumatismo tuberculoso): primer caso reportado en el Perú. Rev Peru Pediatr 59:36–38
Trapiella L, Caminal L, Bernaldo De Quirós JI et al (2001) Poncet's disease in HIV-infected patients. Med Clin (Barc) 117:557
Giner V, García F, Collado A et al (1998) Poncet's disease. Gamma graphic evidence of synovial involvement. Med Clin (Barc) 111:37–38
Wong SK, Yeung SD (2001) Erythema nodosum as the first presenting complaint of asymptomatic pulmonary tuberculosis. Hong Kong J Emerg Med 8:166–168
Heinemann C, Kaatz M, Elsner P (2003) Erythema induratum of Bazin and Poncet's disease—successful treatment with antitubercular drugs. J Eur Acad Dermatol Venereol 17:334–336
Suzuki N, Yasutake T, Ushiyama O et al (1995) Familial Pelger-Huet anomaly accompanied by tuberculosis and complicated by acute polyarthritis. Scan J Rheumatol 24:319–320
Perez C, Torroba L, Gonzalez M et al (1998) Unusual presentation of tuberculous rheumatism (Poncet's disease) with oral ulcers and tuberculid. Clin Infect 26:1003–1004
Chowdhary V, Aggarwal A, Misra R (2002) Multifocal tubercular dactylitis in an adult. J Clin Rheumatol 8:35–37
Rajpal S, Dhingra VK, Chopra KK et al (1999) Poncet’s disease. A unique presentation. Indian J Tubercul 46:133–135
Bahadur P, Seehiaraman ML, Krishnamurthy H (1988) Poncet’s disease. Report of a case. Indian J Tubercul 35:28–29
Ozgul A, Baylan O, Taskaynatan MA et al (2005) Poncet's disease (tuberculous rheumatism): two case reports and review of the literature. Int J Tuberc Lung Dis 9:822–824
Cuende E, Almeida V, Portu J et al (1998) Poncet's disease and papulonecrotic tuberculid in a patient infected with the human immunodeficiency virus. Arthritis Rheum 41:1884–1888
Kwasar M, D´Cruz D, Nathan M et al (2001) Poncet's disease in a patient with AIDS. Rheumatology (Oxford) 40:346–347
Simcock DE, Mukherjee D, Gendi NS (2004) Poncet's disease—a novel cause of non-compliance with anti-tuberculous drugs. Respir Med 98:795–797
Benatar A, Hill ID, Bowie MD (1984) Poncet’s disease. S Afr Med J 65:930–931
Kroot EJ, Hazes JM, Colin EM et al (2007) Poncet's disease: reactive arthritis accompanying tuberculosis. Two case reports and a review of the literature. Rheumatology (Oxford) 46:484–489
Chaudhuri MK, Singh S, Kumar L (1995) Poncet's disease: tuberculous rheumatism. Indian J Pediatr 62:363–365
Gupta KB, Prakash P (2001) Recurrent Poncet’s disease. A rare presentation. Indian J Tubercul 48:31–33
Arora VK, Verma R (1991) Tuberculous rheumatism (Poncet’s disease). Three case reports. Indian J Tubercul 38:229–230
Bhargava AD, Malaviya AN, Kumar A (1998) Tuberculous rheumatism (Poncet’s disease). A case series 45:215–219
Hammeed K, Karim M, Islam N et al (1993) The diagnosis of Poncet's disease. Br J Rheumatol 32:824–826
Qurtom MAF, D´Almeida E, Sattar SA et al (1997) Reactive arthritis due to tuberculous lymphadenitis (Poncet’s disease) in a Sri Lankan woman. Med Principles Pract 6:162–166
Wakhlu A, Agarwal V, Aggarwal A et al (2001) What really is Poncet's disease? Report of a case mimicking sarcoidosis. J Clin Rheumatol 7:411–413
Djuric D, Milosavljevic A, Stanojevic B (1956) Poncet's disease with specific tuberculous arthritis. Srp Arh Celok Lek 84:810–816
Malik SK, Khatri GK, Deodhar SD (1977) Tuberculous rheumatism (Poncet's disease). J Indian Med Assoc 69:201–202
Gemke GR, Aleinikova MS, Iofel GB (1981) Case of esophageal tuberculosis. Klin Med (Mosk) 59:107–109
Utkina NM, Kurmaeva ME (1981) Poncet's rheumatism (an analysis of 102 cases). Sov Med 6:6–8
Makarenko II (1985) Primary chronic tuberculosis with Poncet's rheumatoid syndrome resulting in Landouzy's typhobacillosis. Klin Med (Mosk) 63:131–135
Varela M, Carreño L, Caamaño M (1989) Inguinal tuberculous adenopathies and polyarthritis: is it a case of Poncet's disease? Rev Clin Esp 185:218–219
Pande D, Dubey AP, Choudhury P (1989) Poncet's disease. Indian Pediatr 26:828–830
Bhalerao S, Gautam D, Balani S et al (1992) Poncet's disease. J Assoc Physicians India 40:467–468
Kumar PD, Sasidharan PK, Paul BJ et al (1993) Poncet's disease. J Assoc Physicians India 41:400
Beauvais C, Veillon L, Prier A et al (1993) Present status of Poncet's tuberculous rheumatism. A new case. Rev Rhum Ed Fr 60:919–921
Lesprit P, Lafaurie M, Liote F et al (1996) Tuberculous rheumatism (Poncet's disease) in a patient infected with human immunodeficiency virus. Clin Infect Dis 23:1179–1180
Thakur S, Prasher BS, Sharma V (1996) Poncet's disease—case report. Indian J Med Sci 50:244–246
Sood R, Wali JP, Handa R (1999) Poncet's disease in a north Indian hospital. Trop Doct 29:33–36
Oguntona AS, Adelowo OA, Oghenetega O (2008) Poncet's disease (tuberculous rheumatism) in a Nigerian boy—case report. Niger Med Pract 53:26–27
Dall L, Long L, Stanford J (1989) Poncet's disease: tuberculous rheumatism. Rev Infect Dis 11:105–107
Shrivastav A, Mitra B, Pal J (2009) Images in clinical tropical medicine: reactive arthritis (Poncet's disease) and erythema nodosum accompanying tuberculosis. AmJTrop Med Hyg 80:501–502
Rao H, Sharma LR, Rao N (2002) Tuberculous rheumatism (Poncet's disease). A case report. Indian J Orthop 36:53–54
DeHart DJ (1992) Poncet's disease: case report. Clin Infect Dis 15:560
Heidari B, Bijani K (2003) Poncet's disease: a report of four cases and review of the literature. MJIRI 17:81–83
Roll G, Guzman L (1987) Poncet's rheumatism or tuberculous reactive polyarthritis. Rev Med Chile 115:44–46
Mestitz P (1958) Case of tuberculous rheumatism. Br Med J 1:383–384
Maricic MJ, Alepa FP (1990) Reactive arthritis after Mycobacterium avium-intracellulare infection: Poncet's disease revisited. Am J Med 88:549–550
Isaacs AJ, Sturrock RD (1974) Poncet's disease—fact or fiction? A re-appraisal of tuberculous rheumatism. Tubercle 55:135–142
Sheldon W (1946) Tuberculous rheumatism. Lancet 1:119–121
Dalgleish PG (1952) Tuberculous rheumatism; report of a case. Ann Rheum Dis 11:222–224
Ract A (1966) Tuberculous rheumatism. (Apropos of 2 cases). Rhumatologie 18:139–142
Kling DH, Levine MA (1941) Nondestructive tuberculous polyarthritis versus tuberculous rheumatism (Poncet). Arch Surg 42:956–967
Novaes GS, Kalil G, Borrelli FE (1992) Criteria for diagnosis for Poncet's disease. Rev Bras Reumatol 32:20–26
Wilkinson MC (1967) A note on Poncet's tuberculous rheumatism. Tubercle 48:297–306
Valleala H, Tuuminen T, Repo H et al (2009) A case of Poncet disease diagnosed with interferon-gamma-release assays. Nat Rev Rheumatol 5:643–647
Abadie F, Homse D (2010) Artritis reactiva por tuberculosis-Enfermedad de Poncet. J Clin Rheumatol 16:S101, poster presentation
Puche AM, Montesinos BL, Penades IC et al (2009) Tuberculous osteomyelitis and Poncet disease. An Pediatr (Barc) 71:365–366
Ficko C, Chanson N, Ben M, Rad M et al (2010) Chronic polyarthritis and tuberculosis. Med Mal Infect 40:175–176
Tian J, Chen JW, Gao JS et al (2010) A case of Poncet's disease: retroperitoneal tuberculous lymphadenitis and polyarthritis. Chin Med J (Engl) 123:1952
Lugo-Zamudio GE, Yamamoto-Furusho JK, Delgado-Ochoa D et al (2010) Human leukocyte antigen typing in tuberculous rheumatism: Poncet's disease. Int J Tuberc Lung Dis 14:916–920
Schweitzer LC, Lipnharski F, Prezzi SH (2011) Poncet's arthritis: case report. Rev Bras Reumatol 51:388–393
Garg S, Malaviya AN, Kapoor S et al (2011) Acute inflammatory ankle arthritis in Northern India—Löfgren's syndrome or Poncet's disease. J Assoc Physicians India 59:87–90
Kasivisvanathan V, Connell DW, Molyneaux PL et al (2012) A presentation of Poncet's disease identified following immunosuppressive steroid therapy. Int J Tuberc Lung Dis 16:708–709
Abdulaziz S, Almoallim H, Ibrahim A et al (2012) Poncet's disease (reactive arthritis associated with tuberculosis): retrospective case series and review of literature. Clin Rheum 31:1521–1528
Park SS, Yun CY, Kang EH et al (2010) A case of Poncet's disease in a patient with pulmonary tuberculosis. J Korean Rheum Assoc 17:183–187
Han N, Lee SK, Kim TJ et al (2011) A case of Poncet's disease in a patient with pulmonary tuberculosis accompanying erythema nodosum. Tuberc Respir Dis 71:221–224
van Eden W, Holoshitz J, Nevo Z et al (1985) Arthritis induced by a T-lymphocyte clone that responds to Mycobacterium tuberculosis and to cartilage proteoglycans. Proc Natl Acad Sci USA 82:5117–5120
Bhattacharya A, Ranadive SN, Kale M et al (1986) Antibody-based enzyme-linked immunosorbent assay for determination of immune complexes in clinical tuberculosis. Am Rev Respir Dis 134:205–209
Southwood TR, Hancock EJ, Petty RE et al (1988) Tuberculous rheumatism (Poncet’s disease) in a child. Arthritis Rheum 31:1311–1313
Choi IS, Koh YI (2002) Therapeutic effects of BCG vaccination in adult asthmatic patients: a randomized, controlled trial. Ann Allergy Asthma Immunol 88:584–91
Pearson CM (1956) Development of arthritis, periarthritis and periostitis in rats given adjuvants. Prof Soc Exp Biol Med 91:95–101
Rosler DH, Citera G, Anaya JM et al (1994) Br J Rheumatol 33:692–693
Southwood TR, Gaston JSH (1990) The molecular basis of Poncet’s disease? Br J Rheumatol 29:491
Ahvonen P, Sievers K, Aho K (1969) Arthritis associated with Yersinia enterocolitica infection. Acta Rheum Scand 15:232–253
Gaston JSH, Lillicrap MS (2003) Arthritis associated with enteric infection. Best Pract Res Clin Rheumatol 17:219–239
Toivanen P, Toivanen A (1999) Two forms of reactive arthritis? Ann Rheum Dis 58:737–741
Pacheco-Tena C, Burgos-Vargas R, Vázquez-Mellao J et al (1999) A proposal for the classification of patients for clinic and experimental studies on reactive arthritis. J Rheumatol 26:1338–1346
Kuipers JG, Köhler L, Zeidler H (1999) Reactive or infectious arthritis? Ann Rheum Dis 58:661–664
Aho K, Ahvonen P, Lassus A et al (1973) HLA antigen 27 and reactive arthritis. Lancet 2:157
Leirisalo-Repo M, Hannu T, Mattila L (2003) Microbial factor in spondyloarthropathies: insights from population studies. Curr Opin Rheumatol 15:408–412
Hannu T (2011) Reactive arthritis. Best Pract Res Clin Rheumatol 25:347–357
Acknowledgments
We wish to thank Dr. Leon F. Jaramillo for his aid in translating the Russian papers, and Prof. Anand N. Malaviya for providing information on his case reports.
Disclosures
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rueda, J.C., Crepy, MF. & Mantilla, R.D. Clinical features of Poncet’s disease. From the description of 198 cases found in the literature. Clin Rheumatol 32, 929–935 (2013). https://doi.org/10.1007/s10067-013-2270-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-013-2270-y