Abstract
Background
The utilization of mesh reinforcement of the inguinal area with polypropylene mesh has increased drastically over the last decade. Infertility due to obstructive azoospermia is a rare but serious complication following inguinal hernia repair, especially in young patients. The aim of this study was to evaluate the effect of different mesh structures on integrity of the vas deferens.
Materials and methods
Twenty male Chinchilla rabbits were used. The spermatic cord was dissected free and a Lichtenstein repair was performed with a low-weight polypropylene mesh (UltraPro®) and a heavy-weight polypropylene mesh (Prolene®) on the contralateral side. A vasography was performed after six months in order to investigate obstructions of the vas deferens. Light microscopy of the mesh host tissue interface was also performed and the foreign body reaction analyzed. Spermatogenesis was evaluated using the Johnsen score.
Results
Vasography revealed relevant obstructions (>75% of lumen diameter) located at the mesh margins (50% of Prolene® and 22.2% of UltraPro® mesh samples). Microscopic investigation of the mesh–host tissue interface showed typical formation of foreign body granulomas. The diameters of the foreign body granulomas were significantly reduced in the UltraPro® mesh group (41.7 ± 5.5 μm) compared to the Prolene® mesh group (48.7 ± 7.7 μm). Upon investigating the percentages of apoptotic (TUNEL) and proliferating (Ki67) cells, no significant differences were found. Following Prolene® mesh implantation, a mean Johnsen score of 9.1 ± 1.2 was estimated, which was not significantly different from the UltraPro® mesh samples (8.9 ± 1.4, P > 0.05).
Conclusions
If a mesh material is needed for inguinal hernia repair in young patients, the use of modern low-weight large porous and elastic samples appears to have a beneficial effect on integrity of the vas deferens.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inguinal herniorrhaphy is the operation most commonly performed by general surgeons, with more than 700,000 performed annually in the United States and 200,000 in Germany [1, 2]. An estimated 75–80% of these hernia operations involve the placement, either open or laparoscopically, of a mesh prosthesis to patch a defect in the floor of the inguinal canal. The most widely used prosthetic material in hernia surgery is polypropylene. Foreign body reactions, with fibroblastic ingrowth and a chronic inflammation, are believed to reinforce the abdominal wall and decrease the risk of recurrence. It has been proven that this foreign body reaction is proportionate to the weight and structure of the mesh, and that commonly used meshes contain too much material, producing an exaggerated foreign body reaction/tissue response and leading to clinical complications [3, 4]. To minimize the foreign body reaction and clinical complications, a new type of mesh material has been introduced with a decreased amount of material and larger pores, leading to nearby physiological tissue ingrowth [4–6]. Whereas these so-called light-weight, large pore sized and elastic mesh materials are known to show a favourable outcome in relation to postoperative pain [7–10] compared to conventional heavy-weight, small pore sized and stiff mesh materials, only a few experimental studies have focused on the influence of different mesh materials on the integrity of the vas deferens. With its widespread acceptance and ease of placement, mesh repair is being offered increasingly to young patients, whose fertility status may well be an issue in the future.
To further elucidate the impacts of different polypropylene mesh materials following Lichtenstein hernia repair, an animal study was conducted to investigate the long-term effect on the integrity of the vas deferens and on testicular function.
Materials and methods
Mesh materials
Two different mesh materials were investigated: UltraPro®, a low-weight, large, porous and elastic two-component mesh made of polypropylene and absorbable polyglecaprone monofilaments (Ethicon, Norderstedt, Germany) and Prolene®, a heavy-weight, small, porous and stiff mesh made of polypropylene monofilaments (Ethicon).
Animals
Twenty male Chinchilla rabbits were housed under conditions of constant light and temperature and received a complete diet of feed and water ad libidum throughout the entire study, which was performed according to the NIH guidelines for the use of laboratory animals. All animals received bilateral Lichtenstein hernia repair (n = 40).
Surgical procedure
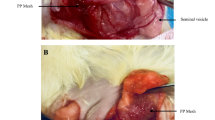
Operations were carried out under general anaesthesia. Following premedication using 0.3 ml/kg buprenorphine applied subcutaneously, an intravenous catheter was placed into an ear vein. Anaesthesia was induced by injecting medetomidine (Dormitor®, 0.3 ml/kg) and 10% 0.2 ml/kg ketamine. Anaesthesia was maintained by performing repeated injections of these medications. Following the induction of anaesthesia, the skin was shaved and disinfected with polyvidone iodine solution. An inguinal incision was performed and the external oblique fascia dissected. Following exploration of the inguinal canal, the cremasteric muscles were resected. A Lichtenstein procedure was carried out using a 4 × 2 cm slitted mesh sample (UltraPro® mesh on one side, Prolene® mesh on the contralateral side). Mesh samples were fixed at the inguinal ligament and the slit of mesh samples was closed using 4/0 Prolene® sutures. External oblique fascia and skin closures were then performed. No antibiotic treatment was given before or during the experiments. Throughout the whole observation period all of the animals were objectively controlled and underwent daily clinical investigation to assess local and systemic complications. Six months after mesh implantation, all of the animals (n = 20) were sacrificed for morphological observations. The abdomen was opened via a median incision for complete exploration. The intraabdominal part of the vas deferens was dissected 2 cm before entering the inguinal canal on both sides, and 5 ml X-ray solution injected for vasography (13.3 g gelatine, 16.6 g red lead = Pb2PbO4, 100 ml aqua). Following ligation of the vas deferens, the whole inguinal canal, including mesh samples as well as testis, was resected and fixed in 10% formaldehyde (Fig. 1).
Assessment of the integrity of the vas deferens
The integrity of the vas deferens was assessed semiquantatively using X-ray vasography. Obstructions of the vas deferens were classified as minor (0–25% reduction of lumen diameter), medium (25–75%) or major (>75%), and examined at the margins of the mesh samples as well as within the mesh area.
Histological analysis
Tissue specimens were embedded in paraffin. Histological investigation was performed on 3 μm sections after haematoxylin and eosin staining (H&E). All sections were processed at the same time to reduce internal staining variations. Spermatogenesis, the main testicular function, was estimated histologically using the Johnsen score (Table 1) [11]. The amount of inflammatory and connective tissue formation at the mesh–host tissue interface was analyzed by measuring the diameters of the foreign body granulomas. After capturing 50 granulomas for each mesh material with a digital camera (400×, Olympus C-3030, Hamburg, Germany), separate measurements of the four quadrants of the granulomas were performed with the help of a digital image analyzing software (Image-Pro Plus, Media Cybernetics, Silver Spring, MD, USA). Immunohistochemistry was done according to the instructions of the manufacturer. In order to detect proliferating cells (Ki67), we used mouse monoclonal antibody MIB-1, 1:10 from Dako (Glostrup, Denmark) and rabbit anti-mouse antibody, 1:300 from Dako (Glostrup, Denmark) as secondary antibody. TUNEL histochemistry for the detection of apoptotic cells was performed with an in situ apoptosis detection kit (APOPTAG, ONCOR, Cat. No. S7100, Heidelberg, Germany). Sections were examined by standard light microscopy (Olympus BX51), and six regions within the interface (400×, area 100 μm × 100 μm) were captured for each sample by a digital camera (Olympus C-3030). The expression of immunohistochemical parameters was classified by two independent, blinded observers. Extent of staining was scored according to the percentage of positive stained cells in the specimen (0–100%).
Statistics
Statistical analysis was carried out using the Statistical Package for Social Sciences (SPSS, Chicago, IL, USA) software. Data were organized according to mesh modification. Analysis of Johnsen score and histological parameters was performed using the Mann–Whitney U test. P values of <0.05 were considered to be significant. All data are presented as mean ± standard deviation if not otherwise mentioned.
Results
Macroscopic observations
The surgical procedure was well tolerated by all animals and the postoperative period was uneventful. None of the animals developed signs of ischaemic orchitis or testicular atrophy.
Integrity of the vas deferens
Following explantation, X-ray vasography showed analyzable results in 16 Prolene® as well as 18 UltraPro® mesh implantations. Relevant obstructions (>75% of lumen diameter) were only located at the mesh margins. Within the mesh area, only 25% (4/16) of the Prolene® samples and 16.7% (3/18) of the UltraPro® samples showed obstructions of 25–75% of the lumen diameter, whereas at the mesh margins 50% (8/16) of the Prolene® and 22.2% (4/18) of the UltraPro® mesh samples induced significant obstructions of more than 75% of the lumen diameter (Fig. 2). Detailed results are given in Table 2.
Histological analysis
Testicular function was estimated histologically. For each testicular sample, ten tubuli seminiferi were classified according to the Johnsen score. Following Prolene® mesh implantation, a mean Johnsen score of 9.1 ± 1.2 was estimated, which was not significantly different from that obtained for the UltraPro® mesh samples (8.9 ± 1.4, P > 0.05).
Microscopic investigation of the mesh–host tissue interface showed typical formation of foreign body granulomas. The diameters of the foreign body granulomas, representing the amount of inflammatory and connective tissue infiltrate, were significantly reduced in the UltraPro® mesh group (41.7 ± 5.5 μm) compared to the Prolene® mesh group (48.7 ± 7.7 μm, P < 0.01, Fig. 3a). Upon investigating the percentages of apoptotic (TUNEL) and proliferating (Ki67) cells, no significant differences were found between the Prolene® mesh sample (TUNEL 18.7 ± 6.9%, Ki67 3.3 ± 1.1%) and the UltraPro® mesh sample (TUNEL 19.5 ± 7.1%, Ki67 3.7 ± 1.2%, Fig. 3b, c).
Discussion
Infertility due to obstructive azoospermia is a rare but serious complication following inguinal hernia repair. Whereas incidences of iatrogenic perioperative injury to the vas deferens during inguinal hernia repair of 0.3% in adults and 0.8–2.0% in childhood are described [12], little is known about the long-term effects of different polypropylene mesh prostheses on the integrity of the vas deferens. Due to the fact that almost 30% of the patients are operated for bilateral hernias and that mesh repair is increasingly being offered to young patients, whose fertility status may well be an issue in the future, this item is of major clinical importance. Aside from case reports, only one large clinical series is reported. Shin et al. investigated 14 cases of azoospermia secondary to inguinal vasal obstruction related to previous mesh herniorrhaphy [13]. They reported on nine patients with bilateral as well as five patients with unilateral obstructions following open or laparoscopic hernia mesh repair. Surgical exploration revealed a dense fibroblastic response encompassing the polypropylene mesh, with either trapped or obliterated vas in all patients.
The first experimental study investigating this matter was performed by Uzzo et al., who compared six dogs operated with a polypropylene mesh to six dogs operated with conventional (Shouldice) repair [14]. They found a decrease in the cross-sectional diameter of the vas deferens on the operated side compared to the control side in both the suture repair and the mesh groups. Furthermore, morphologic changes in the testis were found in three out of six animals in the mesh group. Goldenberg et al. investigated 18 dogs with a follow-up of 60 days, and found a chronic inflammatory reaction in 100% at the mesh side, a reduction of spermatogenesis, as well as a reduction in the diameter of the lumen of the vas deferens at the mesh side [15]. However, studies comparing the effects of different mesh prostheses are rather limited. Peiper et al. investigated spermatic cord perfusion and spermatogenesis in rabbits in a comparison of Lichtenstein hernia repair using UltraPro®, a low-weight, large, porous and elastic mesh, and Marlex®, a heavy-weight, small, porous and stiff mesh, with Shouldice repair [16]. They found a more obvious decrease in spermatic cord perfusion after Marlex® mesh repair than after Shouldice repair. Evaluation of spermatogenesis revealed a certain decrease in Johnsen score in seminiferous tubules after Lichtenstein repair, independent of the kind of mesh used. This was verified within our animal model. Following Prolene® mesh implantation, a mean Johnsen score of 9.1 ± 1.2 was estimated, which was not significantly different from that for UltraPro® mesh samples (8.9 ± 1.4). Berndsen et al. compared a low-weight composite mesh (Vypro II) and a heavy-weight (Prolene) mesh used in Lichtenstein repair in rats [17]. At 90 days after implantation, the median cross-sectional area of the vas deferens was 109 pixels at the Prolene and 158 pixels at the Vypro II mesh side (not a significant difference). Within our study, obstructions were analyzed semiquantitatively, and those in the mesh area were distinguished from those in the mesh margins using vasography. Investigations revealed that obstructions were mainly located at the mesh margins. 50% of the heavy-weight Prolene® as well as 22.2% of the low-weight UltraPro® mesh samples induced significant obstructions of more than 75% of the lumen diameter. Aside from a significantly reduced inflammatory foreign body reaction, the lower number of obstructions observed for the UltraPro® mesh is probably due to its elastic textile properties. One potential explanation for the overall high incidence of obstructions observed is the resection of cremasteric muscle during the operation. Whether intact cremasteric muscle is able to protect the spermatic cord structures needs to be clarified in further experimental trials.
To summarize, great effort has been directed into the challenge of creating a mesh material that optimizes patient outcome. The introduction of low-weight, large, porous and elastic mesh materials has led to improved outcome in terms of postoperative pain and foreign body feeling. However, the influences of different mesh materials on spermatic cord structures have not been studied thoroughly. In this work, for the first time, the locations of induced obstructions were analyzed and investigations were performed that showed a beneficial effect of the application of such mesh materials on the integrity of the vas deferens in this experimental setting. However, animal experiments, particularly rodent animal models, have their natural limitations, and results cannot be extrapolated directly to the situation in humans. In particular, the animals cannot reflect any underlying human disease or comorbidity. Therefore, clinical studies must be conducted to prove the supposed beneficial aspects of low-weight, large, porous and elastic mesh materials on the integrity of the vas deferens.
References
Rutkow IM, Robbins AW (1993) Demographic, classificatory, and socioeconomic aspects of hernia repair in the United States. Surg Clin North Am 73:413–426
Peiper C, Klinge U, Junge K, Schumpelick V (2002) Meshes in inguinal hernia repair. Zentralbl Chir 127:573–577
Klosterhalfen B, Klinge U, Schumpelick V (1998) Functional and morphological evaluation of different polypropylene-mesh modifications for abdominal wall repair. Biomaterials 19:2235–2246
Junge K, Klinge U, Rosch R, Klosterhalfen B, Schumpelick V (2002) Functional and morphologic properties of a modified mesh for inguinal hernia repair. World J Surg 26:1472–1480
Junge K, Klinge U, Prescher A, Giboni P, Niewiera M, Schumpelick V (2001) Elasticity of the anterior abdominal wall and impact for reparation of incisional hernias using mesh implants. Hernia 5:113–118
Klinge U, Klosterhalfen B, Conze J, Limberg W, Obolenski B, Ottinger AP et al (1998) Modified mesh for hernia repair that is adapted to the physiology of the abdominal wall. Eur J Surg 164:951–960
Bringman S, Wollert S, Osterberg J, Smedberg S, Granlund H, Heikkinen TJ (2006) Three-year results of a randomized clinical trial of lightweight or standard polypropylene mesh in Lichtenstein repair of primary inguinal hernia. Br J Surg 93:1056–1059
O’dwyer PJ, Kingsnorth AN, Molloy RG, Small PK, Lammers B, Horeyseck G (2005) Randomized clinical trial assessing impact of a lightweight or heavyweight mesh on chronic pain after inguinal hernia repair. Br J Surg 92:166–170
Horstmann R, Hellwig M, Classen C, Rottgermann S, Palmes D (2006) Impact of polypropylene amount on functional outcome and quality of life after inguinal hernia repair by the TAPP procedure using pure, mixed, and titanium-coated meshes. World J Surg 30:1742–1749
Nienhuijs S, Staal E, Strobbe L, Rosman C, Groenewoud H, Bleichrodt R (2007) Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg 194:394–400
Johnson SG (1970) Testicular biopsy score count—a method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25
Pollak R, Nyhus LM (1983) Complications of groin hernia repair. Surg Clin North Am 63:1363–1371
Shin D, Lipshultz LI, Goldstein M, Barme GA, Fuchs EF, Nagler HM, McCallum S, Niederberger C, Schoor R, Brugh V, Honig S (2005) Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg 241:553–558
Uzzo RG, Lemack GE, Morrissey KP, Goldstein M (1999) The effects of mesh bioprosthesis on the spermatic cord structures: a preliminary report in a canine model. J Urol 161:1344–1349
Goldenberg A, Matone J, Marcondes W, Herbella FA, Farah JF (2005) Comparative study of inflammatory response and adhesions formation after fixation of different meshes for inguinal hernia repair in rabbits. Acta Cir Bras 20:347–352
Peiper C, Junge K, Klinge U, Strehlau E, Krones C, Ottinger A et al. (2005) The influence of inguinal mesh repair on the spermatic cord: a pilot study in the rabbit. J Invest Surg 18:273–278
Berndsen FH, Bjursten LM, Simanaitis M, Montgomery A (2004) Does mesh implantation affect the spermatic cord structures after inguinal hernia surgery? An experimental study in rats. Eur Surg Res 36:318–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Junge, K., Binnebösel, M., Rosch, R. et al. Influence of mesh materials on the integrity of the vas deferens following Lichtenstein hernioplasty: an experimental model. Hernia 12, 621–626 (2008). https://doi.org/10.1007/s10029-008-0400-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10029-008-0400-2