Abstract
Behçet's disease (BD) is an idiopathic multisystem disorder. Involvement of CNS occurs in 4–48% of cases. This study was designed to evaluate the prevalence of subclinical neuropsychiatric affection in asymptomatic Egyptian BD patients using psychometric tests and brain imaging with single photon emission computed tomography (SPECT) and magnetic resonance imaging (MRI), also to assess possibly associated clinical predictive variables. Twenty-five BD patients without overt CNS involvement and ten healthy controls matched for age, education, and sex completed a comprehensive neuropsychological battery including Hamilton scales for anxiety and depression and Wechsler memory scale–revised. Disease activity was assessed using Behçet's Disease Current Activity Form (BDCAF). SPECT was done for all subjects, and 12 patients underwent brain MRI. Compared to controls, 23 (92%) and 24 (96%) patients had anxiety and depression scores respectively above normal range; also, BD patients had significantly lower memory quotient (MQ). SPECT revealed abnormalities in 16/25 (64%), while in 3/12 patients (25%), MRI was abnormal. Subjects with abnormal SPECT had significantly higher ages than those with normal SPECT (P = 0.02) and were more frequently males (P = 0.03). No statistically significant differences between cases with normal or abnormal SPECT were found regarding disease duration, frequency of headache, BDCAF, frequency of active eye disease, major vascular involvement, mean Hamilton anxiety and depression scores, and mean MQ. Early diagnosis of neurological involvement in BD is important in reducing or preventing complications. Neuropsychiatric evaluation and HMPAO brain SPECT were found to be useful for detecting subclinical neurological abnormalities in BD patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Behçet's disease (BD) was originally described by Hulusi Behçet, a Turkish dermatologist, as a tri-syndrome of recurrent outbreaks of aphthous ulcerations of the mouth and genitalia and iritis, often leading to blindness [1]. It is recognized now as having a wide systemic spectrum with CNS involvement that was reported as an initial feature in 5% of BD patients [2]. Although neurological lesions in BD have a poor vital and functional prognosis, recent studies are more optimistic in that respect; compared with older series, neuro-Behçet's disease (NBD) mortality decreased from 25% [2] to 11% [3], with males being more frequently affected than females [4]. The “disease burden” of BD is usually confined to the early years of its course, and in many patients, the disease “burns out.” However, central nervous system involvement and major vessel disease are exceptions [5].
The neurological spectrum of NBD is classified into primary (including subclinical NBD), secondary, and coincidental—unrelated neurological involvement [6]. Its symptoms may vary probably because any part of the central or peripheral nervous systems may be involved, either focally or diffusely. Most patients (80%) have parenchymal brain involvement which mainly affects the brain stem, manifested by cranial neuropathies, ocular motor dysfunction, nystagmus, dysarthria, and ataxia. Other CNS manifestations include meningomyelitis, meningoencephalitis, hemiparesis, bilateral pyramidal signs, sphincter disturbance, and vascular complications (as intracranial hypertension due to dural sinus thrombosis). A subset of patients with parenchymal brain involvement may be clinically silent with non-specific complaints [3, 7]. Cognitive impairment may be rarely the earliest manifestation of neurologic involvement in BD, mainly affecting memory and executive functions [8]. Usually, it is associated with other neurologic symptoms and/or signs [3, 9] while some investigators reported cognitive function abnormalities in BD patients without overt neurologic involvement [10, 11]. Histopathological findings of NBD consist of brain involvement in gray and white matter [1]. Previous studies have reported the use of MRI and CT in BD patients [12–14]. Though CT scans may be strictly normal or show non-specific findings, MRI is more sensitive to pick up white matter and brain stem lesions. It showed pathological findings in up to 70% of BD patients [15, 16]; however, there was no clear correlation between MRI and neurologic findings in some patients [17], while in a significant proportion of patients with clinically evident brain involvement, brain MRI was normal [18–20].
Positron emission tomography reported abnormal glucose metabolism with decreased cerebral blood flow [21], while single photon emission computed tomography (SPECT) studies have already revealed abnormal cortical findings in gray matter with normal MRI [22]. SPECT brain imaging with Tc-99m hexamethyl propyleneamine oxime (HMPAO) was reported as an alternative modality used to assess regional cerebral blood flow (rCBF), and compared with MRI, its images have proven to be more accurate in detecting brain involvement in autoimmune connective tissue disease and to have a better correlation with clinical diagnosis [23, 24]; its use in BD patients was also reported [19, 20, 25]. The frequent detection of abnormalities on neurophysiological studies and neuroimaging in asymptomatic BD patients further suggests that the subgroup of patients with subclinical CNS involvement may not be so uncommon [26].
CNS involvement is a major cause of morbidity and mortality in BD, and approximately 50% of the NBD patients are moderately to severely disabled after 10 years of disease. Also, epidemiological data reported CNS involvement in 26% of BD in Egypt [27]. Besides, there is a lack of reliable laboratory tests to detect CNS involvement, which may be reverted by early institution of corticosteroids or other immunosuppressive agents to obtain the best response and to decrease the risk of fatality [2]. Therefore, this study was designed to evaluate the prevalence of subclinical CNS involvement in asymptomatic Egyptian BD patients using psychometric tests and brain imaging with SPECT and MRI, also to assess possibly associated clinical predictive variables.
Patients and methods
This study comprised 25 adult BD patients (19 males and 6 females; age range, 25–53 years; mean age, 33.8 ± 8.69 years) who were randomly selected among other BD patients attending the outpatient clinic of the Rheumatology Department, Cairo University Hospitals during 12 months consecutively. They all fulfilled the International Study Group criteria for the classification of BD [28]. For each selected patient, previous history did not reveal symptoms of neurological involvement, and neurological examination was normal. Patients suffering from headaches alone, not accompanied by abnormal signs on neurological examination, were not considered to have neurological involvement and thus were not excluded from the study. We followed our committee's ethical guidelines that conform to the provisions of the World Medical Association's Declaration of Helsinki with obtaining informed consent from all participants. Ten healthy controls (7 males, 3 females; age range, 23–50 years; mean age, 31.9 years) also participated in this study. Patients and controls were matched regarding educational level and socioeconomic class. Usually, the number of control cases should be at least equal to the patients' group; however, the financial limitations were an obstacle for the expansion of the control group.
Behçet's Disease Current Activity Form (BDCAF) [29] was used to assess disease activity. Scoring was based on the history of new clinical features present over the preceding 4 weeks prior to assessment. BDCAF scores include oral and genital ulceration; skin, joint, and gastrointestinal involvement; presence of fatigue and headache according to the duration of symptoms. The presence and type of large vessel involvement were documented. We considered eye activity present if there was a history of blurring of vision or if the eye was painful or red with confirmation by a thorough ophthalmological examination.
Psychometric tests
Psychometric tests included the Hamilton scale for anxiety (HARS) [30] and Hamilton scale for depression (HDRS) [31] scores and the Wechsler memory scale–revised (WMS–R) [32]. HARS consists of 14 questions covering different anxiety symptoms. Scores 0–14, 15–24, 25–34, and >35 were judged as normal, mild, moderate, and severe anxiety symptoms, respectively. HDRS consists of 24 questions covering different depressive symptoms. Scores 0–11, 12–17, 18–22, and >23 were considered normal, mild, moderate, and severe depressive symptoms, respectively. WMS–R is the most widely used memory test battery for adults. It consists of subscales for information (I), orientation (O), mental control (MC), logical memory (LM), digit span (DS), visual reproduction (VR), and paired associates language (PALT). Also, it yields a memory quotient (MQ), which is corrected for age and generally approximates the Wechsler Adult Intelligence Scale intelligence quotient [33].
Brain SPECT
All BD subjects and controls underwent brain SPECT using Tc-99m HMPAO, eventually to detect CNS involvement by depicting cerebral blood flow disturbances. A dual head gamma camera (Philips Axis) fitted with a low-energy high-resolution collimator was used. Acquisition began 30–60 min after the IVI of 740 MBq Tc-99m HMPAO while the patient was sitting, eyes open, in a quiet dimly lit room. Image reconstruction was performed through a closed computer program into transaxial, coronal, and sagittal cuts.
Brain MRI
Twelve BD patients underwent brain MRI. It was performed with a 0.5 T Vectra scanner (General Electric Medical Systems, Milwaukee, USA). The images were taken at the standard position immediately after gadolinium injection. The study included axial T1 and T2 spin-echo sequences.
Statistical analysis
Descriptive statistics included frequencies and percentages for each variable while numerical measures were represented as means and standard deviation. Cross tabulation was utilized to describe the relations between variables using the contingency coefficient. Levene's test (F test) for the equality of variances was used and t test for testing the differences between samples. P value <0.05 was considered significant.
Results
Our 25 BD patients (19 males, 76%, and 6 females, 24%) had a median disease duration of 84 months and a mean duration of education of 6.6 ± 5.8 years. Their clinical characteristics are shown in Table 1.
Twenty-two patients (88%) were on oral prednisone (5–20 mg/day; mean, 10.4 ± 7.3) and colchicine, 8 (32%) were on IV cyclophosphamide pulses, 11 (44%) were on azathioprine, 10 (40%) were on oral anticoagulants, 2 (8%) were on nonsteroidal anti-inflammatory drugs, 2 (8%) were on methotrexate (MTX), and one (4%) patient was on chlorambucil.
Psychometric tests
HARS, HDRS, and WMS–R were obtained from all studied subjects. Twenty-three (92%) patients had anxiety scores above the normal range versus none of the control group, with 7 patients having mild, 12 patients having moderate, and 4 patients severe anxiety symptoms. Twenty-four (96%) patients had depression scores that were above the normal range versus none of the controls, with 3 patients having mild, 4 patients having moderate, and 17 patients having severe depressive symptoms. BD patients had a significantly lower memory quotient than the controls (P = 0.04). Except for the scores of O and MC, all other items of WMS–R differed significantly between patients and control (Table 2). No correlation was found between MQ and age (r = −0.02, P = 0.47), disease duration (r = 0.23, P = 0.27), BDCAF (r = −0.07, P = 0.74), HARS (r = −0.32, P = 0.12), and HDRS (r = −0.03, P = 0.09).
Brain imaging
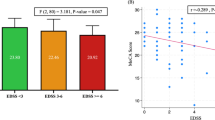
Brain SPECT abnormalities were found in 16 BD patients (64%); 8 patients had mild (50%), 5 had moderate (31%), and 3 had severe hypoperfusion (19%). Hypoperfusion was seen in parietal lobes in 9, the frontal lobe in 7, and the temporal lobe in 3 patients. Three out of 25 had diffuse cortical hypoperfusion, while one had an area of thalamic hypoperfusion. No white matter abnormalities were found (Table 3). All the controls had normal SPECT studies (Fig. 1). MRI findings were abnormal in 3/12 patients (25%); all were males with simultaneous SPECT abnormalities. Nine out of 12 patients had normal MRI; 6/9 had abnormal SPECT findings while the remainders (3/9) had normal SPECT. No gray matter abnormalities were found on brain MRI (Table 3).
Comparison between patients with normal and abnormal SPECT
Patients with abnormal SPECT had significantly higher ages than those with normal SPECT (P = 0.02) and were more frequently males (P = 0.03). No statistically significant differences between subjects with normal and abnormal SPECT were found regarding disease duration, frequency of headache, BDCAF, frequency of active eye disease, major vascular involvement, mean Hamilton anxiety and depression scores, and mean MQ score (Table 4).
Effect of treatment
No correlation was found between the daily steroid dose and MQ (r = −0.063, P = 0.77), HARS (r = 0.01, P = 0.98), or HDRS (r = 0.14, P = 0.98). Although patients with normal SPECT were taking a higher mean daily steroid dose, this difference was not significant (Table 4). Furthermore, there was no statistically significant difference between patients with normal and abnormal SPECT regarding intake of colchicine, MTX, cyclophosphamide, or azathioprine (data not shown).
Discussion
This study was carried out on 25 Egyptian BD patients without overt CNS involvement and showed a high prevalence of neuropsychiatric affection in these subjects compared to controls as detected by psychometric tests (abnormal anxiety and depression scores and most of the WMS–R items) together with brain SPECT abnormalities in 16/25 (64%) patients. Compared with 3/12 (25%) with abnormal brain MRI, 75% (9/12) of this patients' subgroup had hypoperfusion areas in the gray matter on Tc-99m HMPAO SPECT. The prevalence of NBD in BS is around 5% in non-selected large series [3, 34, 35]. Regarding the demographic characteristics of our study population, the male/female ratio was 3.2:1, their mean age (33.8 ± 8.69 years) being comparable to that of the previous reports [3–5, 34, 35]. The reported increased frequency of male involvement may be explained by the higher incidence of systemic complications and more severe disease in men, possibly bringing them to earlier medical attention.
Headache is frequently reported in patients with BD with and without neurological manifestations [3, 4, 36–39] and may accompany exacerbation of systemic manifestations [39]. It has been suggested that headaches may represent vascular or neuronal dysfunction accompanying BD [36]. In a recent series, 63% of BD patients without neurological affection suffered from headaches and were more frequently affected on cognitive measures than those without headache [38]. In the present study, 60% of our patients suffered from recurrent headache (non-specific, mostly paroxysmal migraine-like pain), but without concomitant increase in frequency of abnormal brain SPECT, deducing that headache in our patient group was not associated with subclinical neurological involvement. This is supported by a previous report that only about 10% of patients presenting with an isolated severe headache will turn out to have a neurological syndrome caused by BD [35]. This non-structural headache may be explained as a vascular headache triggered by the immune-mediated disease activity in susceptible individuals [40], and it is one of the items of the BDCAF [29].
Memory impairment was the major finding on neuropsychological testing in a series of NBD patients even with normal MRI [41]. Monastero et al. found 46.1% of BD patients without neurological affection to have cognitive dysfunction with memory being the domain most frequently affected [42]; also, Cavaco et al. found that 40% of BD patients without overt neurological involvement had at least an abnormality on neuropsychological testing [38], and this concurs with our results (Table 2). Similar to previous reports, we showed higher anxiety and depression scores among neurologically silent BD patients compared to controls [42]. Also, Taner et al. found that almost 50% of BD patients had depression and anxiety [43], and another study showed BD to be associated with severe depressive symptoms and lower quality of life [44]. Moreover, we did not find any correlation between anxiety and depression scores and BDCAF scores or MQ or SPECT findings, suggesting that abnormal anxiety and depression scores are related to the psychological stress of a chronic disease rather than direct involvement of CNS. The absence of correlation between the BDCAF score and MQ is in agreement with other reports that found higher levels of cognitive dysfunction in silent NBD patients than in controls, despite their being in the inactive phase of their disease [11, 38]. On the contrary, Monastero et al. [42] showed that BD patients with cognitive impairment had higher BDCAF score; this may be attributed to the difference in their definition of cognitive impairment. However, we found no relation between anxiety, depression scores, and MQ with corticosteroid use agreeing with similar studies [11, 38]. Besides, it has been reported that long-term corticosteroid therapy in patients with rheumatic diseases or bronchial asthma was associated with initial deficits in declarative memory that remained stable at follow-up assessment after 4 years [45].
The presence of abnormal neuroimaging (imaging evidence) using MRI or brain SPECT was among the suggested diagnostic criteria for NBD by Siva and Altıntas [46]. In this study, HMPAO brain SPECT was found to be abnormal in neurologically silent BD patients (64%). Few reports concerning the use of brain SPECT to evaluate rCBF in NBD were published and concluded that Tc-99m HMPAO brain SPECT is a sensitive method for detecting brain involvement in such patients with either silent or apparent CNS affection. Cengiz et al., Garcia-Burillo et al., and Kao et al. [7, 19, 20] reported 50% (6/12), 51.5% (17/33), and 100% (13/13) incidences of SPECT brain abnormalities, respectively; however, the BD populations of Cengiz et al. and Kao et al. were symptomatic while that of Garcia-Burillo et al. was a mixed population. In the latter, the incidence among asymptomatic subjects rises to 57% (12/21). Also, Garcia-Hernandez et al. showed that 61.5% of BD patients without neurological manifestations or with symptoms hard to interpret had abnormal SPECT [47], which is comparable to our results. In the present study, sites of SPECT abnormalities were the parietal, frontal, and temporal zones in accordance with other previous studies [7, 19, 20]. We found a variable correlation between MRI and SPECT findings in the studied subjects; 6/12 had abnormal SPECT and normal MRI while the remainders were congruent (Table 3). In the asymptomatic subgroup of Garcia-Burillo et al., comparable results were raised [19]. Vignola et al. [48] presented seven juvenile NBD patients; 3/7 had normal MRI but pathologic brain SPECT and stated that in the absence of MRI alterations, brain SPECT perfusion defects in BD might be an evidence of CNS involvement. Cengiz et al. [7] reported that the areas of abnormalities detected on SPECT were more extensive than those detected on MRI, and this concurs with the findings in our three BD patients with congruent abnormal SPECT and MRI. The high prevalence of SPECT abnormalities in asymptomatic patients, together with the lack of a correlation with MRI structural abnormalities, suggests a primary blood flow deficit or a local metabolic disturbance [49], probably expressing early, subclinical CNS involvement. This study revealed a significant relation between abnormal SPECT results and age and male gender only (Table 4). An older age at disease onset and a higher male/female ratio had been reported in NBD patients than those without neurological abnormalities [3, 5, 38]. Also, no relationship was observed between duration of the disease from the time of diagnosis and SPECT findings in the Garcia-Burillo et al. study [19].
According to Akman-Demir et al. [3], in a 7-year follow-up study, silent neurological involvement may occur in BD, and patients should undergo periodic neurological evaluation. Although MRI may reveal morphological abnormalities in many patients, predominantly in the white matter, it would be helpful to identify changes before structural damage occurs. Functional neuroimaging with SPECT may display brain perfusion defects at an early stage providing a better understanding of hemocirculatory and metabolic features of the illness as well as monitoring the patient's clinical condition and treatment response. It is evident that CNS involvement in BD must be diagnosed precociously; brain SPECT as a complementary modality can be of great help, and even when being normal, it can exclude presence of cortical involvement [7].
Conclusion
Memory impairment and SPECT abnormalities are frequently found in patients with BD without overt neurological involvement. Since patients with silent NBD tend to progress during follow-up, close clinical monitoring and periodic psychometric testing along with neuroimaging using Tc-99m HMPAO brain SPECT are recommended. The latter could be a standard procedure in evaluating brain involvement in BD patients as a mirror image of active cerebral vasculitis.
References
Shimizu T, Ehrlich GE, lknaba G, Hayashi K (1979) Behçet's disease (Behçet’s syndrome). Semi Arthritis Rheum 8:223–260
Wolf SM, Schotland DL, Phillips LL (1965) Involvement of nervous system in Behçet's syndrome. Arch Neural 12:315–325
Akman-Demir G, Serdaroglu P, Tasçi B, Neuro-Behçet Study Group (1999) Clinical patterns of neurological involvement in Behçet's disease: evaluation of 200 patients. Brain 122:2171–2181
Al-Araji A, Kidd DP (2009) Neuro-Behçet's disease: epidemiology, clinical characteristics, and management. Lancet Neurol 8:192–204
Kural-Seyahi E, Fresko I, Seyahi N et al (2003) The long-term mortality and morbidity of Behçet syndrome: a 2-decade outcome survey of 387 patients followed at a dedicated center. Med Baltimore 82:60–76
Siva A, Saip S (2009) The spectrum of nervous system involvement in Behçet's syndrome and its differential diagnosis. J Neurol 256:513–529
Cengiz N, Sahin M, Onar M (2004) Correlation of clinical, MRI and Tc-99 m HMPAO SPECT findings in neuro Behçet's disease. Acta Neurol Belg 104:100–105
Mimura M, Kato M, Kashima H (2009) Neuro-Behçet's disease presenting with amnesia and frontal dysfunction. Clin Neurol Neurosurg 111:889–892
Ozisik HI, Karlidag R, Hazneci E, Kizkin S, Ozcan C (2005) Cognitive event-related potential and neuropsychological findings in Behçet's disease without neurological manifestations. Tohoku J Exp Med 206:15–22
Monastero R, Camarda C, Pipia C et al (2004) Cognitive impairment in Behçet's disease patients without overt neurological involvement. J Neurol Sci 220:99–104
Erberk-Ozen N, Birol A, Boratav C, Kocak M (2006) Executive dysfunctions and depression in Behçet's disease without explicit neurological involvement. Psychiatry Clin Neurosci 60:465–472
Willeit J, Schmutzhard E, Aichner F, Mayr U, Weber F, Gerstenbrand F (1986) CT and MR imaging in neuro-Behçet disease. J Comput Assist Tomogr 10:313–315
Herskovitz S, Lipton RB, Lantos G (1988) Neuro-Behçet's disease: CT and clinical correlates. Neurology 38:1714–1720
Morrisey SP, Miller DH, Hermaszewski R et al (1993) Magnetic resonance imaging of the central nervous system in Behçet's disease. Eur Neural 33:287–293
Çoban O, Bahar S, Akman-Demir G et al (1996) A controlled study of reliability and validity of MRI findings in neuro-Behçet's disease. Neuroradiology 38:312–316
Koçer N, Islak C, Siva A et al (1999) CNS involvement in neuro-Behçet syndrome: an MR study. AJNR Am J Neuroradiol 20:1015–1024
Avci O, Kutluay E, Argon M, Erdem S, Tahsin Gunes A (1998) Subclinical cerebral involvement in Behçet's disease: a SPECT study. Eur J Neurol 5:49–53
Trotta F, Bajocchi G, Colamussi P, Sandri G, Ciancio G (1998) Cerebral hypoperfusion detected by SPET in early neuro-Behçet syndrome. Nucl Med Commun 19:777–780
García-Burillo A, Castell J, Fraile M et al (1998) Technetium-99m-HMPAO brain SPECT in Behçet's disease. J Nucl Med 39:950–954
Kao CH, Lan JL, ChangLai SP, Chieng PU (1998) Technetium-99m-HMPAO SPECT and MRI of brain in patients with neuro-Behçet's syndrome. J Nucl Med 39:1707–1710
Mineura K, Sasajima T, Kowada M, Shishido F, Uemura K, Nagata K (1989) Sequential PET studies in neuro-Behçet's syndrome. J Neurol 236:367–370
Huang WS, Chiu PY, Kao A, Tsai CH, Lee CC (2002) Decreased cerebral blood flow in neuro-Behçet's syndrome patients with neuropsychiatric manifestations and normal magnetic resonance imaging-a preliminary report. Neuroimaging 12:355–359
Lin WY, Wang SJ, Yen TC, Lan JL (1997) Technetium-99m-HMPAO brain SPECT in systemic lupus erythematosus with CNS involvement. J Nucl Med 38:1112–1115
Kao CH, Lan JL, ChangLai SP, Chieng PU (1998) Technetium-99m-HMPAO brain SPECT in Sjögren's syndrome. J Nucl Med 39:773–777
Mizukami K, Shiraishi H, Tanaka Y et al (1992) CNS changes in neuro-Behçet's disease: CT, MR and SPECT findings. Comput Med Imaging Graph 16:401–406
Tunc T, Ortapamuk H, Naldoken S et al (2006) Subclinical neurological involvement in Behcet's disease. Neurol India 54:408–411
Assaad-Khalil SH, Kamel FA, Ismail EA (1997) Starting regional registry for patients with Behcet's disease in North West Nile Delta region in Egypt. In: Hamza M (ed) Behcet's Disease. Pub Adhoua, Tunisia, pp 173–176
(1990) Criteria for diagnosis of Behçet's disease: International Study Group for Behçet's disease. Lancet 335:1078–1080
Bhakta BB, Brennan P, James TE, Chamberlain MA, Noble BA, Silman AJ (1999) Behçet's disease: evaluation of a new instrument to measure clinical activity. Rheumatology 38:728–733
Hamilton M (1959) The assessment of anxiety states by rating. Br J Psychiatry 32:50–55
Hamilton M (1960) A rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62
Wechsler D (1987) Wechsler memory scale-revised manual. The Psychological Corp. Harcourt Brace Jovanovich, New York, pp 138–141
Kaplan H, Sadock B (2000) Comprehensive text book of psychiatry vol.: 1, 7th edn. Williams and Wilkins, London, Sydney, New York
Kidd D, Steuer A, Denman AM, Rudge P (1999) Neurological complications in Behçet's syndrome. Brain 122:2183–2194
Siva A, Kantarci OH, Saip S et al (2001) Behçet's disease: diagnostic and prognostic aspects of neurological involvement. J Neurol 248:95–103
Monastero R, Mannino M, Lopez G et al (2003) Prevalence of headache in patients with Behçet's disease without overt neurological involvement. Cephalalgia 23:105–108
Kidd D (2006) The prevalence of headache in Behçet's syndrome. Rheumatol Oxf 45:621–623
Cavaco S, da Silva AM, Pinto P et al (2009) Cognitive functioning in Behçet's disease. Ann NY Acad Sci 1173:217–226
Siva A, Altintas A, Saip S (2004) Behçet's syndrome and the nervous system. Curr Opin Neurol 17:347–357
Saip S, Siva A, Altintas A et al (2005) Headache in Behçet's syndrome. Headache 45:911–919
Öktem-Tanör Ö, Baykan-Kurt B, Hakan Gürvit I, Akman-Demir G, Serdaroğlu P (1999) Neuropsychological follow up of 12 patients with neuro-Behçet disease. J Neurol 246:113–119
Monastero R, Cecilia C, Pipia C et al (2004) Cognitive impairment in Behçet's disease patients without overt neurological involvement. J Neurol Sci 220:99–104
Taner E, Coşar B, Burhanoğlu S, Calikoğlu E, Onder M, Arikan Z (2007) Depression and anxiety in patients with Behçet's disease compared with that in patients with psoriasis. Int J Dermatol 46:1118–1124
Uğuz F, Dursun R, Kaya N, Cilli A (2007) Quality of life in patients with Behçet's disease: the impact of major depression. Gen Hosp Psychiatry 29:21–24
Brown ES, Vera E, Frol AB, Woolston DJ, Johnson B (2007) Effects of chronic prednisone therapy on mood and memory. J Affect Disord 99:279–283
Siva A, Altıntas A (2000) Neuro-Behçet syndrome. In: Said G (ed) Intravenous immunoglobulins in the treatment of neurological disorders. Martin Dunitz Publishers, London, pp 115–126
Garcia-Hernandez FJ, Ocana Medina C, Mateos Romero L et al (2002) Usefulness of brain SPECT with HMPAO-99m Tc and psychological tests for diagnosis of neurological involvement in Behçet's disease. Med Clin Barc 119:447–450
Vignola S, Nobili F, Picco P et al (2001) Brain perfusion SPECT in juvenile Neuro-Behçet's disease. J Nucl Med 42:1151–1157
Jacquier-Sarlin MR, Polla BS, Slosman DO (1996) Oxido-reductive state: the major determinant for cellular retention of technetium-99-m-HMPAO. J Nucl Med 37:1413–14116
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zayed, H., Effat, D., Nawito, Z. et al. Silent central nervous system involvement in Egyptian Behçet's disease patients: clinical, psychiatric, and neuroimaging evaluation. Clin Rheumatol 30, 1173–1180 (2011). https://doi.org/10.1007/s10067-011-1725-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-011-1725-2