Abstract
This chapter provides a practical guide to approaching Latent TB Infection (LTBI) assessment and treatment. Identifying and treating LTBI is an important step in Tuberculosis (TB) prevention for TB contacts as well as other patient groups including those that are immunosuppressed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Latent TB infection
- TB contacts
- Anti-TNFα
- Monoclonal antibodies
- Immunosuppression
- TB chemotherapy prophylaxis
- Biologics
- TB reactivation
Introduction
The World Health Organisation (WHO) estimates that one quarter of the world’s population is infected with LTBI. Five to ten percent of individuals with LTBI will develop active disease during their lifetime [1]. Achieving the WHO’s ambitious goal of a reduction in TB related death by 95% by 2035 demands that we address this huge LTBI burden to prevent future active TB cases. The risk of reactivation varies between individuals and the decision to screen for and treat LTBI should focus on groups of individuals who are most likely to benefit or in whom reactivation and development of active disease would cause most harm. Individuals perceive risk differently and so each patient to whom screening is offered and LTBI is identified will have a unique perspective on their risk of reactivation compared to the risks of treatment, and their perspective as well as the experience and views of the responsible clinicians need to be carefully explored. For patients in whom the risk of reactivation is being heightened because of a treatment proposed for another condition (usually immunosuppressive therapy) this risk assessment becomes more complex. The choice to treat LTBI should be reached through shared decision making between the patient and medical professionals.
LTBI is commonly diagnosed by obtaining immunological evidence of prior TB exposure in the form of a positive Interferon Gamma Release Assay (IGRA) and/or tuberculin skin test (TST) result (Table 1). Current tests are not able to distinguish between recent and historical exposure in an individual, although the former confers a higher risk of disease reactivation than the latter. These tests also usually remain positive even after successful latent or active TB treatment.
Immunosuppression increases the likelihood of TB disease (Table 2). Infliximab was introduced in 1999, a revolutionary new treatment for inflammatory bowel disease that targeted the inflammatory cytokine Tumour Necrosis Factor alpha (TNFα). In 2001 the United States Food and Drug Administration (FDA) modified the drug’s labelling to include a boxed warning about infliximab-associated tuberculosis after an analysis identified an excess of reported cases of tuberculosis disease following infliximab infusion. The relatively recent introduction and rapid expansion of the field of biologics treatment that includes anti-TNFα drugs, as well as non-TNFα targeted drugs, now used for many medical conditions presents a challenge in identifying which patients are at risk.
Individuals at Increased Risk of Tuberculosis Reactivation
Contacts of Patients with Active Tuberculosis
The close contacts of patients with pulmonary TB are at risk of TB infection and these individuals should be screened and treated if evidence of LTBI is identified by immunodiagnostic testing [10]. Only patients with pulmonary or upper airway TB are at risk of infecting contacts but in some settings LTBI screening of the contacts of patients with extrapulmonary tuberculosis may be an effective way of identifying groups who share risks for TB exposure [11]. Individuals who have been previously treated for active or latent TB are likely to have persistently positive IGRA or TST results from prior Mycobacterium tuberculosis exposure and repeat testing may not be useful. Instead, a clinical risk assessment that includes infectiousness of the index patient’s disease, duration of exposure and host immune status of the contact will guide whether empirical latent TB treatment is warranted. An 8-h index exposure cut off is used to guide identification of those contacts who require screening, [12] however TB transmission is also possible in shorter time periods varies with environmental, host and disease factors. Large scale whole genome sequencing may elucidate this further.
Children under 15 are particularly at risk of TB infection and progression to active disease following exposure to TB. This is addressed in the chapter on paediatric TB.
New Entrant Screening
In countries with a low incidence of TB disease LTBI screening should be offered to recent arrivals from countries with a high incidence of tuberculosis when the benefits of TB prevention outweigh the risks of LTBI treatment [10].
Patients Receiving Biologic Drug Treatment Including Anti-TNFα Agents
The anti TNFα agents confer varying risks of TB reactivation and this should be considered when selecting an appropriate treatment. Table 3 compares relative risk data to illustrate the variability in TB reactivation risk between treatments. The consensus is that there is a higher TB risk associated with monoclonal anti-TNFα agents, than the soluble receptor etanercept, and a lower or absent risk for non-anti-TNFα targeted biologics [14].
In recent years the field of targeted monoclonal antibodies to treat chronic inflammatory disease and malignancy as well as rarer conditions has expanded to include many agents with differing molecular targets and mechanisms of actions. It is postulated that the pivotal role that TNFα plays in granuloma integrity infers a significantly greater risk of latent TB reactivation when this is impaired in contrast to the effect on cytokines involved in other inflammatory pathways.
In 2018 the European Society of Clinical Microbiology and Infectious Disease (ESCMID) produced a consensus document that summarised the evidence to date with respect to screening recommendations for many biologic agents [14]. Table 4 summarises these recommendations with respect to TB screening and compares them to the Summary of Product Characteristics advice based on clinical trial data provided to the UK Medicines and Healthcare Products Regulatory Agency (MHRA) and/or the European Medicines Agency (EMA) [15].
Anti TNFα agents clearly increase TB risk but this is less clear for other agents. For example, a meta-analysis that reviewed the risk of TB reactivation for non-TNFα targeted agents; IL-6 (tocilizumab), CD20 (rituximab), CD28 (abatacept), IL-12/IL-23, and IL-17 (secukinumab) concluded that the risk of TB reactivation was negligible based on clinical trials which did not report an excess of TB cases compared to the country’s incidence rate. Many of these studies did not include LTBI screening or treatment in their inclusion protocols. A review of 30 clinical trials of Tocilizumab in 15,485 patients with RA with a clinical observation ranging from 14 weeks to 5 years did not report any active TB cases despite the role of IL-6 in T helper cell differentiation, important for antimycobacterial activity. Only sporadic cases of active TB, not exceeding the frequency of the disease in general population, were reported in rituximab and abatacept exposed patients with rheumatoid arthritis, and no cases were associated with ustekinumab and secukinumab in patients with psoriatic arthritis and ankylosis spondylitis [16].
The risk of TB reactivation is likely to be further increased when combined with other immunosuppression such as methotrexate or azathioprine or serial use of more than one biologic agent [17]. It is recommended that that disease registries and adverse effects reporting systems (e.g. the MHRA ‘yellow card’ system https://yellowcard.mhra.gov.uk/ in the UK) are used to disseminate new information about drugs which have been in clinical use for a relatively short time. All patients need to have active TB disease excluded as well as a latent TB assessment prior to starting high risk immunosuppression.
Some case series have reported an increased rate of extra pulmonary disease in patients who are diagnosed with active TB on biologic treatment and others an increased rate of TB reactivation in older subjects with Rheumatoid Arthritis, however these rates are highly variable between large case series and so warrant further investigation [18].
Some recommendations recommend annual or repeat screening on biologic treatment but this has unproven efficacy given the unreliability of IGRA testing in this group and may result in unnecessary interruption of clinically important biologic therapy, and so we recommend repeat screening and clinical evaluation only when a new TB exposure is suspected [18].
Other Medical Conditions that Increase TB Reactivation Risk
Patients living with HIV are at higher risk of TB reactivation, and this is addressed in an earlier chapter “Radiology of Tuberculosis”.
People with diabetes are at three times increased risk of developing active tuberculosis compared with people who do not have diabetes [9]. Patients with renal impairment are also likely to be at increased risk [8] with those requiring dialysis or transplant being at highest risk with adjusted rate ratios of 3.63 (95%CI 1.79–7.33) and 11.35 (95% 2.97–43.41) respectively [19]. This cohort of patients are also likely to have unreliable immunodiagnostic test results due to chronic immunosuppression [20, 21].
Patients with silicosis are at increased risk of TB reactivation [22]. Malnutrition and undernourished states including ileojejunal surgery are also associated with increased risk of TB reactivation [23].
Patients with immune mediated inflammatory diseases such as rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis are at a higher risk of TB reactivation with a peak ranging from 2.0 to 6.4 in rheumatoid arthritis in patients not receiving biologic treatments, lower in ankylosing spondylitis and psoriatic arthritis. This increased rate of reactivation is likely to be related to immunosuppressive drugs used to treat the conditions [18].
Data from a variety of settings demonstrate a higher incidences of active TB in patients with cancer compared to the general population (even when adjusting for age and co-morbidity) with patients with haematological malignancy, head and neck and lung cancer seemingly at highest risk [4]. Pooled study results in 2017 demonstrate an incidence rate ratio of TB of 2.53 for solid organ cancers and for haematological malignancies. The incidence rate ratio in any cancer in children was higher [24]. The UK national guidelines advocates for screening of patients diagnosed with cancer and receiving chemotherapy for LTBI to prevent reactivation [4, 10, 24].
Solid organ transplantation is associated with high levels of immunosuppression and patients are at high risk of TB reactivation. A review of more than 2000 transplantation cases demonstrated TB incidence of 2.6%, significantly higher than the general population and usually occurring in the first year after transplant [25]. Risk increases with lung transplantation, with older age recipients concomitant Hepatitis C, Diabetes, renal failure, higher doses of immunosuppression or lymphocytic depleting antibodies as part of the treatment. Mortality from TB is higher than in the general population (up to 30% in a Spanish cohort) [26]. Active infection can be a result of reactivation from either latent disease in the host (most commonly) or the donor. The American Society of transplantation and the European TBNET consortium recommend screening both host and if possible live donor for LTBI with transplant recipients screened an IGRA, TST and CXR, ideally before receiving immunosuppression. Any positive results require assessment for active TB before commencing LTBI treatment [3, 26]. For patients receiving a stem cell transplant the risk of active TB is appreciably higher (estimates vary from 2 to 40 times) than in the general population [27, 28], with higher mortality associated with the infection and again it is reasonable to assume the majority of that disease is caused by reactivation of latent disease usually in the transplant recipient. TB is a late complication of haemopoetic stem cell transplant and appears to be more likely with significant graft versus host disease. The American and European guidance is allied to the solid organ transplant guidance, proposing assessment for LTBI prior to transplant (ideally prior to immunosuppression) and treatment of LTBI [3, 26], but international agreement is lacking [29].
Risk Assessment for Latent Tuberculosis
Immunological Testing; Interferon Gamma Release Assays and Tuberculin Skin Tests
The most sensitive way to identify all patients with possible latent TB disease is a ‘triple approach’ which includes an Interferon Gamma Release Assay (IGRA) blood test, a tuberculin (Mantoux) skin test and a clinical risk assessment regarding likelihood of prior exposure to tuberculosis infection [30]. The latter is particularly useful for those patients who are already receiving immunosuppression during assessment and are at high risk of false negative IGRA or TST outcomes [31, 32]. Currently, the most commonly available IGRA tests in the UK are the Quantiferon-TB Gold In-Tube and T-Spot.TB tests (see chapter “TB Treatment and Complications”). Both have been demonstrated to predict progression from latent to active TB disease. The T-Spot.TB test may be more sensitive in the immunosuppressed patient group but there are no good head to head studies of this compared to the latest version of the Quantiferon test (QFT Plus) which incorporates CD8 reactivity [32]. Neither test can discriminate between latent and active disease or identify those who are more at risk of TB reactivation. Table 1 presents the number of patients who are not on biologics treatment but have been recently exposed to TB and progress to active disease dependent on their immunodiagnostic test result [2].
Clinical Risk Assessment for Tuberculosis Exposure
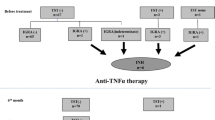
Figure 1 outlines a assessment strategy for LTBI infection. Patients who are already immunosuppressed prior to immunological testing, have a high risk of false negative test results [31, 32]. Table 5 lists conditions and treatments that signify an individual should be considered immunosuppressed at the point of testing for LTBI. In this group a clinical and epidemiological risk assessment based on likelihood of TB exposure is likely to be helpful. The risk assessment consists of identifying epidemiological factors that put the patients at risk of TB exposure (Table 6) alongside co-morbidities and treatments that will also increase TB risk (Table 1). When considering TB treatment, the cumulative risk of disease over a lifetime will vary according to the patients age. The online TST/IGRA Interpreter https://www.tstin3d.com provides useful risk estimates based on age and exposure risks that can be used as a basis for discussions between patients and the medical professionals treating them [34]. These treatment risk estimates can then be balanced against the risks associated with LTBI treatment.
Treatment of Latent Tuberculosis
Since the 1950s when a trial of isoniazid for people with radiographic evidence of previous TB treatment was endorsed by the union of TB and lung disease, treatment has been offered to reduce the risk of TB reactivation [35]. When the index case is likely to be infected with a drug susceptible strain, treatment is composed of regimens of rifamycin and/or isoniazid antibiotics. Proposed adult regimens are as follows (see chapter “HIV and TB” for paediatric and chapter “Multi-Drug Resistant Tuberculosis Management” for adult regimens):
-
1.
Isoniazid 300 mg once daily for 6 months
-
2.
Rifampicin 600 mg (≥50 kg) or 450 mg (<50 kg) plus isoniazid 300 mg once daily for 6 months
-
3.
Rifampicin 600 mg (≥50 kg) or 450 mg (<50 kg) once daily for 4 months
-
4.
Rifapentine 900 mg plus isoniazid 900 mg once weekly for 3 months
In the case of rifampicin or isoniazid monoresistant index cases a preventative regimen containing only the drug to which the index strain was susceptible should be used.
Pyrazinamide containing regimens have been used historically (particularly in patients who are co-infected with HIV) but are longer recommended due to drug intolerance and hepatotoxicity. A weekly rifapentine regimen is an addition to the effective options available for latent tuberculosis and may be more acceptable to patients in terms of tablet burden [36]. A systematic review of factors that increase adherence to LTBI treatment noted that shorter regimes are more likely to be completed [37]. A clinical trial of just 1 month of treatment for patients who are co-infected with HIV (Rifapentine 300 mg (>35 mg) 450 mg (35–40 kg) 600 mg (>45 kg) plus isoniazid 300 mg daily for 1 month (HIV positive patients only) demonstrated to achieve completion rates of 97% and non-inferior to 9 months of INH alone [38]. Drug interactions should be considered when selecting an appropriate regimen and the interaction between the rifamycins and corticosteroids as well as hormonal contraceptive treatments and antiretroviral drugs makes isoniazid monotherapy regimens preferable in these groups of patients.
In the case of exposure to multidrug resistant tuberculosis, the WHO recommend 2 years of close follow up. Fluoroquinolone containing regimens have been used in TB contacts, including those where the index case has multidrug resistant disease, and preliminary results suggest they may be effective, with only pyrazinamide containing regimens being less well tolerated, results of further clinical trials are awaited [39]. See chapter “Contact Investigation” for more details on the process of contact tracing.
Adverse Reactions to LTBI Treatment and Hepatotoxicity
A decision regarding whether to treat or observe LTBI requires accurate information regarding the risks and benefits of therapy to be provided to both healthcare professionals and their patients. The commonest side effects from treatment are nausea, itching and vomiting, and these are usually self-limiting. Polyneuropathy during LTBI chemoprophylaxis is usually caused by isoniazid, it occurs due to inactivation of pyridoxine metabolites and inhibition of the enzyme pyridoxine phosphokinase which is a necessary enzyme to convert pyridoxine to its active form of pyridoxal 5′ phosphate. Seizures and psychosis are rare but important side effects associated with isoniazid, because of depletion of gamma-aminobutyric acid (GABA) which is a pyridoxine dependent pathway. Another rare but important side effect is neutropenia secondary to rifampicin.
Hepatotoxicity is a potentially serious side effect associated with isoniazid, rifamycins and pyrazinamide. Up to 10% of patients taking isoniazid may have an asymptomatic rise in liver function enzymes. The incidence of hepatotoxicity varies widely in different studies with rates from 0.1 to 4% for isoniazid with a death rate from hepatitis of 23.2 per 100,000 in the early studies that guided the American Thoracic Society guidelines on hepatotoxicity [35, 40]. The British Thoracic Society anti-TNFα guidelines reviewed LTBI isoniazid only studies from 1996 to 2002 and calculated a weighted average risk of hepatotoxicity with 6 months of isoniazid of 278 per 100,000 treated patients. Similar methodology calculated a risk of 1766 per 100,000 with the combined 3 month isoniazid and rifampicin regime [41]. This fits with estimates from earlier studies where isoniazid in combination was demonstrated to have a higher incidence of toxicity than isoniazid alone [42].
A recent network analysis has attempted to quantify the risk of hepatotoxicity compared to no treatment in the existing efficacious regimes. Pyrazinamide containing regimes have the highest risk of hepatotoxicity and there was no significant difference in toxicity in single or dual drug regimes, with rates of hepatotoxicity being lower in dual regimes when compared to longer duration (>12 months) isoniazid differing from prior studies that indicated single agent isoniazid was safer [43].
The risk of hepatotoxicity is also dependent on host and treatment factors. There is good evidence that although most hepatotoxicity occurs in the first 16 weeks of TB treatment (for active TB and with isoniazid monotherapy) the duration of treatment affects the risk of hepatotoxicity with higher rates seen in longer regimes [44]. Other well recognised risk factors for hepatotoxicity with LTBI treatment (data mostly taken from isoniazid monotherapy regimes) include alcohol consumption, HIV co-infection, malnutrition, female gender and viral hepatitis infection. Unless preventative treatment is time critical we would advocate treatment and control of viral hepatitis infections prior to starting LTBI treatment. A significant factor relating to the risk of hepatotoxicity is increasing age [44, 45] with the rates in patients over 50 being 2.3 per 1000 treated cases. The UK NICE guidelines recommend LTBI treatment only in those patients under 65 years of age, and only under 35 years of age for new entrant screening where the risk of TB reactivation is likely to be lower because the time since exposure is less certain.
In all patients contemplating LTBI treatment there should be a thorough assessment for risk factors to heighten awareness of hepatotoxicity and education of all patients taking medication will be required, verbal and written information in the patients preferred language [46].
Timing of Starting Immunosuppression and Biologics Treatment
Wherever possible LTBI treatment should begin prior to biologic treatment. In the first reported series of 70 patients receiving infliximab with tuberculosis, 48 of them developed tuberculosis after three or fewer infusions, suggesting that early reactivation is possible [47]. There is insufficient high quality evidence to guide the interval between LTBI treatment and immunosuppression, but expert consensus suggests a 4 week interval between starting LTBI treatment and biologic immunosuppression [48, 49]. A risk: benefit decision will guide whether this interval can be shortened in the case of treatment for severe disease.
Biologic Treatment with Active TB Disease
Biologic treatment should be withdrawn in the case of active disease and guidelines vary regarding when it can be safely restarted, most agreeing that treatment re-start should be defer until TB treatment is successfully completed, with the suggestion that if inflammatory disease is severe and the biologic is low risk they can be restarted after the first 2 months of TB intensive therapy [41, 50].
Adherence and Treatment Support for Patients Taking Latent TB Treatment
The efficacy of treatment LTBI depends on adherence to treatment and all patients should be counselled regarding what to expect when starting treatment, strategies to enhance their adherence and the rationale for treatment [37]. Those patients with risk factors for non-adherence may require enhanced support to complete their LTBI treatment including directly observed treatment [10]. It is useful to explain that the test that confirmed the diagnosis of latent TB infection (the IGRA or TST) will remain positive, particularly in the case of healthcare professionals who may be subject to repeated testing. Asymptomatic patients with LTBI are not unwell or infectious and this should always be explained to patients and their healthcare professionals for clarity.
Successful LTBI assessment and treatment requires collaboration between all relevant heath care professionals. We advocate a patient centred approach that acknowledges a difference in acceptance of risk between individuals.
Acknowledgements and Contributors
With thanks to Mikin Patel for his review of biologics summary product characteristics screening recommendations.
Further Reading
WHO | Global tuberculosis report 2018. In: WHO. http://www.who.int/tb/publications/global_report/en/. Accessed 8 Jun 2019.
Abubakar I, Drobniewski F, Southern J, et al. Prognostic value of interferon-γ release assays and tuberculin skin test in predicting the development of active tuberculosis (UK PREDICT TB): a prospective cohort study. Lancet Infect Dis. 2018;18:1077–87.
Subramanian AK, Theodoropoulos NM, Infectious Diseases Community of Practice of the American Society of Transplantation. Mycobacterium tuberculosis infections in solid organ transplantation: guidelines from the infectious diseases community of practice of the American Society of Transplantation. Clin Transplant. 2019;e13513.
Dobler CC, Cheung K, Nguyen J, Martin A. Risk of tuberculosis in patients with solid cancers and haematological malignancies: a systematic review and meta-analysis. Eur Respir J. 2017;50:1700157.
Brassard P, Lowe A-M, Bernatsky S, Kezouh A, Suissa S. Rheumatoid arthritis, its treatments, and the risk of tuberculosis in Quebec, Canada. Arthritis Rheum. 2009;61:300–4.
Horsburgh CR, Rubin EJ. Latent tuberculosis infection in the United States. N Engl J Med. 2011;364:1441–8.
Jick SS, Lieberman ES, Rahman MU, Choi HK. Glucocorticoid use, other associated factors, and the risk of tuberculosis. Arthritis Care Res. 2006;55:19–26.
Min J, Kwon SK, Jeong HW, Han J-H, Kim YJ, Kang M, Kang G. End-stage renal disease and risk of active tuberculosis: a nationwide population-based cohort study. J Korean Med Sci. 2018. https://doi.org/10.3346/jkms.2018.33.e341.
Jeon CY, Murray MB. Diabetes mellitus increases the risk of active tuberculosis: a systematic review of 13 observational studies. PLoS Med. 2008;5:e152.
National Institute of Clinical Excellence. Tuberculosis NICE guidance [NG33]. 2016. https://www.nice.org.uk/guidance/ng33/chapter/recommendations#close-contacts. Accessed 1 May 2019
Humphreys A, Abbara A, Williams S, John L, Corrah T, McGregor A, Davidson RN. Screening contacts of patients with extrapulmonary TB for latent TB infection. Thorax. 2018;73:277–8.
WHOrganization. Tuberculosis and air travel : guidelines for prevention and control. World Health Organization; 2008.
Souto A, Maneiro JR, Salgado E, Carmona L, Gomez-Reino JJ. Risk of tuberculosis in patients with chronic immune-mediated inflammatory diseases treated with biologics and tofacitinib: a systematic review and meta-analysis of randomized controlled trials and long-term extension studies. Rheumatol Oxf Engl. 2014;53:1872–85.
Fernández-Ruiz M, Meije Y, Manuel O, Akan H, Carratalà J, Aguado JM, Delaloye J. ESCMID study group for infections in compromised hosts (ESGICH) consensus document on the safety of targeted and biological therapies: an infectious diseases perspective (Introduction). Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2018;24(Suppl 2):S2–9.
Electronic Medicines Compendium (eMC). In: eMC. https://www.medicines.org.uk/emc. Accessed 1 May 2019.
Cantini F, Nannini C, Niccoli L, Petrone L, Ippolito G, Goletti D. Risk of tuberculosis reactivation in patients with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis receiving non-anti-TNF-targeted biologics. Mediat Inflamm. 2017;2017:8909834.
Lorenzetti R, Zullo A, Ridola L, Diamanti AP, Laganà B, Gatta L, Migliore A, Armuzzi A, Hassan C, Bruzzese V. Higher risk of tuberculosis reactivation when anti-TNF is combined with immunosuppressive agents: a systematic review of randomized controlled trials. Ann Med. 2014;46:547–54.
Goletti D, Petrone L, Ippolito G, Niccoli L, Nannini C, Cantini F. Preventive therapy for tuberculosis in rheumatological patients undergoing therapy with biological drugs. Expert Rev Anti-Infect Ther. 2018;16:501–12.
Al-Efraij K, Mota L, Lunny C, Schachter M, Cook V, Johnston J. Risk of active tuberculosis in chronic kidney disease: a systematic review and meta-analysis. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2015;19:1493–9.
Southern J, Sridhar S, Tsou C-Y, Hopkins S, Collier S, Nikolayevskyy V, Lozewicz S, Lalvani A, Abubakar I, Lipman M. Discordance in latent tuberculosis (TB) test results in patients with end-stage renal disease. Public Health. 2019;166:34–9.
Rogerson TE, Chen S, Kok J, Hayen A, Craig JC, Sud K, Kable K, Webster AC. Tests for latent tuberculosis in people with ESRD: a systematic review. Am J Kidney Dis Off J Natl Kidney Found. 2013;61:33–43.
Cowie RL. The epidemiology of tuberculosis in gold miners with silicosis. Am J Respir Crit Care Med. 1994;150:1460–2.
Pickleman JR, Evans LS, Kane JM, Freeark RJ. Tuberculosis after jejunoileal bypass for obesity. JAMA. 1975;234:744.
Cheng MP, Chakra CNA, Yansouni CP, Cnossen S, Shrier I, Menzies D, Greenaway C. Risk of active tuberculosis in patients with cancer: a systematic review and metaanalysis. Clin Infect Dis. 2017;64:635–44.
Abad CLR, Razonable RR. Mycobacterium tuberculosis after solid organ transplantation: a review of more than 2000 cases. Clin Transpl. 2018;32:e13259.
Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40:990–1013.
Roy V, Weisdorf D. Mycobacterial infections following bone marrow transplantation: a 20 year retrospective review. Bone Marrow Transplant. 1997;19:467–70.
Fan W-C, Liu C-J, Hong Y-C, Feng J-Y, Su W-J, Chien S-H, Chen T-J, Chiang C-H. Long-term risk of tuberculosis in haematopoietic stem cell transplant recipients: a 10-year nationwide study. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2015;19:58–64.
Russo RL, Dulley FL, Suganuma L, França IL, Yasuda MAS, Costa SF. Tuberculosis in hematopoietic stem cell transplant patients: case report and review of the literature. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2010;14(Suppl 3):e187–91.
Singanayagam A, Manalan K, Sridhar S, et al. Evaluation of screening methods for identification of patients with chronic rheumatological disease requiring tuberculosis chemoprophylaxis prior to commencement of TNF-α antagonist therapy. Thorax. 2013;68:955–61.
Edwards A, Gao Y, Allan RN, et al. Corticosteroids and infliximab impair the performance of interferon-γ release assays used for diagnosis of latent tuberculosis. Thorax. 2017;72:946–9.
Wong SH, Gao Q, Tsoi KKF, Wu WKK, Tam L, Lee N, Chan FKL, Wu JCY, Sung JJY, Ng SC. Effect of immunosuppressive therapy on interferon γ release assay for latent tuberculosis screening in patients with autoimmune diseases: a systematic review and meta-analysis. Thorax. 2016;71:64–72.
Public Health England. Immunisation against Infectious Diseases. Chapter 6 Contraindications and special considerations. 2017.
Menzies D, Gardiner G, Farhat M, Greenaway C, Pai M. Thinking in three dimensions: a web-based algorithm to aid the interpretation of tuberculin skin test results. Int J Tuberc Lung Dis Off J Int Union Tuberc Lung Dis. 2008;12:498–505.
(1982) Efficacy of various durations of isoniazid preventive therapy for tuberculosis: five years of follow-up in the IUAT trial. International Union Against Tuberculosis Committee on Prophylaxis. Bull World Health Organ. 60:555–64.
Sterling TR, Villarino ME, Borisov AS, et al. Three months of rifapentine and isoniazid for latent tuberculosis infection. N Engl J Med. 2011;365:2155–66.
Stuurman AL, Vonk Noordegraaf-Schouten M, van Kessel F, Oordt-Speets AM, Sandgren A, van der Werf MJ. Interventions for improving adherence to treatment for latent tuberculosis infection: a systematic review. BMC Infect Dis. 2016;16:257.
Swindells S, Ramchandani R, Gupta A, et al. One month of rifapentine plus isoniazid to prevent HIV-related tuberculosis. N Engl J Med. 2019;380:1001–11.
Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis Off Publ Infect Dis Soc Am. 2017;64:1670–7.
Saukkonen JJ, Cohn DL, Jasmer RM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med. 2006;174:935–52.
British Thoracic Society Standards of Care Committee. BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF- treatment. Thorax. 2005;60:800–5.
Steele MA, Burk RF, DesPrez RM. Toxic hepatitis with isoniazid and rifampin. A meta-analysis. Chest. 1991;99:465–71.
Zenner D, Beer N, Harris RJ, Lipman MC, Stagg HR, van der Werf MJ. Treatment of latent tuberculosis infection: an updated network meta-analysis. Ann Intern Med. 2017;167:248.
Yew WW, Leung CC. Antituberculosis drugs and hepatotoxicity. Respirol Carlton Vic. 2006;11:699–707.
Fountain FF, Tolley E, Chrisman CR, Self TH. Isoniazid hepatotoxicity associated with treatment of latent tuberculosis infection: a 7-year evaluation from a public health tuberculosis clinic. Chest. 2005;128:116–23.
TB Alert Latent TB. In: Truth TB. https://www.thetruthabouttb.org/latent-tb/. Accessed 2 May 2019.
Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. Tuberculosis associated with infliximab, a tumor necrosis factor α–neutralizing agent. N Engl J Med. 2001;345:1098–104.
Iannone F, Cantini F, Lapadula G. Diagnosis of latent tuberculosis and prevention of reactivation in rheumatic patients receiving biologic therapy: international recommendations. J Rheumatol Suppl. 2014;91:41–6.
Holroyd CR, Seth R, Bukhari M, et al. The British Society for Rheumatology biologic DMARD safety guidelines in inflammatory arthritis. Rheumatol Oxf Engl. 2019;58:e3–e42.
Cantini F, Nannini C, Niccoli L, et al. Guidance for the management of patients with latent tuberculosis infection requiring biologic therapy in rheumatology and dermatology clinical practice. Autoimmun Rev. 2015;14:503–9.
Levin SN, Kaplan TB. Infectious complications of novel multiple sclerosis therapies. Curr Infect Dis Rep. 2017;19:7.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martin, L., Russell, G. (2021). Anti-tumour Necrosis Alpha Factor Treatment, Immunosuppression and Chemotherapy Prophylaxis. In: Kon, O.M. (eds) Tuberculosis in Clinical Practice. Springer, Cham. https://doi.org/10.1007/978-3-030-75509-6_17
Download citation
DOI: https://doi.org/10.1007/978-3-030-75509-6_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-75508-9
Online ISBN: 978-3-030-75509-6
eBook Packages: MedicineMedicine (R0)