Abstract
A 5-year-old girl with right atrial isomerism, complete atrioventricular septal defect, hypoplastic left ventricle, double outlet right ventricle, and mixed-type total anomalous pulmonary venous connection with totally occluded left pulmonary veins presented at our center for fenestrated total cavo-pulmonary connection with an extra cardiac conduit at the age of 3 years. Eleven months after the Fontan completion, she developed protein-losing enteropathy (PLE). Spontaneously closed fenestration was thought to be the cause of the PLE, and she underwent revision of fenestration at the age of 5 years. After the operation, PLE did not improve, and newly developed hypoxemia impaired her systemic ventricular function, leading to the initiation of veno-arterial extracorporeal membrane oxygenation (ECMO) with the Endumo® system 18 days after the operation to treat her hemodynamic instability. Although the ECMO circuit was changed three times during the first 8 days, the fourth circuit could be used for 74 days without hemolysis and serum leakage, until the patient unfortunately died 82 days after the operation due to multi-organ failure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Text
Extracorporeal membrane oxygenation (ECMO) is now generally used for post-operative cardiopulmonary resuscitation in pediatric patients with congenital heart disease. Whereas prolonged ECMO support for >48 h is known to be a risk factor for successful ECMO weaning, there are some patients whose cardiopulmonary functions recover and who are weaned from ECMO following recovery [1–3]. Therefore, for successful weaning it is essential to avoid crucial hemorrhagic complications, such as intracranial hemorrhage, cerebral thromboembolic infarction, and pneumonic hemorrhage, during the supporting period. Prolonged usage of ECMO support also requires frequent circuit exchange for plasma leakage from the oxygenator membrane, thrombus formation in the pump circuit, or hemolysis, all of which cause hemodynamic instability and/or coagulopathy, especially in younger populations [4, 5], even if the new circuit was already filled with a blood solution.
To treat those issues, our center has been using a new pediatric ECMO system, Endumo® (Heiwa Bussan, Tokyo, Japan), since 2009 [6]. Here we report our clinical experience of a case involving 74 days of continuous usage of the Endumo® system without circuit exchange.
Case
A 5-year-old girl (body weight 13.0 kg, body surface area 0.56 m2) with right atrial isomerism, complete atrioventricular septal defect, hypoplastic left ventricle, double outlet right ventricle, and mixed-type total anomalous pulmonary venous connection with totally occluded left pulmonary veins underwent fenestrated total cavo-pulmonary connection with extra-cardiac conduit at the age of 3 years. Since 1 year after the operation, however, she has suffered from persistent protein-losing enteropathy (PLE).
Because her fenestration in the Fontan pathway had spontaneously closed, revision of fenestration was performed at the age of 5 years. After the operation, the PLE did not improve, and newly developed hypoxemia impaired her systemic ventricular function. We therefore initiated veno-arterial ECMO support with the Endumo® system at 18 days after the operation to treat her hemodynamic instability. A 12-Fr heparin-coated cannula for femoral arterial cannulation (Edwards Lifesciences, Irvine, CA) was cannulated from the right internal carotid artery, and a 14-Fr heparin-coated cannula for femoral venous cannulation (Edwards Lifesciences) was cannulated from the right internal jugular vein. These cannulae were connected to the ECMO circuit with non-heparin-coated connectors. A non-heparin-coated shunt tube equipped with heparin-coated t-shaped stopcocks connected the arterial and venous tubes.
Heparin was continuously administered to control the activated coagulation time to between 180 and 200 s. At 2, 3, and 8 days after the initiation of ECMO support, the ECMO circuits were changed to decrease pump flow and hemolysis. For the third circuit exchange, the oxygenator was upsized from BIOCUBE®2000 (membrane surface area 0.4 m2) to BIOCUBE®4000 (membrane surface area 0.8 m2) (BIOCUBE®; Protedyne Corp., Windsor, CT). With these changes, the patient could be stably supported with a total pump blood flow of around 1.0 l/min (1.4 l/min including shunt circuit flow), urine output of around 2–5 ml/kg/h, and average rotating speed of 2,645 rounds per minute without circuit exchange.
During ECMO support, however, she developed pneumonia followed by acute respiratory distress syndrome, and subsequent pneumothorax and a respiratory tract hemorrhage as a consequence of aggressive endotracheal suctioning and frequent alveolar recruitment. To improve only one right lung condition and support Fontan circulation, we used a low dose of sedation, the so-called “awake ECMO,” in an attempt to wake her up. Under “awake ECMO,” the cough reflex was retrieved and mechanical positive pressure ventilation was temporarily weaned off.
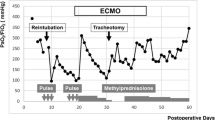
Ultimately, the fourth circuit could be used for 74 days without hemolysis or plasma leakage, until the patient unfortunately died 82 days after the operation due to multi-organ failure. Changes of blood biochemical examination during ECMO support are presented in Fig. 1. Until multi-organ failure developed on ECMO day 67, the serum lactate dehydrogenase level gradually decreased after the third circuit exchange, remaining at <1,000 IU/l, and the serum total bilirubin level did not increase. The elevation of serum C reactive protein level was indicative of the deterioration of pneumonia.
During ECMO support, there was no thromboembolic episode. Brain and whole body computed tomography scans also showed no evidence of thromboembolism. Although her family declined an autopsy, the inspection of the postmortem ECMO circuit after careful irrigation with saline showed that a small amount of thrombus had formed around the outport of the oxygenator and heat exchanger; no thrombus formation was observed in the centrifugal pump (Fig. 2). The whitish thrombus at the outport of oxygenator increased quite slowly during entire support period without mobility.
The oxygenator (a) and heat exchanger (b) of the fourth circuit after careful irrigation. A small thrombus formation was present around the outlet portion. No thrombus formation was observed in the centrifugal pump (c). Although whitish thrombus was formed at the outport of the oxygenator, it increased quite slowly during the entire support period
Comment
The Endumo® system is expected to promise superior antithrombogenicity and device durability [6]. The entire circuit, with the exception of the centrifugal pump, was coated by T-NCVC coating®, which provides stable and long-term antithrombotic activity. Combining the BIOCUBE® oxygenator and ROTAFLOW® centrifugal pump (MAQUET Cardiopulmonary AG, Hirrlingen, Germany) provides satisfactory results and continuation of the gas exchange and pump flow. The advantage of this system in clinical situations for treating pediatric patients has been reported [7].
Here we report our clinical experience with 74-day usage of the Endumo® system without circuit exchange. During stable ECMO support, we attempted “awake ECMO” to improve “only” her right lung. “Awake ECMO” has been recently tried for patients awaiting lung transplantation under ECMO support to prevent respiratory tract infection or pneumothorax and also to strengthen respiratory muscles [8, 9]. The recovery of her lung condition was unfortunately insufficient to rescue her life. The cause of her death was multi-organ failure due to septicemia—death was not the result of prolonged pressure afterload by long-term veno-arterial ECMO support. On the other hand, weaning from mechanical positive airway ventilation was possible under the stable and long-term veno-arterial ECMO support without circuit exchange.
A total of four ECMO circuits were used to provide cardiopulmonary support. The first two circuits were exchanged to enable a decrease of pump flow; nevertheless adequately sized venous and arterial cannulae were used. Therefore, an attempt was made to upsize the oxygenator at the second circuit exchange. Oxygenator size mismatch usually causes inadequate oxygenation, which sometimes results in decreased pump flow in clinical situations. The reason why this occurs is still a matter of concern. Thrombus formation was not observed in both circuits; however, minor thrombus scattering might be present if the oxygenator situation was resolved.
The postmortem inspection of the ECMO circuit revealed a moderate amount of thrombus formation in the heat exchanger. The heat exchanger in the Endumo® system is a well-known site of thrombus formation due to structural issues. However, body temperature control by the heat exchanger is essential in the pediatric patient for management during ECMO. Further improvement in the design of the heat exchanger to reduce thrombus formation is required.
The ROTAFLOW® centrifugal pump is often selected for long-term usage in ECMO support because of its good durability [6, 10]. Its spiral housing is believed to provide an ideal distribution of blood flow and to minimize hemolysis and thrombus formation. To our knowledge, however, this report is the first to demonstrate the absence of thrombus formation in this centrifugal pump after more than 2 months of usage in a clinical setting.
At present, there are no clinically available ventricular assist devices for pediatric patients in Japan. The shortage of donor heart transplantation in pediatric patients is quite serious. Moreover, PLE after the Fontan operation is not an indication for heart transplantation to date. In such a situation, recovery of the patient’s cardiopulmonary function is the only approach to wean the patient from ECMO support. The usage of a durable and safe ECMO system allows the treating physician(s) to initiate various clinical treatments for the improvement of the patient’s cardiopulmonary system and may possible rescue more patients developing cardiogenic shock after congenital heart surgery in the near future.
References
Chrysostomou C, Morell VO, Kuch BA, O’Malley E, Munoz R, Wearden PD. Short- and intermediate-term survival after extracorporeal membrane oxygenation in children with cardiac disease. J Thorac Cardiovasc Surg. 2012;12:1391–8.
Almond CS, Singh TP, Gauvreau K, Piercey GE, Fynn-Thompson F, Rycus PT, Bartlett RH, Thiagarajan RR. Extracorporeal membrane oxygenation for bridge to heart transplantation among children in the United States: analysis of data from the organ procurement and transplant network and extracorporeal life support organization registry. Circulation. 2011;25:2975–84.
Thourani VH, Kirshbom PM, Kanter KR, Simsic J, Kogon BE, Wagoner S, Dykes F, Fortenberry J, Forbess JM. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) in pediatric cardiac support. Ann Thorac Surg. 2006;82:138–44.
Urlesberger B, Zobel G, Zenz W, Kuttnig-Haim M, Maurer U, Reiterer F, Riccabona M, Dacar D, Gallisti S, Leschnik B, Muntean W. Activation of the clotting system during extracorporeal membrane oxygenation in term newborn infants. J Pediatr. 1996;129:264–8.
Muntean W. Coagulation and anticoagulation in extracorporeal membrane oxygenation. Artif Organs. 1999;23:979–83.
Nishinaka T, Tatsumi E, Taenaka Y, Katagiri N, Ohnishi H, Shioya K, Matsuda T. At least 34 days of animal continuous perfusion by a newly developed extracorporeal membrane oxygenation system without systemic anticoagulants. Artif Organs. 2002;26:548–51.
Hoashi T, Kagisaki K, Yamashita K, Tatsumi E, Nishigaki T, Yoshida K, Hayashi T, Ichikawa H. Early clinical outcomes of new pediatric extra-corporeal life support system (Endumo® 2000) in neonates and infants. J Artif Organs 2013;16:267–72.
Fuehner T, Kuehn C, Hadem J, Wiesner O, Gottlieb J, Tudorache I, Olsson KM, Greer M, Sommer W, Welte T, Haverich A, Hoeper MM, Warnecke G. Extracorporeal membrane oxygenation in awake patients as bridge to lung transplantation. Am J Respir Crit Care Med. 2012;185:763–8.
Olsson KM, Simon A, Strueber M, Hadem J, Wiesner O, Gottlieb J, Fuehner T, Fischer S, Warnecke G, Kühn C, Haverich A, Welte T, Hoeper MM. Extracorporeal membrane oxygenation in non-intubated patients as bridge to lung transplantation. Am J Transplant. 2010;10:2173–8.
Mender N, Podechtl F, Feil G, Hiltmann P, Sebending F. Seal-less centrifugal blood pump with magnetically suspended rotor: rot-a-flot. Artif Organs. 1995;19:620–4.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Kusajima, K., Hoashi, T., Kagisaki, K. et al. Clinical experience of more than 2 months usage of extracorporeal membrane oxygenation (Endumo®4000) without circuit exchange. J Artif Organs 17, 99–102 (2014). https://doi.org/10.1007/s10047-013-0747-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10047-013-0747-8





