Abstract
Reactivation of latent tuberculosis (TB) is an established risk of anti-tumour necrosis factor α (anti-TNF) therapy. We report five cases of active TB occurring in 703 patients treated with anti-TNF therapy over a 10-year period in a central London hospital and review our screening practices for identifying latent TB prior to anti-TNF treatment. Four patients were receiving adalimumab and one patient etanercept at the time of TB diagnosis. Four of the five patients were born in countries with a high TB prevalence. Two of the five patients were healthcare workers. All patients had normal chest radiographs prior to anti-TNF treatment. Our data emphasise that country of origin is important in the determining risk of latent TB and that a normal chest radiograph does not exclude latent TB.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anti-tumour necrosis factor α (anti-TNF) therapy has revolutionised the treatment of the inflammatory arthritides, reducing both symptoms and radiological progression of disease [1]. Reactivation of latent tuberculosis (TB) is an established risk of anti-tumour necrosis factor α (anti-TNF) therapy. The British Thoracic Society (BTS), in conjunction with the British Society for Rheumatology and the British Society of Gastroenterology, have produced guidelines on TB risk assessment prior to initiation of anti-TNF therapy. These emphasise that TB risk is associated with country of origin [2].
London has a large immigrant population. It has a TB incidence of 44.3 per 100,000/year and accounts for 39% of TB cases in the UK [3]. Therefore, the risk of TB associated with anti-TNF might be expected to be higher for Londoners than for the UK overall. The Rheumatology Department of University College Hospital serves an ethnically diverse population in central London. We report our experience of TB in patients receiving anti-TNF and TB screening in patients due to commence anti-TNF.
Methods
We report all patients under the care of the Rheumatology Department of University College Hospital, London, who developed active TB whilst receiving anti-TNF therapy. All patients receiving anti-TNF therapy are reviewed on a 3–6-monthly basis in a dedicated anti-TNF clinic by one of the authors (S. Moore), and details of any adverse events are recorded on a database. In addition, we report a review of our screening practices for identifying latent TB prior to anti-TNF therapy over a 4-year period.
Results
Cases of active TB in patients receiving anti-TNF therapy
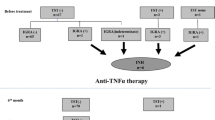
Seven hundred and three patients have received anti-TNF therapy in our department since the year 2000, some of whom have received multiple anti-TNF agents over time. One hundred and ten have received infliximab, 369 etanercept, and 379 adalimumab. Active TB occurred in five patients receiving anti-TNF. All cases occurred between June 2006 and June 2010. Four were receiving adalimumab and one etanercept at the time of TB diagnosis. One patient, who had been receiving adalimumab for 5 months at the time of TB diagnosis, had received infliximab for 6 months prior to commencing adalimumab. This patient was symptomatic for 2 months before the diagnosis of TB was made. The other four patients had not received any other anti-TNF drug in the past. All were diagnosed with TB within 12 months of starting anti-TNF (range 1–11 months, mean 7 months).
All patients were diagnosed by a TB specialist, with microbiological confirmation in three cases and high clinical and radiological suspicion in two. All had rheumatoid arthritis (RA). Two were born in Bangladesh, one in Afghanistan, one in South Africa, and one in UK. Two were healthcare workers, including the British-born patient. All five patients had a normal chest radiograph prior to starting anti-TNF, and in four, this was performed within the time-window specified in the BTS guidelines (<3 months before starting anti-TNF). None were prescribed TB chemoprophylaxis or referred to a TB specialist prior to starting anti-TNF.
Screening for latent TB prior to anti-TNF treatment
Given that five patients developed TB after starting anti-TNF therapy between June 2006 and June 2010, we reviewed the clinic records of all rheumatology patients (n = 308) assessed and considered eligible for anti-TNF during this time period. Clinic records were evaluated for evidence of a TB risk assessment, including documentation of country of birth, as recommended in the BTS guidelines. Thirty-six patients (11.7%) had their birthplace documented (12 UK, 9 Indian subcontinent, 5 Middle East, 4 mainland Europe, 3 China, 3 Africa). Two hundred and ninety-five patients (95.8%) had a chest radiograph prior to anti-TNF, and this was within the recommended timeframe in 219 (74.2%). Tuberculin skin testing (TST) was not performed; BTS guidelines do not recommend their use in patients receiving immunosuppressants.
Twenty-three of 308 patients (7.5%) assessed between June 2006 and June 2010 were referred to a TB specialist for the following reasons: abnormal chest radiograph (n = 16), previous TB (n = 4), TB exposure (n = 1), previous positive TST (n = 1), and previous reaction to BCG vaccination (n = 1). No patient was referred because of country of origin. Four of these patients received isoniazid chemoprophylaxis for latent TB; three due to chest radiographs suggestive of previous TB and one due to a history of untreated childhood TB. The time between starting chemoprophylaxis and starting anti-TNF was 2 months in two patients, 3 months in one patient, and 6 months in one patient. None of the four patients treated for latent TB have developed active TB thus far (two are receiving etanercept and two adalimumab, mean follow-up 12.5 months).
Discussion
Our data emphasise that country of origin is important in determining risk of latent TB; four of the five patients who developed TB on anti-TNF were born in countries with a high TB prevalence [4]. The short time from commencing anti-TNF to TB diagnosis is consistent with reactivation of latent TB rather than de novo infection. Rheumatologists should have a low threshold for referring patients who were born in countries with a high prevalence to a TB specialist. The low percentage of patients in whom country of origin was recorded suggests there was insufficient emphasis on this in our risk stratification process. Occupational exposure is also an important risk factor for latent TB, with two of the five TB cases occurring in healthcare workers. Our findings highlight that a normal chest radiograph does not exclude latent TB and should not discourage referral to a TB specialist if risk factors are present.
Active TB developed in five of 703 patients (0.7%) treated with anti-TNF. The British Society for Rheumatology Biologics Registry reported active TB in 40 of 10,712 RA patients (0.37%) treated with anti-TNF [5]. The increased frequency of TB in our cohort may reflect the inadequacies in our screening process highlighted above, and the increased prevalence of TB in the London population compared to the UK as a whole [3].
A smaller proportion of our patients received TB prophylaxis prior to anti-TNF than reported in other European series; only four of 308 patients (1.3%) assessed for anti-TNF between June 2006 and June 2010 received TB prophylaxis, compared with 12.6% of patients who received adalimumab in the ReAct study [6]. This discrepancy is likely to reflect both insufficient attention to country of origin in our TB risk stratification process and the use of TSTs in the ReAct study. Of patients in the ReAct study, 12.6% had a positive TST at baseline, whereas only 3% had a chest radiograph suggestive of previous TB.
We report no patients who developed TB whilst receiving anti-TNF between 2000 and 2006, despite lack of screening for latent TB in the early years of anti-TNF use. We are confident this is not due to underreporting as all patients on anti-TNF are reviewed regularly in a dedicated clinic. This observation may be due to chance as the absolute number of patients who developed active TB was small. Another explanation would be a change in the demographics of the patients receiving anti-TNF over time, with more patients from countries with a high prevalence of TB in recent years. However, we do not have data to confirm or refute this hypothesis. There has also been a small increase in the background UK population incidence of TB over time [3].
We report more TB with adalimumab than etanercept despite similar numbers of patients taking each drug, in keeping with studies of drug-specific TB risk [5, 7]. We suggest it is preferable to use etanercept following TB chemoprophylaxis, and in patients from countries with a high prevalence of TB. There were no cases of TB in patients receiving infliximab, but fewer patients received this drug. It is interesting to note that one patient who was diagnosed with active TB 5 months into treatment with adalimumab had previously received infliximab for 6 months without symptoms of TB.
BTS guidelines state that in patients receiving immunosuppressant medication (which is the majority of patients in the UK who are considered for anti-TNF), a TST is unhelpful for identifying latent TB. Instead, an individual risk assessment should be performed; if the annual risk of TB is greater than the risk of hepatotoxicity from TB chemoprophylaxis, then chemoprophylaxis should be given. This calculation is based on country of birth, time since entry into the UK, and age. The pitfalls of this ‘epidemiological’ approach to determining TB risk are unnecessary exposure to hepatotoxic drugs and delay in starting anti-TNF (hence, more erosive joint disease) in some individuals from high prevalence areas.
Reliable tests for latent TB are needed. BTS recommendations not to use TSTs in patients taking immunosuppressants contrast those of the American College of Rheumatology [8] and are unique in Europe. The rationale for the BTS recommendations is twofold. Firstly, a negative TST result does not reliably exclude latent TB in immunosuppressed patients. Secondly, a positive result may be due to previous immunisation with the BCG vaccine, which has been widely used in the UK. In contrast, the USA has never implemented mass BCG immunisation, and there is a wide variation of national BCG vaccination policies within mainland Europe [9]. There has been considerable interest in the role of interferon-gamma release assays (the results of which are not affected by BCG vaccination), but further research into their sensitivity and specificity in patients receiving immunosuppressants is required before their use can be recommended [8, 10].
References
Goekoop-Ruiterman YP, de Vries-Bowstra JK, Allaart CF et al (2008) Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): A randomised controlled trial. Arthritis Rheum 58:S126–S135
Omerod LP, Milburn HJ, Gillespie S, Ledingham J, Rampton D (2005) BTS recommendations for assessing risk, and for managing M. tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax 60:800–805
Anderson C, Moore J, Kruijshaar M, Abubakar I. (2008) Tuberculosis in the UK. Annual Report on Tuberculosis Surveillance in the UK 2008. London: Health Protection Agency Centre for Infections.
Global tuberculosis control: epidemiology, financing, strategy: WHO report 2009.
Dixon WG, Hyrich KL, Watson KD, Lunt M, Galloway J, Ustianowski A (2010) Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 69:522–528
Burmester GR, Mariette X, Montecucco C et al (2007) Adalimumab alone and in combination with disease-modifying antirheumatic drugs for the treatment of rheumatoid arthritis in clinical practice: the research in active rheumatoid arthritis (ReAct) trial. Ann Rheum Dis 66:732–739
Tubach F, Salmon D, Ravaud P et al (2009) Risk of tuberculosis is higher with anti-tumour necrosis factor monoclonal antibody therapy than with soluble tumour necrosis factor therapy: the three-year prospective French research axed on tolerance of biotherapies registry. Arthritis Rheum 60:1884–1894
Saag KG, Teng GG, Patkar NM et al (2008) American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying anti-rheumatic drugs in rheumatoid arthritis. Arthritis Rheum 59:762–784
Infuso O, Falzon D (2006) European survey of BCG vaccination policies and surveillance in children, 2005. Euro Surveill 11:6–11
Sauzullo I, Mengoni F, Scrivo R et al (2010) Evaluation of QuantiFERON-TB Gold In-Tube in human immunodeficiency virus infection and in patients candidate for anti-tumour necrosis-alpha treatment. Int J Tuberc Lung Dis 14:834–840
Disclosures
None
Author information
Authors and Affiliations
Corresponding author
Additional information
Satveer Mankia and James E. Peters contributed equally to the writing of the manuscript.
Rights and permissions
About this article
Cite this article
Mankia, S., Peters, J.E., Kang, S. et al. Tuberculosis and anti-TNF treatment: experience of a central London hospital. Clin Rheumatol 30, 399–401 (2011). https://doi.org/10.1007/s10067-010-1605-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-010-1605-1