Abstract
Online hemodiafiltration (OL-HDF) is a blood purification therapy based on diffusion and ultrafiltration and is classified into two types according to the mode of addition of the substitution fluid: pre-dilution OL-HDF (pre-HDF) and post-dilution OL-HDF (post-HDF); we previously reported that pre-HDF is more biocompatible. However, we used lower blood and substitution flow rates in that study and may not have accurately simulated the treatment conditions used in Europe. In this study, we compared the biocompatibilities of the treatment conditions of pre-HDF, commonly used in Japan, and post-HDF, commonly used in Europe, to determine the most biocompatible treatment conditions. We compared the biocompatibilities of pre-HDF and post-HDF using high blood flow rates and high substitution fluid volumes, and also compared the results with those of our previous study. We enrolled six stable patients undergoing maintenance dialysis at our clinic for this study. After the patients underwent hemodialysis (HD), post-HDF, and pre-HDF treatment, the biocompatibilities (based on the serum levels of high-sensitivity C-reactive protein, interleukin-6, pentraxin-3, β-thromboglobulin, and soluble P-selectin, and the results of the lymphocyte blastogenesis test using phytohemagglutinin and concanavalin A as mitogens) and removal performances (removal performance for urea, creatinine, β2-microglubulin [MG], and α1-MG, and albumin leakage) were determined. There were no significant differences in the biocompatibility parameters evaluated among the three treatment modes. Post-HDF was associated with significantly higher removal rates of β2-MG than HD. Post-HDF was associated with significantly higher removal rate of α1-MG, and also significantly higher albumin leakage, than HD and pre-HDF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Online hemodiafiltration (OL-HDF) is a blood purification therapy based on diffusion and ultrafiltration and is classified into two types: pre-dilution online HDF (pre-HDF), in which the substitution fluid is added before the filter, and post-dilution online HDF (post-HDF), in which the substitution fluid is added after the filter. In Japan, mainly pre-HDF is used as OL-HDF. Kikuchi et al. reported that pre-HDF with a high substitution fluid volumes of ≥40 L improves the survival rate as compared to hemodialysis (HD) [1]. In Japan, as of the end of 2017, 29.4% of all dialysis patients underwent HDF, and 70.9% of these patients received pre-HDF [2]. HDF is superior to HD in terms of the removal performance for low-molecular-weight protein (LMWP) solutes such as β2-microglobulin [β2-MG; molecular weight (MW), 11,800] and α1-microglobulin (α1-MG; MW, 33,000) on account of the filtration using high volumes of substitution fluid. β2-MG is known to cause dialysis-related amyloidosis [3] and frequently manifests as pruritus [4]. Moreover, a high rate of removal of α1-MG is associated with a reduction in the incidence of complications such as restless legs syndrome [5]. In contrast, in Europe, OL-HDF is usually performed with higher blood flow rates (300–400 mL/min [6], 392 mL/min, mean [7]), in the post-HDF mode, and with higher flow rates of the substitution fluid than those used in Japan [6, 7]. This could perhaps be attributable to the different types of hemodiafilters used between Europe and Japan.
Biocompatibilities of different membrane materials [8, 9] and different treatment methods [10, 11] have been investigated. We previously evaluated the biocompatibility of pre-HDF vs. post-HDF, and concluded that pre-HDF was more biocompatible than post-HDF, based on the levels of inflammatory markers and responses in the lymphocyte blastogenesis test [11]. The treatment conditions used in that study were a blood flow rate of 200 mL/min and substitution fluid volume of 50 L/session for pre-HDF and 10 L/session for post-HDF, all of which were lower than the values used in Europe. In this study, we compared the biocompatibility of the treatment conditions of pre-HDF, commonly used in Japan, and post-HDF, commonly used in Europe. Our objective was to determine the most biocompatible treatment conditions, because we believe that it is important to administer the treatment under optimal conditions. Thus, in this study, we compared the biocompatibilities of the treatment conditions used for HD, pre-HDF, and post-HDF, using higher blood flow rates and substitution fluid volumes as compared to those used in our previous study.
Materials and methods
Patients
The patient inclusion criteria for this study were as follows:
・Good vascular access.
・Good hemodynamic stability during treatment.
・Patients not scheduled to be hospitalized during the study period, with preserved ADLs.
Study design
We enrolled stable patients undergoing maintenance dialysis at our clinic for this study. The study was conducted between May 2014 and July 2014. All the patients included in this study had been receiving pre-HDF under individualized treatment conditions for at least 1 month prior to participation in this study.
After enrollment, each of the patients received one of the three modes of treatment in the following sequence: HD (washout period), post-HDF, pre-HDF, post-HDF, and HD. Each of these treatments was adopted for 2 weeks. Blood sampling was performed on the day of the final treatment (Fig. 1). The following filters were used in the present study: APS-21E (Asahi Kasei Medical Co., Ltd., Tokyo, Japan) for HD, ABH-21P (Asahi Kasei Medical) for pre-HDF, and ABH-21F (Asahi Kasei Medical) for post-HDF. The filter specifications are shown in Table 1. ABH-P is currently not available in the market; in its place, ABH-PA, which is based on ABH-P, and has optimized hollow fiber and housing, is available. The differences between ABH-P and ABH-PA are as follows (respectively); membrane thickness, 45 μm vs. 43 μm; hollow fiber, straight vs. wavy; hollow fiber density, 70% vs. 60%; in addition, the housing is also improved in ABH-PA.
The three modes of treatment were performed using the following: HD, a dialyzer used frequently in Japan; pre-HDF, a recommended hemodiafilter; post-HDF a hemodiafilter designed for post-HDF, with a thicker inner diameter than that for pre-HDF; the membrane material used was the same for all three.
The treatment conditions were as follows: blood flow rate (QB), 300 mL/min; total dialysate flow rate (total QD), 600 mL/min; treatment time, 4 h; substitution fluid volume, 60 L/session for pre-HDF and 20 L/session for post-HDF (Table 2). The blood flow rate does not completely mimic that used in Europe in this study. The vascular access used is an arteriovenous fistula in 91.5% of men and 84.6% of women in Japan [2]; therefore, we considered that the upper limit of the blood flow rate for stable treatment is 300 mL/min. If the blood flow rate is in excess of 300 mL/min, some problems may arise with blood removal, recirculation may occur, and the venous pressure may increase. We set the blood flow rate at 300 mL/min in this study in consideration of the above points.
Parameters
Pre-treatment blood samples were collected using an arterial puncture needle prior to connection to the circuit. Post-treatment blood samples were collected through the arterial sampling port of the dialysis circuit after completion of treatment, the dialysate flow was terminated, and the blood was circulated at a flow rate of 100 mL/min for 1 min.
Biocompatibility was evaluated by measuring the serum levels of high-sensitivity C-reactive protein (CRP), interleukin-6 (IL-6), the plasma levels of pentraxin-3 (PTX-3), β-thromboglobulin (β-TG), and soluble P-selectin, and the lymphocyte blastogenesis response (elicited using phytohemagglutinin [PHA] and concanavalin A [Con A] as mitogens). High-sensitivity CRP was measured by latex agglutination assay, IL-6 and β-TG by enzyme immunoassay, and PTX-3 by enzyme-linked immunosorbent assay. The rate of change (CR) of each biocompatibility parameter was calculated using the following formula (Eq. 1) after correction of the post-treatment values for the hematocrit (Hct).
where Hctpre is the pre-treatment Hct value, Hctpost is the post-treatment Hct value, Cpre is the pre-treatment solute concentration, and Cpost is the post-treatment solute concentration.
The lymphocyte blastogenesis response was evaluated by measuring the 3H-thymine uptake by the isolation culture method. In this test, lymphocytes are incubated without any stimulation, or with PHA (which particularly activates CD4 + T cells) or Con A (which particularly activates CD8 + T cells) stimulation. The Stimulation Index (SI) was calculated as the ratio of the response to each stimulation (PHA or Con A) relative to the response in the absence of stimulation, and the change in SI before and after treatment was evaluated.
Removal performances for urea and creatinine, as small-molecule solutes and for β2-MG and α1-MG as LMWPs, were evaluated. The removal rate of each LMWP was calculated using the following formula (Eq. 2) after correction of the post-treatment values of LMWP concentration for the Hct.
For measurement of albumin leakage, a part of the waste dialysate fluid was stored at the rate of 2 L/h [12] from the initiation to termination of the treatment; the entire volume of the stored waste fluid was mixed well, and a part of it was used for the measurements.
Statistical analyses
For statistical analyses of the biocompatibilities and solute removal performances, one-way analysis of variance, followed by Tukey’s test, was used, with the significance level set at < 5%.
Results
We enrolled six stable patients (five men and one woman) who were receiving maintenance dialysis at our clinic for this study. The mean patient age was 54.7 ± 14.6 years; the mean dialysis vintage was 96.8 ± 82.6 months, and the underlying disease was diabetic nephropathy in four patients, chronic glomerulonephritis in one patient, and vesicoureteral reflux in one patient (Table 3).
Biocompatibility
No significant changes were observed in the CRs of inflammatory markers (high-sensitivity CRP, IL-6, and PTX-3) in any of the three modes of treatment. No major changes of the high-sensitivity CRP level were observed after treatment as compared to the levels before treatment, while the serum IL-6 levels decreased after the treatment in all the three treatment modes. In contrast, the plasma PTX-3 levels increased after the treatments (Fig. 2).
Levels of inflammatory markers [high-sensitivity C-reactive protein (CRP), interleukin 6 (IL-6), and pentraxin-3 (PTX-3)]. The rates of change were calculated after correction of post-treatment values for the hematocrit (Hct). No significant differences were observed in any of the parameters evaluated among the treatment conditions (n = 6, mean ± SD)
No significant differences were observed in the CRs of the platelet function parameters (β-TG and soluble P-selectin) in any of the three treatment modes. The plasma β-TG levels decreased after the treatment in all three treatment modes, whereas no significant changes in the P-selectin levels were observed (Fig. 3).
Levels of platelet markers [β-thromboglobulin (TG) and soluble P-selectin] and the lymphocyte function testing [lymphocyte blastogenesis test using phytohemagglutinin (PHA) and concanavalin A (Con A) as mitogens]. The rates of change were calculated after correction of post-treatment values for the hematocrit (Hct). The rates of change of the stimulation index (SI) (with stimulation/without stimulation) for each stimulus (PHA or Con A) were evaluated. No significant differences were observed in any of the parameters evaluated among the treatment conditions (n = 6, mean ± SD)
There was also no significant difference in the SI of lymphocyte function (PHA and Con A) in any of the three modes. In contrast, the SI for lymphocyte blastogenesis response increased after treatment as compared with that before treatment in all three modes (Fig. 3).
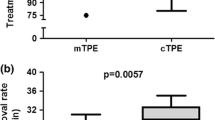
Comparison of the removal performances (Fig. 4)
Removal rates of urea, creatinine, β2-microglobulin (β2-MG), and α1-microglobulin (α1-MG), and albumin leakage. Although no significant differences for the small-molecule solutes (urea and creatinine) were observed among the treatment modes, post-hemodiafiltration (HDF) was associated with significantly higher removal rates of low-molecular-weight proteins (LMWPs) (β2-MG, α1-MG), and significantly higher albumin leakage than pre-HDF and hemodialysis (HD) (n = 6, mean ± SD)
There were no significant differences in the removal performances for small-molecule solutes among the treatment modes.
Post-HDF was associated with a significantly higher removal rate of β2-MG than HD. Post-HDF was also associated with a significantly higher removal rate of α1-MG than HD and pre-HDF. Furthermore, post-HDF was also associated with significantly higher albumin leakage than HD and pre-HDF.
Discussion
Our previous study is the only one, until date, to have examined how the difference in the mode of addition of substitution fluid in pre-HDF and post-HDF affects the biocompatibility of the treatment [11]. In the present study, we used higher blood and substitution fluid flow rates as compared to those used in our previous study. The present study is highly novel, given that the number of patients undergoing online HDF in Japan is increasing every year, the number of patients opting for post-HDF is increasing, and higher blood flow rates are being used. Another important feature of this study was the evaluation of a greater number of markers as compared to that in our previous study, with a special focus placed on platelet function and inflammatory markers.
We examined how the mode of dilution might influence the biocompatibility of online HDF by evaluating various markers. High-sensitivity CRP (MW: 105,000) is a protein whose blood concentration increases in association with inflammation or tissue damage. IL-6 (MW: 21,000) is an inflammatory cytokine whose concentration increases in the presence of bio-incompatibility of dialysis membranes. PTX-3 (MW: 40,000, forming an octamer [13]) is an acute inflammatory protein expressed in the vascular endothelial cells, macrophages, and other cells, and its expression increases in a manner dependent on inflammatory stimuli. β-TG (MW: 35,400) is a platelet-specific protein present in the alpha granules of platelets and is released into the circulation in association with platelet activation. Therefore, β-TG is used as an indicator of platelet activation. Soluble P-selectin (MW: 137,000) is present in the plasma and is used as an indicator of platelet activation and vascular endothelial cell activation [14]. The lymphocyte blastogenesis test is performed to evaluate the responses of lymphocytes to stimuli. Among the biocompatibility parameters evaluated in this study, IL-6 and β-TG, based on their molecular weight, are molecules that can be removed and produced during treatment.
There were no significant differences in any of the biocompatibility parameters evaluated in this study between pre-HDF and post-HDF. In contrast, our previous study concluded that pre-HDF was preferable to post-HDF based on the responses in the lymphocyte blastogenesis test, as well as intercellular adhesion molecule-1 and IL-6 measurements [11].
Under the conditions of the present study, the blood flow rate at the filter inlet [QB + substitution fluid flow rate (QS)] was 550 mL/min (QB: 300 mL/min, QS: 250 mL/min) for pre-HDF and 300 mL/min for post-HDF. The corresponding values in the previous study were 410 mL/min (QB: 200 mL/min, QS: 210 mL/min) for pre-HDF and 200 mL/min for post-HDF. The filtration fractions (FF), a parameter reflecting the blood flow rate calculated using formula (Eq. 3), of post-HDF were 31.8 ± 1.0% (1st post-HDF) and 32.1 ± 0.9% (3rd post-HDF).
where QF is the filtration flow rate and substitution flow rate.
The increased blood flow rates at the filter inlet in both pre-HDF and post-HDF as compared to those in the previous study might have imposed a greater stress on the blood cells, resulting in poorer biocompatibility. The European Renal Association–European Dialysis and Transplant Association group (ERA–EDTA) proposed that post-HDF should be performed at a FF of 20–25% [15], a value that was exceeded in the present study. In this study, the blood flow rates were still lower than those used in Europe, and it is considered that this could explain the higher values of FF obtained by us [31.8 ± 1.0% (1st post-HDF) and 32.1 ± 0.9% (3rd post-HDF)] as compared to the values proposed by the ERA–EDTA (20–25%). Higher values of FF are associated with excessive blood concentration and could have adverse effects on the body. In this study, the blood flow rate, FF, and filtration volume were higher during post-HDF, and the blood flow rate at the filter inlet was higher during pre-HDF. This suggests that there may be a larger number of factors influencing the biocompatibility of post-HDF as compared to pre-HDF; nevertheless, the results between pre-HDF and post-HDF were comparable.
The limitations of this study were that it was a single-center study, with a small sample size. In addition, post-HDF was associated with a significantly higher removal rate of α1-MG than pre-HDF. For comparison of the changes between IL-6 and β-TG, which are molecules produced and removed simultaneously during HDF, differential removal performances could interfere with accurate evaluation of these parameters.
Conclusion
Under the conditions of the present study, i.e., with increased flow rates of blood and substitution fluid, there was no difference in the biocompatibility between pre-HDF and post-HDF.
Future studies should include flow-cytometric analysis of CD62P, a direct indicator of platelet activation, to evaluate how the different modes of addition of substitution fluid and operating conditions, such as the blood flow rate, might affect the biocompatibilities of pre-HDF and post-HDF. When performed under appropriate conditions (i.e., of blood flow rate, substitution fluid flow rate, membrane material, and structure of the hemodiafilters), it appears that pre-HDF and post-HDF may have an equivalent level of biocompatibility. Increases in blood and/or substitution fluid flow rates could have a substantial effect on the biocompatibility in both modes of HDF. Biocompatibility may also vary depending on the membrane material and structure. The present results suggest the need to set appropriate treatment conditions in terms of the biocompatibility when performing HDF and that both pre-HDF and post-HDF can be performed effectively by comprehensively considering various factors, including the hemodiafilter types and patient condition.
References
Kikuchi K, Hamano T, Wada A, Nakai S, Masakane I. Predilution online hemodiafiltration is associated with improved survival compared with hemodialysis. Kidney Int. 2019;95:929–38.
Nitta K, Goto S, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, Nakai S, Goto S, Wada A, Hamano T, Hoshino J, Joki N, Abe M, Yamamoto K, Nakamoto H. 2018 annual dialysis data report, JSDT renal data registry. J Jpn Soc Dial Ther. 2018;51:699–766 (in Japanese).
Gejyo F, Yamada T, Odani S, Nakagawa Y, Arakawa M, Kunitomo T, Kataoka H, Suzuki M, Hirasawa Y, Shirahama T, Cohen AS. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–6.
Andoh T, Maki T, Li S, Uta D. β2-Microglobulin elicits itch-related responses in mice through the direct activation of primary afferent neurons expressing transient receptor potential vanilloid 1. Eur J Pharmacol. 2017;810:134–40.
Sakurai K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013;35:64–8.
Canaud B, Chenine L, Renaud S, Leray H. Optimal therapeutic conditions for online hemodiafiltration. Contrib Nephrol. 2011;168:28–38.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macià J, Carreras J, Soler J, Torres F, Campistol JM, Martinez-Castelao A, ESOHOL Study Group. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Kawabata K, Nagake Y, Shikata K, Fukuda S, Nakazono H, Takahashi M, Ichikawa H, Makino H. Soluble P-selectin is released from activated platelets in vivo during hemodialysis. Nephron. 1998;78:148–55.
Ojeda R, Arias-Guillén M, Gómez M, Vera M, Fontseré N, Rodas L, Filella X, Reverter JC, Lozano F, Villamor N, Maduell F. Study of biocompatibility of membranes in online hemodiafiltration. Blood Purif. 2020;49:400–8.
van den Oever GM, Grooteman MP, Bartels PC, Blankestijn PJ, Bots ML, van den Dorpel MA, Schoorl M, Schoorl M, Ter Wee PM, Nubé MJ. Post-dilution hemodiafiltration and low-flux hemodialysis have dissimilar effects on platelets: a side study of CONTRAST. Nephrol Dial Transplant. 2009;24:3461–8.
Sakurai K, Saito T, Yamauchi F, Asahi D, Hosoya H. Comparison of the effects of predilution and postdilution hemodiafiltration on neutrophils, lymphocytes and platelets. J Artif Organs. 2013;16:316–21.
Yamashita A, Yoshimoto T, Yoshimoto K, Shiraishi K, Sakai T, Sakai K. Methodological consideration on measurement of removed solute amount. J Jpn Soc Dial. 1982;15:803–7 (in Japanese).
Inforzato A, Rivieccio V, Morreale AP, Bastone A, Salustri A, Scarchilli L, Verdoliva A, Vincenti S, Gallo G, Chiapparino C, Pacello L, Nucera E, Serlupi-Crescenzi O, Day AJ, Bottazzi B, Mantovani A, De Santis R, Salvatori G. Structural characterization of PTX3 disulfide bond network and its multimeric status in cumulus matrix organization. J Biol Chem. 2008;283:10147–61.
Dunlop LC, Skinner MP, Bendall LJ, Favaloro EJ, Castaldi PA, Gorman JJ, Gamble JR, Vadas MA, Berndt MC. Characterization of GMP-140 (P-selectin) as a circulating plasma protein. J Exp Med. 1992;175:1147–50.
Chapdelaine I, de Roij van Zuijdewijn CL, Mostovaya IM, Lévesque R, Davenport A, Blankestijn PJ, Wanner C, Nubé MJ, Grooteman MP, Blankestijn PJ, Davenport A, Basile C, Locatelli F, Maduell F, Mitra S, Ronco C, Shroff R, Tattersall J, Wanner C, EUDIAL Group. Optimization of the convection volume in online post-dilution hemodiafiltration: practical and technical issues. Clin Kidney J. 2015;8:191–8.
Acknowledgements
This study received no external funding and was self-funded by Dr. Kenji Sakurai.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no conflicts of interests to declare.
Ethics approval
Informed consent for participation was obtained from all the subjects enrolled in this research, which was conducted with the approval of our institutional committee for research in humans.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kurihara, Y., Hosoya, H., Kishihara, R. et al. Comparison of the effects of pre-dilution and post-dilution online hemodiafiltration on the levels of inflammatory markers, lymphocytes, and platelets. J Artif Organs 25, 59–65 (2022). https://doi.org/10.1007/s10047-021-01281-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-021-01281-5