Abstract
Although the innovation has come in ECMO field, many problems remain unresolved. One of the main problems is about long-term durability and biocompatibility. Another is the system’s size, weight, and its complicated equipment. For the former problem, we have previously developed ECMO system which consists of a tiny, hydrodynamically levitated centrifugal pump (BIOFLOAT-NCVC), a membrane oxygenator with hollow polyolefin fibers (BIOCUBE-NCVC), and the circuit treated with a heparin-bonding material (T-NCVC coating), and reported three cases of animal experiments for 30-day heparin-free drive. For the latter problem, we have integrated these elements to the compact system with sensors of temperature, pressure, and SvO2, and blood flow. Its installation area is 595 cm2, weighs 8.9 kg with attachable oxygen cassette, and battery which could last an hour at least. To evaluate the biocompatibility of this system, this ECMO was installed in four goats. Scheduled duration was 14 days. Heparin was continuously infused to control their ACT between 150 and 200 s except one 2-week experiment without systemic heparinization. All of the four goats survived till the scheduled termination. Function of the pump and the oxygenator during ECMO was stable. No obvious adverse events were observed. All lab data were of normal range after 1 week. Small infarctions were found at kidneys, but they were not clinically significant. No thrombus was found in the pump system. The oxygenators were extremely clean except a little thrombus formation; while, the heparin-free examination revealed acceptable cleanliness. The present study revealed good anti-thrombogenicity of this ultra-compact durable ECMO system with heparinization. Our system encourages awake and extubated management, rehabilitation, inter-hospital transfer, and prehospital initiation of ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extracorporeal Membrane Oxygenation (ECMO) is the form of cardiopulmonary bypass which is indicated to cardiogenic shock, severe respiratory failure, or cardiopulmonary arrest, etc. [1]. Although innovation has come in this field, many problems remain unresolved. Main problem is about long-term durability or biocompatibility, especially thrombogenicity. Another problem is the system’s size and weight, which prevents recent innovative management during ECMO which includes letting patients awake, extubated, rehabilitation including, standing, walking [2, 3] or advanced usage like patients’ transfer equipped with ECMO [4, 5], and prehospital initiation of ECMO [6,7,8].
For the former problem, we have previously developed ECMO system which consists of a hydrodynamically levitated centrifugal pump (BIOFLOAT-NCVC) [9], a membrane oxygenator with hollow polyolefin fibers (BIOCUBE-NCVC) [10, 11], and a circuit treated with a heparin-bonding material (T-NCVC coating) [10, 11]. Its preclinical study has already been done for without the administration of heparin for 30 days, and revealed its good performance [9].
For the latter problem, ECMO system needs to be more compact and lighter, and portable so as to hang out by oneself, and simpler, which means that the system has less lines and cables without essential ones. Embedded sensors and displays are also needed to continuously monitor pressures and body blood temperatures and oxygenations. Oxygen gas cylinder and batteries are also needed to achieve hospital transfer.
To achieve this matter, we have integrated these technical elements into the compact system with sensors of temperature, pressure, and SvO2, and a display of these information, portable oxygen cassette, and battery. Its weight is less than 10 kg.
The purpose of this study is to evaluate the long-term biocompatibility of this ultra-compact ECMO system in a series of chronic animal experiments.
Materials and methods
ECMO system
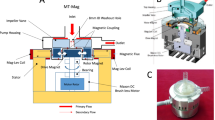
Figure 1 shows macro-inspection of the system. All of the elements were compactly arranged in this system. Its size was 595 cm2 in area, 405 mm in height, and its total weight is 8.9 kg with the gas cylinder unit and 6.6 kg without it. So, it is easy to lift or carry on one’s tod. Disposable unit could be easily mounted to the driver unit within a minute without any instruments, which is designed for quick priming for an emergent initiation of ECMO.
The ECMO system has three parts: disposable unit, driver unit, and gas cylinder unit. The disposable unit consists of the blood pump, the oxygenator, and some sensors’ interface which contact blood. The driver unit consists of the external motor unit of the pump system and sensors’ transducers and cables, and the display and the battery. The gas cylinder unit contains oxygen cylinder.
Centrifugal blood pump
The minute description about the blood pump system is in reference 9. In brief, the pump system consists of a disposable tiny pump head (a priming volume of 18 ml), and an external driving motor unit. The impeller was designed to levitate hydrodynamically, which enhanced the pump’s durability and anti-thrombogenicity.
Oxygenator, cannula, and surface coating technique
A membrane oxygenator with hollow polyolefin fibers (BIOCUBE 6000, Nipro Corporation, Japan) was used. The surface area of the hollow fibers in this oxygenator was 1.3 m2. Both the inflow and outflow cannulas, which were originally developed for femoral cannulation, were made of polyurethane and had been reinforced with wire to prevent kinking. All of the surfaces of the circuit were treated with a heparin-bonding material (T-NCVC coating) to impart anti-thrombogenicity.
Implanted sensors and display
Figure 2 shows where and what kind of the sensors are implanted. Sensors of pressure, temperature, were implanted in series with ECMO circuit. These sensors were made not to protrude nor dent, without generating blood stagnation or turbulence. Flowmeter and SO2 sensor was attached to inflow tube. All of these sensors’ transducer is implanted on driver unit. On top of the driver unit is touch panel display which indicates these information in real time. So, no additional cables or lines to transport such information are needed.
Battery and oxygen supply
A lithium ion battery is built in the driver unit. The gas cylinder unit has capacity of 150-L oxygen of standard conditions and is easily detachable. It could be used not merely when a patient is in bed, with external supply of oxygen and electrical energy, but also when a patient is out of bed, with these oxygen and energy units which could last 1 h.
The entire system to achieve portability
Figure 1 shows macro-inspection of the system. All of the elements descripted above were compactly arranged in this system. Its size was 595 cm2 in area, 405 mm in height, and its total weight is 8.9 kg. So, it is easy to lift or carry on one’s tod. The gas cylinder unit could be detached easily for bedside use with external oxygen supply, which result in lighter (6.6 kg) device. Disposable unit could be easily mounted to the driver unit within a minute without any instruments, well designed for quick priming in an emergent initiation of ECMO.
Animals and surgical procedure
Four Saanen goats (42–61 kg) were used. The protocols for the surgical procedures, postoperative care, and the termination of the animals were approved by the institutional committee on animal experiments. All of the animals received humane care in accordance with the “Guide for Care and Use of Laboratory Animals” (NIH publication 86-23, revised 1996) during the experiments. Anesthesia was induced with ketamine (8–10 mg/kg IM) and maintained via mechanical ventilation with 1.0% isoflurane in oxygen gas (50–100%). The right carotid artery and the right jugular vein were exposed. After the initial infusion of heparin (100 I U/kg), the inflow cannula was inserted via the vein, through the right atrium, and the end of cannula was placed between posterior vena cava and the diaphragm under fluoroscopic guidance. The outflow cannula was inserted into the artery. Then, the whole ECMO system (i.e. the pre-primed centrifugal pump and the membrane oxygenator) were connected to the cannulas. An additional (not included in the ECMO system) ultrasonic flowmeter probe (ME-9PXL, Transonic Systems Inc., US) was attached to the inflow cannula. Both the pump and oxygenator were fixed to a harness that had been specially designed for the animal experiments. The animals were extubated after they became conscious. A schematic view of the experiments is shown in Fig. 3.
Postoperative care
The scheduled experimental duration was over 14 days. The rotational speed of the pumps was adjusted to maintain the flow of the ECMO between 2.0 and 3.0 L/min. ACT were checked every day and continuous administration of heparin was performed to control their ACT between 150 and 200 s in the three of the four experiments. In the other experiment, heparin was not used without initial shot during the surgery. V/Q ratio of ECMO was 1.
Measurements for device performance
Bypass flow and pressures of the three points of the device (before the pump, between the pump and the oxygenator, and after the oxygenator) were continuously monitored and recorded with the software Labchart 8. The pressure drop across the oxygenator was daily evaluated from these measurements to estimate flow path blockage by thrombus formation. O2 and CO2 transfer rate was examined by the blood gas analysis of these pressure lines, just after the initiation of the ECMO, and at postoperative days 1, 3, 7, and just before the termination.
Biocompatibility measurements
Blood samples were collected before the implantation of the device, after its implantation, and at postoperative days 1, 3, 7, and just before the termination. The goats’ plasma-free hemoglobin, red and white cell counts, and biochemical parameters were measured. Pathological dissection was done to explore the evidence of thromboembolism or bleeding of the internal organs. Also, the dissection of the entire system was done to explore the formation of thrombi.
Results
All of the four goats survived till the scheduled termination and no device exchange was necessary. None of the animals presented any signs of thromboembolic complications.
Overall performance of this ECMO
The data of the pumps’ and the oxygenators’ performance are shown in Table 1 and Fig. 4. The pumps and the oxygenators were operated stably. The ECMO flow was controlled to targeted flow rate with little manipulation of pump speed. There were no mechanical problems. The O2 transfer rate and CO2 transfer rate remain stable during the experiment. The animals’ blood data indicated that the oxygenator has worked sufficiently during the course of the experiments.
Coagulation status, hemolysis and other blood sample data
Table 2 is the animals’ hematological and biochemical data. The activated clotting time (ACT) was controlled from 150 to 200 s among the three with heparin administration. The other heparin-free animal exhibited 100–120 s of ACT. The animals’ plasma-free hemoglobin increased immediately after the implantation of the device, but returned to the normal range within a week. All of the animals’ biological data remained within their normal ranges throughout the experiment.
Pathological exploration
Macro-inspection of the internal organs revealed small infarctions at kidneys (Fig. 5). But it is not clinically significant because values of BUN and creatinine were not elevated and their urination was normal without macroscopic hematuria. No other evidence of thromboembolism nor bleeding was found in the all animals. The cannulation site was clean.
Macro-inspection of dissected elements of the systems and organs depicted number on columns of sensors are corresponding to numbers depicted in Fig. 2
Dissection of the ECMO system
Figure 5 shows macroscopic inspection of elements of the system.
Macroscopic examinations of the explanted blood pumps did not detect any thrombus formation on the surface that came into contact with blood, e.g., in the blade passages or the washout hole in the impeller. There were no remarkable findings like scratches on the pump housing, which indicates that the impeller had not come into mechanical contact with the pump housing.
In heparin (+) group, the oxygenators were extremely clean with little thrombus formation; while, the heparin-free examination revealed acceptable cleanliness. Thrombus formation was observed between the casing and the fiber bundle near the corners of both the inlet and outlet sides due to low flow velocity near the casing wall.
Other elements of the ECMO circuit including tube, sensors, and their junctions were free of thrombi.
The inflow cannulas were deformed in all cases probably due to external forces associated with neck movement of the animals. This was considered to be inevitable in the present animal model. All of the inflow and outflow cannulas were free from thrombi, except for a small ring thrombus observed in the edge of the outflow port.
Discussion
The needs for long-term durable ECMO
The need for durable mechanical circulatory support systems, including ECMO systems, has been growing as such systems are increasingly being used to aid the recovery of cardiopulmonary function or bridges to devices such as implantable LVAD [1]. There are several reports about long-term ECMO support. Hoashi et al. [12] reported on prolonged cardiopulmonary support in neonates and infants with a median support period of 21.7 days. Kusajima et al. [13] described the continuous use of a single ECMO circuit for 74 days, which did not result in hemolysis or serum leakage.
Technical elements to achieve anti-thrombogenicity in this ECMO system
We have previously developed hydrodynamically levitated centrifugal pump, membrane oxygenator with hollow polyolefin fibers, and coating technique, and demonstrated its biocompatibility in 30-day animal experiments. All of these elements are installed on the present system [9,10,11]. Especially, the pump of the system which employs hydrodynamic levitation systems is expected to exhibit superior anti-thrombogenicity compared to conventional pivot-bearing pumps. The mechanism is that some of the fluid that passes through the impeller returned to the impeller inlet, washing out the corners of the blood path. This washout flow is driven by the pressure difference created by the impeller. Indeed, the explanted blood pumps did not have any clot formation on their inner surfaces.
Furthermore, the sensors of pressures and blood temperatures have tube-like form and do not protrude nor dent against blood stream and are equipped in serial manner without any branch lines. These sensors were also free from thrombi even under the heparin-free ECMO drive and, thus, demonstrate good anti-thrombogenicity.
Compact, integrated design to achieve portability
The system is 595 cm2 of installation area (about A4 size), weighs 8.9 kg with portable oxygen cassette, and battery which could last an hour at least stand alone and with sensors of pressure, temperature, SvO2, and flowmeter, and display which could monitor these parameters simultaneously in one display above the system without any additional cables or lines. This is quite small and simple but an integrated structure considering conventional ECMO system consisting of a big pump and oxygenator, and long circuit lines, many branch lines, cables of sensors, and independent displays to monitor these bioinformation.
Concerning installing compact ECMO system, hydrodynamic levitation-based pumps can have simple structures and be controlled with simple circuits because there is no need to monitor the position of the pump’s impeller. This is superior to magnetic levitation-based pump (another non-contacting levitation systems), which tends to be bigger. Furthermore, motor driver unit of the pump is implanted on the driver unit of this system to achieve compactness. Priming volume of the pump itself is only 18 mL.
Clinical revolution of the management of ECMO
These advantages make patient management easier, especially in the situation of extubating management, rehabilitation like standing, walking inside or outside of hospital, patient’s intra-hospital transfer, inter-hospital transfer with ambulance car, helicopter, and aircraft, or even prehospital initiation of ECMO.
Conclusion
Chronic animal experiments aimed at evaluating the biocompatibility of a newly developed integrated, compact ECMO system. All of four animals survived for the scheduled two-week study period without major complications. All of the pump surfaces and pump circuit that came into contact with blood were free from thrombi, while there are some in artificial lung and no signs of adverse effects on end-organ function were observed. The newly developed portable ECMO system demonstrated satisfactory biocompatibility.
References
Paden ML, Conrad SA, Rycus PT, Thiagarajan RR, ELSO Registry. Extracorporeal life support organization registry report 2012. ASAIO J. 2013;59:202–10.
Peek GJ, Mugford M, Tiruvoipati R, Wilson A, Allen E, Thalanany MM, Hibbert CL, Truesdale A, Clemens F, Cooper N, Firmin RK, Elbourne D. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicenter randomized controlled trial. Lancet. 2009;374:1351–63.
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, Haverich A, Hoeper MM, Warnecke G. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–8.
Ranney DN, Bonadonna D, Yerokun BA, Mulvihill MS, Al-Rawas N, Weykamp M, Gunasingha RM, Bartz RR, Haney JC, Daneshmand MA. Extracorporeal membrane oxygenation and interfacility transfer: a regional referral experience. Ann Thorac Surg. 2017;104:1471–8.
Charon C, Allyn J, Bouchet B, Nativel F, Braunberger E, Brulliard C, Martinet O, Allou N. Ten thousand kilometre transfer of cardiogenic shock patients on venoarterial extracorporeal membrane oxygenation for emergency heart transplantation: Cooperation between Reunion Island and Metropolitan France. Eur Heart J Acute Cardiovasc Care. 2017. https://doi.org/10.1177/2048872617719652 (Epub ahead of print).
Lamhaut L, Hutin A, Puymirat E, Jouan J, Raphalen JH, Jouffroy R, Jaffry M, Dagron C, An K, Dumas F, Marijon E, Bougouin W, Tourtier JP, Baud F, Jouven X, Danchin N, Spaulding C, Carli P. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: an observational study and propensity analysis. Resuscitation. 2017;117:109–17.
Haas NL, Coute RA, Hsu CH, Cranford JA, Neumar RW. Descriptive analysis of extracorporeal cardiopulmonary resuscitation following out-of-hospital cardiac arrest—an ELSO registry study. Resuscitation. 2017;119:56–62.
Lamhaut L, Hutin A, Deutsch J, Raphalen JH, Jouffroy R, Orsini JP, Baud F, Carli P. Extracorporeal cardiopulmonary resuscitation (ECPR) in the prehospital setting: an illustrative case of ECPR performed in the Louvre museum. Prehosp Emerg Care. 2017;21:386–9.
Tsukiya T, Mizuno T, Takewa Y, Tatsumi E, Taenaka Y. Preclinical study of a novel hydrodynamically levitated centrifugal pump for long-term cardiopulmonary support. In vivo performance during percutaneous cardiopulmonary support. J Artif Organs. 2015;18:300–6.
Eya K, Tatsumi E, Taenaka Y, Takewa Y, Wakisaka Y, Toda K, Nakatani T, Masuzawa T, Baba Y, Miyazaki K, Nishimura T, Ohno T, Ahn JM, Takano H. Development of a membrane oxygenator for long-term respiratory support and its experimental evaluation in prolonged ECMO. ASAIO J. 1996;42:M832–M836836.
Nishinaka T, Tatsumi E, Taenaka Y, Katagiri N, Ohnishi H, Shioya K, Fukuda T, Oshikawa M, Sato K, Tsukiya T, Homma A, Takewa Y, Takano H, Sato M, Kashiwabara S, Tanaka H, Sakai K, Matsuda T. At least 34 days of animal continuous perfusion by a newly developed extracorporeal membrane oxygenation system without systemic anticoagulants. Artif Organs. 2002;26:548–51.
Hoashi T, Kagisaki K, Yamashita K, Tatsumi E, Nishigaki T, Yoshida K, Hayashi T, Ichikawa H. Early clinical outcomes of new pediatric extracorporeal life support system (Endumo® 2000) in neonates and infants. J Artif Organs. 2013;16:267–72.
Kusajima K, Hoashi T, Kagisaki K, Yoshida K, Nishigaki T, Hayashi T, Ichikawa H. Clinical experience of more than 2 months usage of extracorporeal membrane oxygenation (Endumo® 4000) without circuit exchange. Artif Organs. 2014;17:99–102.
Acknowledgements
This study was supported by research funding based on joint research agreement with NIPRO Co., Ltd.. This research was also supported by AMED under Grant number 16lk0103019h0003.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Akiyama, D., Katagiri, N., Mizuno, T. et al. Preclinical biocompatibility study of ultra-compact durable ECMO system in chronic animal experiments for 2 weeks. J Artif Organs 23, 335–341 (2020). https://doi.org/10.1007/s10047-020-01180-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-020-01180-1