Abstract
Purpose
In this double-blind prospective randomized trial, our objective was to investigate the effect of antibiotic prophylaxis in patients undergoing elective inguinal hernia surgery with mesh repair in a large-volume tertiary referral trauma center.
Methods
Eligible patients were assigned randomly to either an antibiotic prophylaxis group or a control group. Patients in the prophylaxis group were given 1 g cefazolin by IV bolus injection whereas the placebo control group received an equal volume of sterile saline preoperatively. A Lichtenstein repair was done in all cases. The patients were examined for surgical site infection (SSI) and other postoperative local complications before discharge, and reexamined 3, 5, 7, and 30 days after discharge.
Results
Groups were well matched for age, sex, coexisting diseases, ASA scores, type of hernia, type of anesthesia, duration of surgery. Incidence of infection was 7% in the control group (7/100) and 5% in the prophylaxis group (5/100) (P = 0.38). All the infections were superficial and responded well to drainage and proper antibiotic therapy. All other postoperative complications were similar in the two groups.
Conclusions
In our settings antibiotic prophylaxis has no significant effect on the incidence of SSI in elective repair of inguinal hernias with mesh. The most effective way to reduce the incidence of infection in prosthetic repair may be a specific center for treatment of abdominal wall hernias.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Routine antibiotic prophylaxis after inguinal hernia repairs is controversial. mainly because of the diverse incidence of postoperative wound infection in control groups in prospective trials [1]. Last updated Cochrane meta-analysis concluded that “administration of antibiotic prophylaxis for elective inguinal hernia repair cannot be universally recommended” [2]. They also stated that “antibiotic prophylaxis cannot either be recommended against when high rates of wound infection are observed” [2]. A European Hernia Society guideline also states that “In clinical settings with low rates (<5%) of wound infection, there is no indication for the routine use of antibiotic prophylaxis in elective open groin hernia repair in low-risk patients” [3]. However, in many institutions, antibiotic prophylaxis is still used for high-risk patients or in the centers with a high incidence of infection (>5%).
In this double-blind prospective randomized trial, our objective was to investigate the possible benefit from antibiotic prophylaxis in a large volume tertiary referral trauma center in which all kinds of emergency and elective cases, for example hernia repairs, are admitted into the same operating rooms.
Patients and methods
This prospective randomized controlled trial was conducted at Diskapi Teaching and Research Hospital. The ethics committees of the hospital approved the study and all patients gave informed consent. The trial was registered on http://www.controlled-trials.com (ISRCTN85660082).
Selection of the patients
A sample size of 200 patients (100 per group) was chosen to give 70% power at 0.05 two-tailed level of significance, assuming that a 7% incidence of wound infection in the no-antibiotic group would fall to 1% when antibiotics were used. A 7% incidence of wound infection was selected because of similar or higher incidence in prospective trials without prophylactic antibiotics in Turkey [4, 5]. Patients with primary unilateral inguinal hernias who were electively prepared for tension-free mesh repair during the study period were candidates for the trial. Patients who underwent unilateral primary hernia repair with a previously repaired and left for interval repair on the contralateral side were also included into the study. Exclusion criteria for the patients were: age under 18, recurrent hernias, simultaneous bilateral repairs, incarcerated or strangulated hernias requiring emergency repair, coagulation disorder or anticoagulant medication (i.e. acetylsalicylic acid, clopidogrel, warfarin), history of allergy, sensitivity, or anaphylaxis to cephalosporin antibiotics, antibiotic therapy within 72 h before operation, cardiac valvular problems that require specific perioperative antibiotic regimen, presence of infection at the time of operation, pregnancy or lactation, immunosuppressive diseases (i.e. newly diagnosed or uncontrolled diabetes mellitus, malignancy, HIV), glucocorticoid medication, giant scrotal hernias, prosthetic valves or joints, drain usage, and patients did not accept the registry and randomization.
Randomization
Eligible patients were assigned double-blinded, randomly, to either an antibiotic prophylaxis group or a control group. Patients were randomized by use of sealed envelopes which included equal numbers of patients to be randomized either to the control arm or to the antibiotic prophylaxis arm.
Antibiotic prophylaxis
The antibiotic prophylaxis group received 1 g cefazolin (cephazolin sodium; Eczacibasi, Istanbul, Turkey) by IV bolus injection whereas the placebo control group received sterile saline of equal volume. The anesthesiologist administered the trial medication (antibiotic or sterile saline in coded syringes) when the patient entered the operating room or, at the latest, at the induction of anesthesia. No topical antibiotic or antiseptic agents were used within the surgical field after the repair had been completed. None of the patients in either group was given any additional antibiotic postoperatively.
Surgery
The operations were performed either by supervised surgical residents or staff surgeons, all of whom were blinded to the study group. Skin was shaved just before or in the operating room and prepared by use of povidone–iodine. Anesthesia type was not standardized. All patients underwent open tension-free mesh herniorrhaphy using a monofilament polypropylene mesh (Trulene mesh-sutures, Bangalore, India) in a standardized Lichtenstein technique. Mesh was secured in place with monofilament 2-0 polypropylene sutures (Sterilen; Steril Saglik Malzemeleri, Ankara, Turkey). Skin was closed with continuous subcuticular 3-0 polyglactine (Rapidlak; Orhan Boz, Ankara, Turkey).
Follow-up and intervention
Patient demographics, coexisting diseases, ASA class, type of anesthesia, type of hernia, duration of surgery (DOS), and length of hospital stay (LOS) were recorded. The data were collected from the patients’ records and operation reports. All the patients were mobilized on the day of operation and their wounds were inspected daily until discharge. On the first postoperative day non-steroidal anti-inflammatory drugs were given twice a day unless there was a contraindication. Dressings of the operation on the surgical incision site were changed on the first day and were totally removed on the third postoperative day. Surgical wound and inguinal area were inspected before discharge and reexamined on 3, 5, 7, and 30 days after discharge. The surgeon who performed the follow-up was blinded to the study and frequently was not the surgeon who performed the operation. In cases of missing observations, the patients were contacted by telephone and invited for physical examination. None of the control visits was performed by telephone interview.
Wound infections were classified as superficial incisional surgical site infection (SSSI) and deep surgical site infection (DSSI). As defined by the Centers for Disease Control, SSSI was an infection occurring within 30 days of the operation involving only the skin or subcutaneous tissue and DSSI was an infection involving fascial and muscle layers and also implant (graft) up to a year after the operation [6, 7]. In cases of infection, appropriate microbial cultures were obtained. Surgical site infection (SSI) as deep or superficial was confirmed with microbial culture for identification of the microorganism and therapeutic antibiotic regimen was given according to the antibiogram results. In cases of seroma, aspiration was performed under sterile conditions and drainage fluid was also sampled for microbial culture. In addition to wound infection, all postoperative local and systemic complications were also recorded throughout the follow-up period.
Primary and secondary outcomes
SSSI and DSSI incidence were primary outcomes. The day the infection was first recognized, microbial culture for identification of the microorganism, type of the treatment, and the final result of the wound and prosthetic material were secondary outcomes to be compared between the two groups.
Statistical analysis
The results were analyzed statistically by use of SPSS for Windows software (SPSS, Chicago, Illinois, USA). Comparisons of categorical variables between the two groups were performed by use of the chi-squared test with the Yates correction. The Kolmogorov–Smirnov test was used to test for normal distribution of the numeric variables. The Student t test and Mann–Whitney U tests were used to compare the parametric and nonparametric variables between the two groups. Binary logistic regression was performed with the backward conditional method to analyze the significant independent predictors of infection as the dependent variable. P values less than 0.05 were regarded as significant. Data are presented as number of patients (%), mean ± SD or median (minimum−maximum) where appropriate.
Results
Two hundred (100 antibiotic group, 100 control group) patients who had primary, unilateral inguinal hernia mesh repair were analyzed in this study between July 2008 and October 2010. No patient was lost to follow-up. Patients who did not come to their control visit (this was generally the fifth control visit which was 30 days after the operation) were invited for physical examination in the same the or one day later. There was no mortality in the follow-up period. Groups were well matched for age, sex, coexisting diseases, ASA scores, type of hernia, type of anesthesia, DOS except for LOS (Table 1). The mean LOS was slightly longer in the control group than in the antibiotic group (P = 0.044). All patients in the antibiotic prophylaxis group (100/100) and 96 patients (96/100) in the control group stayed for only one day in the hospital. None of the patients who stayed longer than one day developed SSI.
Perioperative allergic reaction was recorded in one patient only. After the trial had been completed, however, we found this patient was in the control group and this side effect was thought to be related to the anesthetic drugs and concluded there was no adverse reaction to cefazolin. The characteristics and incidence of postoperative complications in the two groups are shown in Table 2. Twelve SSIs were recorded (6%). There was no DSSI in either group. All the infections were superficial. The incidence of infection was 7% in the control group (7/100) and 5% in the prophylaxis group (5/100). The number of patients which must be treated to prevent one infection was 48. No patient required debridement or graft removal. All patients who developed SSSI responded well to drainage and appropriate antibiotic therapy. The incidence of all postoperative complications, including wound infection, was similar in the two groups. Seromas were diagnosed and treated with fine-needle aspiration. All fluid samples were cultured for microbial culture even if the fluid was clear–serous from a well-healing incision. Details of the 12 patients with SSSI (five antibiotic prophylaxis, seven control) are summarized in Table 3. However nine seromas (six from the control group and three from the placebo group) required multiple aspirations. Two of these nine seromas eventually developed SSSI (one from the antibiotic group and one from the placebo group) and fluid samples from these patients also became purulent in their subsequent aspirations.
When age, gender, type of hernia, American Society of Anesthesiologists (ASA) class, duration of operation, presence of seroma, presence of hematoma/ecchymosis, comorbidities, hernia site, type of anesthesia, DOS, LOS and prophylactic antibiotics usage were accepted as covariates and infection was the dependent variable in the binary logistic regression analysis by the backward conditional method; presence of seroma (P = 0.046) and hematoma (P = 0.039) reached statistical significance as the independent variables predicting infection in inguinal hernias with mesh repair.
Discussion
Since the 1990s, use of prosthetic material in inguinal hernia repair has increased, and tension-free repair has become the most popular technique [8–10]. When a prosthesis is implanted, for example in joint replacement and in cardiac or vascular implantation, the benefit of antibiotic prophylaxis has been proved [11, 12]. In this study, we analyzed the effect of antibiotic prophylaxis in a referral trauma center in which all kinds of emergency and elective cases were performed. Although the incidence of infection after our inguinal hernia repairs was high, antibiotic prophylaxis had a small, statistically insignificant effect on prevention of infection in this study (5% vs. 7%).
The 7% incidence of wound infection in our control group was between results reported after two prospective randomized trials in our country—9 and 6.6% [4, 5]. In a prospective study from England, meshes were implanted in 2,432 (91.3%) of 2,665 patients and the incidence of SSI was 7.6% (63/827) when no prophylaxis was given [13].
SSI risk in inguinal hernia repair usually originates from contaminants of the operating theater environment, the surgical team or, most commonly, skin flora; the most common pathogen of SSI is Staphylococcus aureus [14]. Not surprisingly, S. aureus was isolated from all of our SSIs. Intravenous administration of a single dose of cephalosporin, frequently cefazolin, has been generally recommended just before incision in elective clean surgical procedures when using a prosthetic material and in clean contaminated procedures [14]. Cefazolin was chosen because of its known activity against S. aureus and S. epidermidis, historically the most common agents isolated from infected hernia incisions. Morales et al., Aufenacker et al., Celdran et al. and, Perez et al. used cefazolin for antimicrobial prophylaxis of inguinal hernia repair with mesh in their trials [15–18].
Elective inguinal hernia repair is a good example of a clean operative procedure. Its acceptable postoperative incidence of infection is approximately 2% [19–21]. With such low incidence, antibiotic prophylaxis is not appropriate, because of its cost, the risk of toxic and allergic side effects, and the risk of emergence of resistant micro-organisms [22]. Actual incidence of infection in most hospitals is not approximately 2%, and an incidence of ≥9% is not rare [23–26]. Many surgeons may therefore feel obliged to do something to reduce the current incidence of infection in their settings, even though previous studies have hardly shown any benefit of antibiotic prophylaxis. Because multiple hospital and patient-related factors may effect the incidence of infection [7], strict exclusion criteria were used for standardization of patient and hospital-related factors in our study.
In cases of SSI and, especially, DSSI, the risk of recurrence should also be evaluated. There is an increased risk of recurrence after wound infection of classic herniorrhaphy repairs [27]. However, the occurrence of infections in prosthesis repairs does not increase the incidence of recurrence, even if the mesh is removed, because the fibrotic reaction around the posterior wall of the inguinal canal may prevent recurrence [17, 28]. A multicenter trial which investigated SSI in ventral incisional hernia concluded that open surgical technique and the medical center rather than patient co-morbidities or hernia characteristics are associated with the occurrence of postoperative SSI [29]. Although SSI after other abdominal wall hernia repairs and laparoscopic repairs are beyond the scope of our study, medical center and type of surgery both seem to affect SSI. Therefore, all medical centers should investigate their own incidence of infection.
Inappropriate follow-up or unrecorded data may cause under-reporting of infection after hernia surgery. In general practice the patients are discharged 24 h after the operation. In cases of local wound complications, especially infection and seroma, treatment was performed in the emergency department or in outpatient settings. For most (72%) of these patients diagnosis was in the 4–6 weeks follow-up period after discharge [30]. Bailey et al. [24] also revealed a difference between recorded incidence of wound infection in inguinal hernia repairs in hospital records and in community surveillance reports (3% vs. 9%). Assessment of SSI by surgeons is, therefore, not always precise and the actual incidence may be higher. In this study surgical wound and inguinal area were inspected before discharge and re-examined 3, 5, 7, and 30 days after discharge by surgical residents; all wound infections were diagnosed after discharge. Fortunately, infection in inguinal hernia surgery is not as devastating as in cardiothoracic, orthopedic, vascular, and neurosurgery operations. All our infected patients were treated with drainage and appropriate antibiotic therapy, and no patient required hospitalization, debridement, or graft removal. We accept that our follow-up was too short for DSSI which requires at least a year. Yerdel et al. (n = 280) and Aufenacker et al. (n = 1,040) reported three (two from placebo) and two (one from each) cases, respectively, in whom they had removed the mesh [5, 16]. Antibiotic prophylaxis in prevention of DSSI and mesh removal must be questioned. On the other hand, it is not obvious if the antibiotic prophylaxis is effective during all the follow-up period, especially if an implant (mesh) replacement (one-year follow-up was required) was performed. Indeed prophylaxis was found to be effective for prevention of infection for only one week after intervention [31]. Nonetheless our late SSI and recurrence will be measured after one postoperative year for each patient and will be announced in a further report.
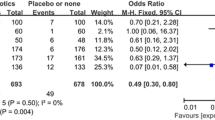
Although high incidence of SSI (≥9%) after inguinal hernioplasties is not infrequent [23–26], our incidence, especially in the prophylaxis group, was somewhat higher than that in previous randomized controlled trials (RCTs). In the studies of Yerdel et al. and Celdran et al. the incidence of infection after antibiotic prophylaxis was around zero (0.7 and 0%) but that after placebo was high (9 and 8.2%) [5, 17]. Both studies were concluded early for ethical reasons related to the high incidence of infection in their control groups, and they recommended antibiotic prophylaxis. However a meta-analysis including these two trials which reviewed the effectiveness of antibiotic prophylaxis in prevention of wound infection after mesh repair did not favor routine use of antibiotic prophylaxis, because four RCTs did not demonstrate any significant benefit from prophylaxis [32]. In this review six RCTs which met the criteria were analyzed. Not surprisingly, these trials were also six of seven trials of inguinal mesh hernia repair reviewed in the last updated Cochrane meta-analysis of antibiotic prophylaxis for hernia repair (thirteen RCTs were analyzed of which seven were mesh repairs) [3]. In these six trials overall incidence of infection was 1.5% (18/1,230) and 3% (38/1,277) for antibiotic and placebo groups, respectively. Both were lower than in our trial (5% vs. 7%). Nine of the infections were DSSI, three in the antibiotic group and six in the placebo group, some of which required treatments such as debridement, multiple drainage, and even graft removal. Encouraging aspects of our trial were the absence of DSSI and that all SSSI responded well to proper antibiotics with or without drainage. Nevertheless we must still struggle to reduce our SSI with a multidisciplinary effort; we also plan similar RCTs in which local antibiotics or combined therapy are examined.
The shortcomings of our study are the lack of data about the nutritional status, obesity, and smoking status of patients, all of which are patient-related factors that may affect the risk of development of wound infection [7].
In fact, the incidence of infection in specific hernia centers where only elective hernia repairs are conducted is not higher than 1% [33]. Our results were worse than those of specific centers and most general hospitals. A strict prophylaxis and preoperative care protocol could not reduce our incidence of infection. This is probably because of regional and institutional factors. In our setting, inguinal hernia repairs are scheduled after major abdominal surgery and, when necessary, even contaminated trauma cases are admitted between the elective cases. We therefore plan to establish a specific hernia center in our hospital in a separate place with separate entry.
Consequently, we were not able to demonstrate any significant benefit of antibiotic prophylaxis in elective tension-free mesh repair of inguinal hernia. We believe the most effective way to reduce our incidence of infection in prosthetic inguinal hernia repair in our hospital would be to improve infection-control procedures and, perhaps, establish a specific in-hospital hernia-repair center.
References
Sanchez-Manuel FJ, Seco-Gil JL (2004) Antibiotic prophylaxis for hernia repair. Cochrane Database Syst Rev (4):CD003769
Sanchez-Manuel FJ, Lozano-García J, Seco-Gil JL (2007) Antibiotic prophylaxis for hernia repair. Cochrane Database Syst Rev (3):CD003769
Simons MP, Aufenacker T, Bay-Nielsen M, Bouillot JL, Campanelli G, Conze J, de Lange D, Fortelny R, Heikkinen T, Kingsnorth A, Kukleta J, Morales-Conde S, Nordin P, Schumpelick V, Smedberg S, Smietanski M, Weber G, Miserez M (2009) European hernia society guidelines on the treatment of inguinal hernia in adult patients. Hernia 13:343–403
Cingi A, Manukyan MN, Güllüoğlu BM, Barlas A, Yeğen C, Yalin R, Yilmaz N, Aktan AO (2005) Use of resterilized polypropylene mesh in inguinal hernia repair: a prospective, randomized study. J Am Coll Surg 201:834–840
Yerdel MA, Akin EB, Dolalan S, Turkcapar AG, Pehlivan M, Gecim IE, Kuterdem E (2001) Effect of single-dose prophylactic ampicillin and sulbactam on wound infection after tension-free inguinal hernia repair with polypropylene mesh: the randomized, double-blind, prospective trial. Ann Surg 233:26–33
Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control 20:271–274
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Hospital infection control practices advisory committee. Infect Control Hosp Epidemiol 20:250–280
Bay-Nielsen M, Kehlet H, Strand L, Malmstrøm J, Andersen FH, Wara P, Juul P, Callesen T, Danish Hernia Database Collaboration (2001) Quality assessment of 26, 304 herniorrhaphies in Denmark: a prospective nationwide study. Lancet 358:1124–1128
Hair A, Duffy K, McLean J, Taylor S, Smith H, Walker A, MacIntyre IM, O’Dwyer PJ (2000) Groin hernia repair in Scotland. Br J Surg 87:1722–1726
EU Hernia Trialists Collaboration (2002) Repair of groin hernia with synthetic mesh: meta-analysis of randomized controlled trials. Ann Surg 235:322–332
Hill C, Flamant R, Mazas F, Evrard J (1981) Prophylactic cefazolin versus placebo in total hip replacement. Lancet 1:795–796
Kaiser AB, Petracek MR, Lea JV (1987) Efficacy of cefazolin, cefamandole and gentamicin as prophylactic agents in cardiac surgery: results of a prospective, randomized, double-blind trial in 1030 patients. Ann Surg 206:791–797
Taylor EW, Duffy K, Lee K, Hill R, Noone A, Macintyre I, King PM, O’Dwyer PJ (2004) Surgical site infection after groin hernia repair. Br J Surg 91:105–111
Terzi C (2006) Antimicrobial prophylaxis in clean surgery with special focus on inguinal hernia repair with mesh. J Hosp Infect 62:427–436
Morales R, Carmona A, Paga′n A (2000) Utilidad de la profilaxis antibio′tica en la reduccio′n de la infeccio′n de herida en la reparacio′n de la hernia inguinal o crural mediante malla de prolipropileno [utility of antibiotic prophylaxis in reducing wound infection in inguinal or femoral hernia repair using polypropylene mesh]. Cir Esp 67:51–59
Aufenacker TJ, van Geldere D, van Mesdag T, Bossers AN, Dekker B, Scheijde E, van Nieuwenhuizen R, Hiemstra E, Maduro JH, Juttmann JW, Hofstede D, van Der Linden CT, Gouma DJ, Simons MP (2004) The role of antibiotic prophylaxis in prevention of wound infection after Lichtenstein open mesh repair of primary inguinal hernia: a multicenter double-blind randomized controlled trial. Ann Surg 240:955–961
Celdrán A, Frieyro O, de la Pinta JC, Souto JL, Esteban J, Rubio JM, Señarís JF (2004) The role of antibiotic prophylaxis on wound infection after mesh hernia repair under local anesthesia on an ambulatory basis. Hernia 8:20–22
Perez AR, Roxas MF, Hilvano SS (2005) A randomized, double-blind, placebo-controlled trial to determine effectiveness of antibiotic prophylaxis for tension-free mesh herniorrhaphy. J Am Coll Surg 200:393–398
Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH (1993) Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg 128:79–88
Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE Jr, Sweet RL, Wenzel RP (1994) Quality standard for antimicrobial prophylaxis in surgical procedures. Infectious Diseases Society of America. Clin Infect Dis 18:422–427
Woods RK, Dellinger EP (1998) Current guidelines for antibiotic prophylaxis of surgical wounds. Am Fam Physician 57(11):2731–2740
Waldvogel FA, Vaudaux PE, Pittet D, Lew PD (1991) Perioperative antibiotic prophylaxis of wound and foreign body infections: microbial factors affecting efficacy. Rev Infect Dis 13:782–789
Taylor EW, Byrne DJ, Leaper DJ, Karran SJ, Browne MK, Mitchell KJ (1997) Antibiotic prophylaxis and open groin hernia repair. World J Surg 21:811–815
Bailey IS, Karran SE, Toyn K, Brough P, Ranaboldo C, Karran SJ (1992) Community surveillance of complications after hernia surgery. BMJ 304:469–471
Santos KRN, Neto GPB, Fonseca LS, Filho PPG (1997) Incidence surveillance of wound infection in hernia surgery during hospitalization and after discharge in a university hospital. J Hosp Infect 36:229–233
Shankar VG, Srinivasan K, Sistla SC, Jagdish S (2010) Prophylactic antibiotics in open mesh repair of inguinal hernia—a randomized controlled trial. Int J Surg 8:444–447
Glassow F (1964) Is postoperative wound infection following simple inguinal herniorrhaphy a predisposing cause of recurrent hernia? Can Med Assoc J 91:870–871
Gilbert AI, Felton LL (1993) Infection in inguinal hernia repair considering biomaterials and antibiotics. Surg Gynecol Obstet 177:126–130
Kaafarani HM, Kaufman D, Reda D, Itani KM (2010) Predictors of surgical site infection in laparoscopic and open ventral incisional herniorrhaphy. J Surg Res 163:229–234
Ranaboldo CJ, Karran SE, Bailey IS, Karran SJ (1993) Antimicrobial prophylaxis in ‘clean’ surgery: hernia repair. J Antimicrob Chemother 31:35–41
Sanderson PJ (1999) Assessing the role of prophylactic antibiotics in clean surgery. J Hosp Infect 42:7–9
Aufenacker TJ, Koelemay MJ, Gouma DJ, Simons MP (2006) Systematic review and meta-analysis of the effectiveness of antibiotic prophylaxis in prevention of wound infection after mesh repair of abdominal wall hernia. Br J Surg 93:5–10
Kark AE, Kurzer MN, Belsham PA (1998) Three thousand one hundred seventy-five primary inguinal hernia repairs: advantages of ambulatory open mesh repair using local anesthesia. J Am Coll Surg 186:447–456
Conflict of interest
None of the authors declare conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ergul, Z., Akinci, M., Ugurlu, C. et al. Prophylactic antibiotic use in elective inguinal hernioplasty in a trauma center. Hernia 16, 145–151 (2012). https://doi.org/10.1007/s10029-011-0881-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-011-0881-2