Abstract
Online hemodiafiltration (OL-HDF) is a treatment modality using diffusion and ultrafiltration. There are two types of dilution methods in OL-HDF: pre-dilution, which is commonly provided in Japan, and post-dilution, which is commonly provided in Europe. The optimal OL-HDF method for individual patients is not well studied. In this study, we compared the clinical symptoms, laboratory data, spent dialysate, and adverse events of pre- and post-dilution OL-HDF. We conducted a prospective study of 20 patients who underwent OL-HDF between January 1, 2019 and October 30, 2019. Their clinical symptoms and dialysis efficacy were evaluated. All patients underwent OL-HDF every 3 months in the following sequence: first pre-dilution, post-dilution, and second pre-dilution. We evaluated 18 patients for the clinical study and 6 for the spent dialysate study. No significant differences in spent dialysates regarding small and large solutes, blood pressure, recovery time, and clinical symptoms were observed between the pre- and post-dilution methods. However, the serum α1-microglobulin level in post-dilution OL-HDF was lower than that in pre-dilution OL-HDF (first pre-dilution: 124.8 ± 14.3 mg/L; post-dilution: 116.6 ± 13.9 mg/L; second pre-dilution: 125.8 ± 13.0 mg/L; first pre-dilution vs. post-dilution, post-dilution vs. second pre-dilution, and first pre-dilution vs. second pre-dilution: p = 0.001, p < 0.001, and p = 1.000, respectively). The most common adverse event was an increase in transmembrane pressure in the post-dilution period. Compared to pre-dilution, the post-dilution method decreased the α1-microglobulin level; however, there were no significant differences in clinical symptoms or laboratory data.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
End-stage renal disease (ESRD) requiring maintenance dialysis has been associated with shorter survival and poorer quality of life, despite improvements in medical treatment. Hemodiafiltration (HDF) is a type of blood purification therapy that combines diffusive and convective transport and renal replacement therapy that can remove β2-microglobulin (β2-MG) [1] and various cytokines compared to hemodialysis (HD) [2, 3]. Recent studies have reported the increased use of online hemodiafiltration (OL-HDF) worldwide due to its potential to prevent future dialysis-related complications—such as intradialytic hypotension and dialysis-related amyloidosis—and improve the quality of life and survival outcomes of patients with ESRD [4,5,6]. In Japan, an additional fee for national reimbursement of OL-HDF in 2012 resulted in an increased proportion of patients receiving OL-HDF (2012, 4.8%; 2018, 27.3%) [7].
Pre-dilution OL-HDF with a low blood flow rate (Qb) and a protein leakage hemodiafilter has been widely accepted in Japan because it is less likely to cause blood concentration and allows for a high substitution fluid rate (Qs) [8,9,10]. However, in Europe and other countries, post-dilution OL-HDF with a high Qb and non-protein leakage hemodiafilter is mainly performed. Post-dilution OL-HDF with high Qs and Qb improves prognoses and becomes enhanced with increasing Qs [11,12,13]. However, post-dilution OL-HDF has not been accepted in Japan because high Qb is not preferred and albumin leakage is difficult to control. Sakurai et al. previously showed that pre-dilution OL-HDF was superior to post-dilution OL-HDF in terms of biocompatibility [14]. However, they also reported that post-dilution OL-HDF with the appropriate hemodiafilters efficiently removed low-molecular-weight protein (LMWP), achieved mild albumin leakage without high Qb and Qs, and was comparable with pre-dilution OL-HDF in terms of biocompatibility [15]. However, few studies have examined the association between dialysis-related clinical symptoms and the dilution method.
The clinical differences between the pre- and post-dilution methods of OL-HDF in Japan are unclear and require investigation to achieve clinically optimal OL-HDF. Therefore, we examined the clinical symptoms, laboratory data, spent dialysate data, and adverse events associated with the pre- and post-dilution methods of OL-HDF.
Materials and methods
Ethical approval
This study and protocol was approved by the Tsuchiya General Hospital Institutional Review Board on human research (approval number: E180618-1), and all enrolled subjects gave their informed consent. This study was performed in accordance with the principles of the Declaration of Helsinki (as revised in Tokyo in 2004).
Study design and population
This prospective, single-center study enrolled 20 patients who underwent OL-HDF at the Nakajima-Tsuchiya Clinic of the Akane Foundation. Eligibility criteria included patients aged ≥ 20 years who were receiving pre-dilution OL-HDF three times a week, 4 h per session for > 3 months. The exclusion criteria were as follows: serious cardiovascular complications (New York Heart Association grade ≥ III); serious liver dysfunction (aspartate aminotransferase or alanine aminotransferase ≥ 100 IU/L); respiratory failure requiring oxygen administration; malignancy not treated radically; severe cognitive decline (Hasegawa dementia scale-revised ≤ 23); and investigators’ judgment of patient eligibility. The discontinuation criteria during the study period included withdrawal of consent, inability to continue this study due to adverse events, death, pregnancy, and investigators’ judgment for study termination.
The study period ranged from January 1, 2019 to October 30, 2019. The first month comprised the observation period, and HDF was performed under the same conditions as those used before this study. Thereafter, OL-HDF was performed using the first pre-dilution method for 3 months, post-dilution method for 3 months, and second pre-dilution method for 3 months. In this study, OL-HDF was standardized to the following conditions: three times per week for 4 h per session; FIX-210Seco® (asymmetric triacetate membrane; Nipro Co., Osaka, Japan) used for the hemodiafilter; Qb of 250 mL/min; total dialysate flow rate (Qd) of 500 mL/min, including Qs; Qs of 200 mL/min (total of 48 L per session) with the pre-dilution method; and Qs of 60 mL/min (total of 14.4 L per session) with the post-dilution method (Supplementary Fig. 1). These OL-HDF conditions were adjusted according to each patient’s state, such as vital signs during OL-HDF, transmembrane pressure (TMP), and laboratory data. The HDF conditions and medications were adjusted according to the condition of each patient. A GC-110N® dialysis machine (JMS Corp., Hiroshima, Japan) was used for HDF. The Kindaly 4E® (Fuso Pharmaceutical Industries Ltd., Osaka, Japan) was used as the dialysate, and the theoretical values of the dialysate after adjustment were as follows: sodium (Na), 140 mEq/L; potassium (K), 2.0 mEq/L; calcium (Ca), 2.75 mEq/L; magnesium (Mg), 1.0 mEq/L; chloride (Cl), 112.25 mEq/L; CH3COO, 8 mEq/L; HCO3, 27.5 mEq/L; and C6H12O6, 125 mg/dL. The dialysate and substitution fluid quality were based on the 2016 Japanese Dialysate Water Quality Standards [16].
Data collection
We collected data regarding sex, age, dialysis vintage, HDF vintage, HDF modality, reasons for conversion to HDF from HD, body mass index, smoking status, medical history, comorbidities, vascular access, primary cause of ESRD, history of renal treatment for peritoneal dialysis (PD) or kidney transplantation, Charlson comorbidity index (CCI) score [17] at the study initiation, and HDF conditions during the observation period. We performed assessments for the first OL-HDF session in the last week of each treatment period.
The study outcomes were pre-HDF and post-HDF blood pressure, recovery time (RT), visual analog scale (VAS) score, and laboratory data. Blood pressure was measured in the sitting position. In the next session of the assessment, the amount of time required by the patients to fully recover from fatigue (RT) after the end of the assessment session was reported in minutes. Itchiness, restless leg syndrome, pain in the bones and joints, cramps, post-dialysis malaise, sleep disorders, and moodiness were examined using VAS, with scores ranging from 0 to 10 points. The following laboratory data were evaluated: normalized protein catabolic rate, geriatric nutritional risk index. dialysis efficiency (Kt/V), urea reduction ratio, C-reactive protein, total protein, albumin, blood urea nitrogen, creatinine (Cre), uric acid (UA), Na, K, Cl, Ca, phosphorus (P), white blood cells, red blood cells, hemoglobin. hematocrit, platelets, iron, total iron binding capacity, unsaturated iron binding capacity, ferritin, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglycerides, brain natriuretic peptide, corrected calcium, transferrin saturation, human atrial natriuretic peptide, whole parathyroid hormone, α1-microglobulin (α1-MG), and β2-MG. Adverse events were recorded throughout the study period.
Collection of spent dialysates
We collected and examined the total spent dialysate under OL-HDF conditions in 10 of the 20 eligible OL-HDF patients. The spent dialysate was partially stored throughout the assessment session at a rate of 20 mL/min (total of 4.8 L per session); thereafter, the total amount stored was well-mixed. Subsequently, a portion of the stored spent dialysate was measured. Moreover, the blood urea nitrogen (molecular weight: 28), Cre (113), UA (168), and P (30) levels were used to evaluate the removal of small-molecular-weight solutes (SMWS), while the β2-MG (11,800) and α1-MG (33,000) levels were used to evaluate the removal of LMWP. The reduction rate (RR), removal amount (RA) including albumin leakage, clear space (CS), and clear space rate (CSR) [18] were also examined. The RRs of β2-MG and α1-MG were corrected using the hematocrit level to exclude the effects of the blood concentration, and the RA included the amount of albumin leakage. The equations for RR, RA, CS, and CSR (Supplementary Fig. 2) were determined to be similar to the spent dialysate examination for other eligible HDF patients in this study.
Statistical analysis
All results are presented as numbers and percentages or as means and standard deviations. Multiple comparison tests using a linear mixed model were performed for the first pre-dilution and post-dilution, the post-dilution and second pre-dilution, and the first pre-dilution and second pre-dilution. Additionally, multiple comparison tests using the Bonferroni corrections were performed [19]. The p-values were corrected by tripling the original p-value, and when the corrected p-value was > 1, it was recorded as p = 1.000. SPSS software (SPSS version 25; SPSS Inc., Chicago, IL, USA) was used to perform multiple comparison tests, and a corrected p < 0.05 was defined as statistically significant. To determine the number of adverse events, the number of incidents per patient per month was calculated.
Results
Of the 20 eligible HDF patients, 2 discontinued the study due to poor compliance with oral medications during the observation period and prolonged hospitalization with ileus during the first pre-dilution period. Eighteen patients underwent HDF treatment during the study period, of which only one had an increased Qb (300 mL/min) due to hyperkalemia during the post-dilution period, and the condition persisted during the second pre-dilution period.
Patient characteristics are shown in Table 1. Of the 18 eligible patients, 14 (78%) were men and 4 (22%) were women. The dialysis and HDF vintages were 18.1 ± 7.1 and 13.3 ± 6.6 months, respectively, and the most common primary disease with ESRD was chronic glomerulonephritis (72%). Five patients (28%) had a history of PD, while only one patient (6%) had a history of kidney transplantation. The CCI score was 3.0 ± 1.2 points.
In the linear mixed model, blood pressures before and after HDF and RT were not significantly different during the study period. Similarly, VAS assessments for itchiness, restless legs syndrome, bone and joint pain, cramps, post-dialysis malaise, sleep disorders, and moodiness did not yield significantly different scores during the study period (Table 2).
Table 3 shows the laboratory data. Only the α1-MG level was significantly different during the first pre-dilution versus post-dilution and post-dilution versus second pre-dilution assessments. The α1-MG levels were 124.8 ± 14.3 mg/L (first pre-dilution), 116.6 ± 13.9 mg/L (post-dilution), and 125.8 ± 13.0 mg/L (second pre-dilution) (first pre-dilution vs. post-dilution, post-dilution vs. second pre-dilution, and first pre-dilution vs. second pre-dilution: p = 0.001, p < 0.001, and p = 1.000, respectively). The β2-MG levels were 25.3 ± 3.1 mg/L (first pre-dilution), 24.5 ± 2.7 mg/L (post-dilution), and 25.9 ± 3.1 mg/L (second pre-dilution) (first pre-dilution vs. post-dilution, post-dilution vs. second pre-dilution, and first pre-dilution vs. second pre-dilution: p = 0.139, p = 0.003, and p = 0.414, respectively), and there was a significant difference between the post-dilution and second pre-dilution levels.
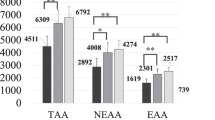
Of the 10 eligible HDF patients who underwent the spent dialysate examination, 4 were excluded due to partially missing spent dialysate data. Therefore, six eligible HDF patients were included in the spent dialysate examination. The characteristics of the patients who underwent the spent dialysate examination are shown in Supplementary Table 1. There were no significant differences in the RR, CS, CSR, and RA, including albumin leakage; however, there was a significant difference in the RR of P (Fig. 1 and Supplementary Table 2).
Spent dialysate data. A Reduction rate. Only phosphorus level was significantly different between the first and second pre-dilution periods (p = 0.012). B Removal amount. C Clear space. D Clear space rate. BUN blood urea nitrogen, Cre creatinine, HDF hemodiafiltration, P phosphorus, UA uric acid, α1-MG α1-microglobulin, β2-MG β2-microglobulin
Adverse events are described in Supplementary Table 3. There were no serious adverse events related to switching to different treatment modes. The most common adverse events were dialysis hypotension during the first pre-dilution period and increased TMP during the post-dilution period. Dialysis hypotension was treated by decreasing the rate of fluid removal, intravenous drip, or antihypertensive medication.
Discussion
In this study, we investigated the clinical symptoms, laboratory data, spent dialysate data, and adverse events of HDF associated with the pre-dilution and post-dilution methods. Consequently, we found no difference between pre-dilution and post-dilution OL-HDF regarding the CS, CSR, blood pressure, RT, and clinical symptoms in terms of VAS scores. Our data also showed that the serum α1-MG level significantly decreased in the post-dilution OL-HDF. This finding suggests that post-dilution OL-HDF provides more efficient serum α1-MG removal than pre-dilution OL-HDF; however, there was no significant difference in clinical symptoms.
The CS shows the body fluid volume at which the concentration of the solute of interest becomes zero by the treatment. The CSR calculates the ratio of clear space to total body fluid volume [18]. For 10 of the20 HDF patients, we planned to examine the spent dialysate; however, only 6 of the 10 eligible HDF patients could be examined. Under the OL-HDF conditions in this study, the CS and CSR did not differ significantly during the spent dialysate examination performed during the three periods. In future studies, the CS and CSR should be investigated for various treatment modalities including HDF.
It is challenging to choose the optimal OL-HDF conditions to achieve the best quality of life and survival outcomes for each dialysis patient. Various dialysis-related symptoms, such as depression [20], sleep disorders [21], itchiness [22], retinal hypotension [23], and RT after dialysis [24], were observed and are risk factors for mortality. The prevalence of moderate or severe itchiness is 40–50%, which is relatively high [22], while the prevalence of sleep disorders is 49% [21]. Restless leg syndrome, irritability, and skin pigmentation are not risk factors for mortality; however, they are uncomfortable. Sakurai et al. reported that the aggressive removal of many LMWPs while allowing 3–5 g of albumin leakage improved severe restless leg syndrome; the target RR was > 35% for α1-MG, which is a surrogate marker for evaluating LMWP removal [25,26,27]. During this study, the RT and VAS results of subjective evaluations of dialysis-related symptoms were not significantly different. Future studies are needed to determine the association between HDF conditions and clinical symptoms.
Grosjean et al. reported that the retinol binding protein 4 (RBP4) is a LMWP that is associated with insulin resistance and metabolic syndrome. RBP4 has also been reported to be positively correlated with total cholesterol and triglycerides. In fact, ESRD patients have fourfold higher RBP4 than the general population. Treatment with HDF and renal transplantation reduce serum RBP4 levels [28]. In this study, RBP4 was not measured and the use of dyslipidemic drugs was not restricted; in four patients, dyslipidemic drugs were added or increased. Further study of the association between HDF and RBP4 is needed.α1-MG has a short half-life of only a few hours and exists in the blood at the same rate in the free form and the bound form with IgA, albumin, or prothrombin [29]. Its bioactivity as a potent radical scavenger and heme-binding protein has recently attracted considerable attention [30]. For dialysis patients, a large amount of oxidized α1-MG is molecularly degraded, and although its blood concentration is high [31], it is suspected that α1-MG does not function properly as a radical scavenger. It has been suggested that the active removal of α1-MG promotes α1-MG turnover and recovers its original function as a radical scavenger [30]. In a recent Japanese study, Kurihara et al. found that the RR of α1-MG and leakage of Alb were significantly higher in post-dilution OL-HDF, with no significant difference in biocompatibility parameters between HD, pre-dilution OL-HDF, and post-dilution OL-HDF [32]. Okada et al. also showed that the RR of α1-MG was higher with a minimum substitution volume of 6 L/session in post-dilution OL-HDF than with a maximum substitution volume of 48 L/session in pre-dilution OL-HDF [33]. During this study, only serum α1-MG was significantly decreased with the post-dilution method than that with the first and second pre-dilution methods. The RA of α1-MG was not significantly different; however, it was higher with the post-dilution method than with the pre-dilution method, according to the spent dialysate examination. In fact, compared to the pre-dilution method, the post-dilution method with a substitution volume of ≥ 6 L/session increased the RR of α1-MG [33]. Therefore, in this study, the post-dilution method decreased serum α1-MG by approximately 10 mg/L lower than the pre-dilution method. The active removal of α1-MG may have improved its original function. Additionally, all six patients who underwent the spent dialysate examination had albumin leakage > 6 g during the three periods. The RRs of α1-MG were 40.3 ± 4.7% during the first pre-dilution period, 38.4 ± 6.1% during the post-dilution period, and 36.3 ± 6.6% during the second pre-dilution period; furthermore, this study achieved > 35% RRs of α1-MG. However, no significant difference was observed in restless leg syndrome, which is associated with α1-MG. The results of this study suggested that the degree of restless leg syndrome was mild, or that the 3-month periods may have been too short to observe changes in restless leg syndrome. Our results also found that the RR of α1-MG and Alb leakage were consistent with those of previous studies [32, 33], and serum α1-MG decreased significantly. In this study, we found no significant differences in clinical symptoms due to α1-MG. Therefore, the clinical significance of α1-MG requires further research.β2-MG is a precursor of amyloid fibrils in dialysis-related amyloidosis. In fact, according to previous studies, pre-dialysis β2-MG levels of ≤ 27.5 mg/L [34] and ≤ 32.2 mg/L [35] suggested a survival benefit. The Japanese Society for Dialysis Therapy recommends that the pre-dialysis serum β2-MG level should be ≤ 30 mg/L [36], which our HDF conditions in this study adhered to.
The CCI score in this study was 3.0 ± 1.2 points lower than the 6.5 ± 2.8 points for induction dialysis patients in Japan in 2007 [37], due to eligible patients who tolerated outpatient dialysis and HDF. There were no obvious adverse events associated with pre-existing conditions, except for ileus in one patient. During the first pre-dilution period, dialysis hypotension was more frequent. Therefore, the treatment for four eligible participants was adjusted for dry weight or antihypertensive medications during the observation and first pre-dilution periods. With the post-dilution method, the substitution volume is limited by the Qb due to the blood concentration in the hemodiafilter. When the substitution volume is excessively increased, there are risks of excessive albumin leakage and blood concentration [38]. For the post-dilution method in this study, we selected the FIX hemodiafilter, which tends to cause less TMP elevation [39]; however, in actuality, TMP elevation occurred with fouling, which is clogging of the hemodiafilter due to protein adhesion. Therefore, we had to reduce the Qs. The actual substitution fluid volumes were 47.0 ± 0.4 L per session during the first pre-dilution period, 13.9 ± 1.1 L per session during the post-dilution period, and 46.8 ± 0.5 L per session during the second pre-dilution period. Therefore, it is necessary to determine the HDF conditions without TMP elevation.
Our study had some limitations. First, it was a single-center study with a small sample size. Therefore, it is unclear whether these results can be generalized to other facilities or OL-HDF methods outside Japan. However, the strength of this study is that it provides a detailed and exploratory examination, including clinical symptoms and adverse events. Second, the VAS scores and RTs were self-reported by participants; therefore, the possibility of participant bias could not be ruled out because they could not be blinded. However, in this study, no significant differences in the subjective evaluations were observed. Third, blood was not collected from the venous circuit (the outlet of dialyzers) or arterial circuit (the inlet of dialyzers) 60 min after the start of dialysis. Therefore, the clearance of each solute was not calculated in the present study. However, we collected all drainage fluids and found no significant differences in the CS and CSR among the three periods.
Conclusion
There were no significant differences in the CS, CSR, blood pressure, and clinical symptoms when using the pre- and post-dilution methods for OL-HDF. However, the serum α1-MG levels were significantly lower with the post-dilution method than with the pre-dilution method. Future studies should clarify the differences between the pre-dilution and post-dilution methods for the selection of optimal dialysis conditions for each patient.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Hoshino J, Yamagata K, Nishi S, Nakai S, Masakane I, Iseki K, et al. Carpal tunnel surgery as proxy for dialysis-related amyloidosis: results from the Japanese society for dialysis therapy. Am J Nephrol. 2014;39:449–58.
Panichi V, Rizza GM, Paoletti S, Bigazzi R, Aloisi M, Barsotti G, et al. Chronic inflammation and mortality in haemodialysis: effect of different renal replacement therapies. Results from the RISCAVID study. Nephrol Dial Transplant. 2008;23:2337–43.
Panichi V, Paoletti S, Consani C. Inflammatory pattern in hemodiafiltration. Contrib Nephrol. 2008;161:185–90.
Susantitaphong P, Siribamrungwong M, Jaber BL. Convective therapies versus low-flux hemodialysis for chronic kidney failure: a meta-analysis of randomized controlled trials. Nephrol Dial Transplant. 2013;28:2859–74.
Nistor I, Palmer SC, Craig JC, Saglimbene V, Vecchio M, Covic A, et al. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis. 2014;63:954–67.
Wang AY, Ninomiya T, Al-Kahwa A, Perkovic V, Gallagher MP, Hawley C, et al. Effect of hemodiafiltration or hemofiltration compared with hemodialysis on mortality and cardiovascular disease in chronic kidney failure: a systematic review and meta-analysis of randomized trials. Am J Kidney Dis. 2014;63:968–78.
Nitta K, Abe M, Masakane I, Hanafusa N, Taniguchi M, Hasegawa T, et al. Annual dialysis data report 2018, JSDT Renal Data Registry: dialysis fluid quality, hemodialysis and hemodiafiltration, peritoneal dialysis, and diabetes. Ren Replace Ther. 2020;6:51.
Tsuchida K, Minakuchi J. Clinical benefits of predilution on-line hemodiafiltration. Blood Purif. 2013;35(Suppl 1):18–22.
Masakane I, Kikuchi K, Kawanishi H. Evidence for the clinical advantages of predilution on-line hemodiafiltration. Contrib Nephrol. 2017;189:17–23.
Kawanishi H. Development of online hemodiafiltration in Japan. Ren Replace Ther. 2021;7:51.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97.
Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96.
Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202.
Sakurai K, Saito T, Yamauchi F, Asahi D, Hosoya H. Comparison of the effects of predilution and postdilution hemodiafiltration on neutrophils, lymphocytes and platelets. J Artif Organs. 2013;16:316–21.
Sakurai K, Saito T, Yamauchi F, Hosoya HH, Kurihara Y, Yoshida K, et al. Comparison of the effects of pre-dilution and post-dilution on-line hemodiafiltration on inflammatory markers, lymphocytes and platelets. Nephrol Dial Transplant. 2018;33:186–9.
Mineshima M, Kawanishi H, Ase T, Kawasaki T, Tomo T, Nakamoto H. 2016 update Japanese Society for Dialysis Therapy Standard of fluids for hemodialysis and related therapies. Ren Replace Ther. 2018;4:15.
Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Yamashita AC. The clear space index. Contrib Nephrol. 2017;189:197–203.
Gelman A, Hill J, Yajima M. Why we (usually) don’t have to worry about multiple comparisons. J Res Educ Eff. 2012;5:189–211.
Lopes AA, Albert JM, Young EW, Satayathum S, Pisoni RL, Andreucci VE, et al. Screening for depression in hemodialysis patients: associations with diagnosis, treatment, and outcomes in the DOPPS. Kidney Int. 2004;66:2047–53.
Elder SJ, Pisoni RL, Akizawa T, Fissell R, Andreucci VE, Fukuhara S, et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol Dial Transplant. 2008;23:998–1004.
Narita I, Alchi B, Omori K, Sato F, Ajiro J, Saga D, et al. Etiology and prognostic significance of severe uremic pruritus in chronic hemodialysis patients. Kidney Int. 2006;69:1626–32.
Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–20.
Rayner HC, Zepel L, Fuller DS, Morgenstern H, Karaboyas A, Culleton BF, et al. Recovery time, quality of life, and mortality in hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2014;64:86–94.
Maduell F, Ojeda R, Arias-Guillen M, Bazan G, Vera M, Fontsere N, et al. Assessment of dialyzer surface in online hemodiafiltration; objective choice of dialyzer surface area. Nefrologia. 2015;35:280–6.
Sakurai K. Biomarkers for evaluation of clinical outcomes of hemodiafiltration. Blood Purif. 2013;35(Suppl 1):64–8.
Maduell F, Arias-Guillen M, Fontsere N, Ojeda R, Rico N, Vera M, et al. Elimination of large uremic toxins by a dialyzer specifically designed for high-volume convective therapies. Blood Purif. 2014;37:125–30.
Grosjean F, Esposito P, Maccarrone R, Libetta C, Dal Canton A, Rampino T. RBP4: a culprit for insulin resistance in end stage renal disease that can be cleared by hemodiafiltration. Biomed Res Int. 2017; p. 7270595. https://doi.org/10.1155/2017/7270595.
Akerstrom B, Maghzal GJ, Winterbourn CC, Kettle AJ. The lipocalin alpha1-microglobulin has radical scavenging activity. J Biol Chem. 2007;282:31493–503.
Rutardottir S, Karnaukhova E, Nantasenamat C, Songtawee N, Prachayasittikul V, Rajabi M, et al. Corrigendum to “Structural and biochemical characterization of two heme binding sites on alpha1-microglobulin using site directed mutagenesis and molecular simulation” [BBAPAP 1864/1 (2016) Page 29 to 41]. Biochim Biophys Acta Proteins Proteom. 2020;1868: 140394.
Kristiansson A, Bergwik J, Alattar AG, Flygare J, Gram M, Hansson SR, et al. Human radical scavenger alpha1-microglobulin protects against hemolysis in vitro and alpha1-microglobulin knockout mice exhibit a macrocytic anemia phenotype. Free Radic Biol Med. 2021;162:149–59.
Kurihara Y, Hosoya H, Kishihara R, Yoshinaga M, Iwadate Y, Yamauchi F, et al. Comparison of the effects of pre-dilution and post-dilution online hemodiafiltration on the levels of inflammatory markers, lymphocytes, and platelets. J Artif Organs. 2022;25:59–65.
Okada K, Michiwaki H, Mori H, Tashiro M, Inoue T, Shima H, et al. Removal performance of pre- and post-dilution online hemodiafiltration using identical hemodiafilters in the same patients. J Artif Organs 2022 [ahead of print].
Cheung AK, Rocco MV, Yan G, Leypoldt JK, Levin NW, Greene T, et al. Serum beta-2 microglobulin levels predict mortality in dialysis patients: results of the HEMO study. J Am Soc Nephrol. 2006;17:546–55.
Okuno S, Ishimura E, Kohno K, Fujino-Katoh Y, Maeno Y, Yamakawa T, et al. Serum beta2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24:571–7.
Watanabe Y, Kawanishi H, Suzuki K, Nakai S, Tsuchida K, Tabei K, et al. Japanese society for dialysis therapy clinical guideline for “Maintenance hemodialysis: hemodialysis prescriptions.” Ther Apher Dial. 2015;19 Suppl 1:67–92.
Nakai S, Masakane I, Shigematsu T, Hamano T, Yamagata K, Watanabe Y, et al. An overview of regular dialysis treatment in Japan (as of 31 December 2007). Ther Apher Dial. 2009;13:457–504.
Sakurai K, Hosoya H, Kurihara Y, Yamauchi F, Suzuki A, Kurosawa K, et al. Evaluation of low-volume post-dilution online hemodiafiltration with Japanese high-performance hemodiafilters. J Artif Organs. 2020;23:234–9.
Albalate Ramon M, Martinez Miguel P, Bohorquez L, de Sequera P, Bouarich H, Perez-Garcia R, et al. Asymmetric cellulose triacetate is a safe and effective alternative for online haemodiafiltration. Nefrologia (Engl Ed). 2018;38:315–20.
Acknowledgements
The authors thank Kayo Nishino, Junko Yamamoto, and Yoshihisa Taka for data collection and management.
Funding
This study received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by YK. The first draft of the manuscript was written by YK and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kawai, Y., Maeda, K., Moriishi, M. et al. Comparison of the pre-dilution and post-dilution methods for online hemodiafiltration. J Artif Organs 27, 48–56 (2024). https://doi.org/10.1007/s10047-023-01391-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-023-01391-2