Abstract
Our study was conducted to compare self-fixing lightweight polyester mesh (group I) to the standard heavy weight polypropylene mesh (group II) using tension-free Lichtenstein hernioplasty as regard to the effect of mesh implantation and perimesh fibrosis on testicular blood flow. 80 patients with uncomplicated inguinal hernia were divided in two groups. Doppler ultrasonography measured testicular volume, testicular artery velocity preoperative and 3rd month post operative. Blood flow in the testicles was represented by resistive index (RI). No case of testicular atrophy occurred in either group, however, in both groups a significant postoperative decrease in testicular volume (p = 0.001 in group I and p < 0.001 in group II) was accompanied by a significant increase in RI as compared to their pre-operative values (p < 0.001 in group I and p = 0.009 in group II). Comparing the two groups, patients in group I showed higher values of decrease in testicular volume accompanied by more increase in RI values postoperatively compared to group II patients, but these values did not reach a significant value (p = 0.107, p = 0.136). There was a significant increase in the number of post-operative varicocele and hydrocele in group I compared to group II. Mesh implantation has an effect on testicular size and blood flow by decreasing the testicular size and increasing the RI. This effect was more obvious in the parietex progrip. Although there is an indirect relation between RI and the sperm count, testicular blood flow alone is not enough to judge fertility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As recurrences have decreased with the introduction of mesh hernia repairs, more interest is now directed to other consequences of hernia surgery. Although it is presumed to be a rare complication, a few reports suggested that the use of mesh for male inguinal hernia repair could cause male infertility [1, 2]. Patients considered being at the greatest risk are fertile men (18–60 years of age) undergoing bilateral mesh repair for inguinal hernias and those who undergo unilateral repair with impairment of the contra-lateral testis [2].
Although the mesh used in open tension-free inguinal hernioplasty is described as an inert material, all meshes cause an initial followed by chronic inflammatory tissue response in the recipient after implantation [3,4,5]. The chronic inflammatory reaction that incorporates the spermatic cord and the mesh appears 1–12 weeks after surgery, and this inflammatory reaction is observed both after anterior and posterior (preperitoneal) hernia repairs [6,7,8]. This might result in the compression of the testicular arteries or obstruction of the veins which is believed to be an important cause of ischemic orchitis after tension-free mesh repair [9]. Moreover, as the mesh is placed in close contact with the vas deferens, the fibrotic reaction may cause thickening of the wall of the vas deferens with narrowing and obstruction of the lumen at the site of which might result in fertility problems [6, 10, 11].
Various studies have shown that mesh itself, mesh localization, and perimesh fibrosis may affect the testicular blood flow [12]. The most important factor in preserving testicular volume and testicular function is the maintenance of arterial circulation [13]. The best way to assess whether or not the testicular volume and testicular function is affected is by measuring the RI (the resistive index) or the vascular resistance [13]. The normal range of the RI in the distal portion of the testicular artery is (0.63–1). [14] The peak systolic velocity (PSV) and the end-diastolic velocity (EDV) can be measured, and the RI can be calculated as follows: RI = PSV − EDV/PSV [6]. Increasing RI implies an increase in vascular impedance.
Studies demonstrated that the RI can be an indicator for detecting male subfertility. In patients with low sperm counts, the RI was significantly increased compared with patients with normal sperm counts. This relationship suggests that the intratesticular RI can be used as a means to quantify testicular perfusion and, indirectly, assess gonadal function. Hence, testicular perfusion is related to testicular function [15].
Aim of the work
The aim of this prospective study is to compare self-fixing lightweight polyester (Parietex™ progrip™) mesh versus the standard heavy weight polypropylene mesh in tension-free Lichtenstein inguinal hernia repair as regard to the effect of mesh implantation and perimesh fibrosis on testicular blood flow.
Patients and methods
This study was conducted at Alexandria Main University hospital, Alexandria, Egypt. This is a 1000-bed teaching hospital owned by the Faculty of Medicine of the University of Alexandria. The ethics committee and review board in our institute approved the study and treatment protocol. An informed consent was obtained from all patients who agreed to participate in the study.
This study is a prospective, single-center study. Patients with primary uncomplicated inguinal hernia undergoing Lichtenstein tension-free hernioplasty were allocated to use either self-fixing, lightweight polyester (parietex progrip®) or the standard heavyweight polypropylene mesh. This self-gripping mesh (Parietex™ ProGrip™) manufactured by Sofradim production (Group Covidien, Trévoux, France) is made of lightweight iso-elastic large-pore knitted polyester fabric that incorporates resorbable micro grips to provide self-gripping fixation during the first few months after implantation. The micro grips are club-shaped 1-mm projections that are made of biodegradable polylactic acid (PLA). The micro grips integrate into the tissue for 0.5 mm below the lower rim of the mesh and provide stronger tissue incorporation at 5 days than fixation with staples [16]. Fixation is, therefore, greatly facilitated without the requirement for sutures that can penetrate underlying tissues and damage cutaneous nerves. The mesh is secured around the spermatic cord with a self-gripping flap. After resorption of the PLA grips, the remaining lightweight mesh (40 g/m2) ensures long-term posterior wall reinforcement [17]. The prolene mesh used in our study is PROLENE™ (ETHICON). It is a nonabsorbable monofilament-knitted mesh with a pore size of 0.8 mm, a weight of 80–100 g/m2 and a tensile strength of 156.5 N/cm.
The primary end point is the effect of mesh implantation and perimesh fibrosis on testicular blood flow as measured by RI. The secondary end point includes operative time, mesh fixation time, morbidities, and hospital re-admission.
Study population
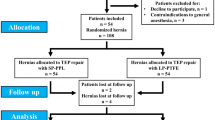
From January 2015 to April 2016, a total of 146 patients were admitted to our surgery department for elective repair of primary uncomplicated inguinal hernia. Fifty patients did not meet inclusion criteria while 16 patients declined to participate in the study. The remaining 80 patients were included in the study.
Inclusion criteria
Male patient with uncomplicated primary inguinal hernia.
Exclusion criteria
Patients with irreducible, obstructed, strangulated or recurrent hernia.
Sample size was calculated using Epi-save software to conduct a clinical trial study to compare self-fixing lightweight polyester (Parietex™ progrip™) mesh with the standard heavyweight polypropylene mesh in tension-free Lichtenstein inguinal hernia repair as regard to the effect of mesh implantation and perimesh fibrosis on testicular blood flow. Blood flow in the testicles was represented by vascular resistance or resistive index (RI) which was used as the main parameter for calculation of sample size of the study.
Sample size was estimated to be 23 patients in each group (total of 46 patients) included in the study to detect SI change by 0.05 from 0.8 ± 0.06 among patients using the standard heavyweight polypropylene mesh [13] to 0.75 ± 0.06 among patients subjected to self-fixing lightweight polyester (Parietex™ progrip™) mesh. The estimated sample size is made at an assumption of 95% confidence level and 80% power of study.
Treatment protocol
Patient’s data were recorded at time of admission with particular stress upon risk factors of the hernia as (chronic cough, chronic constipation etc.), physical examination, laboratory and radiological investigation in the form of Doppler ultrasonography to measure testicular volume, testicular artery velocity and spermatic cord thickness performed pre-operatively.
Color Doppler U/S was performed with the patients in supine position. The patients were asked to hold the penis supra-pubically in a temperature-controlled room after resting for at least 10 min with the transducer placed gently on the scrotum. Measurements of the testis were recorded in the longitudinal, transverse, and anteroposterior axis; the approximate volume for ellipsoid structures was calculated by multiplying the result by 0.52 [18]. After evaluation of testicular parenchyma and testicular volume, the intratesticular blood flow and testicular artery was identified at 1 cm superior to the pole of the testes in the spermatic cord and peak systolic velocity (PSV), and end diastolic velocity (EDV) were detected [19]. The resistive index (RI) calculated as (RI = systolic peak velocity − end diastolic peak velocity/systolic peak velocity).
Operative details
Three experienced hernia surgeons carried out operations under general anesthesia. Antibiotic prophylaxis was performed routinely with amoxicillin + clavulanic acid (Augmentin 1.2 g). All patients were placed in the supine position with arms abducted 90° to the body. After 5–8-cm, inguinal skin incision was made and the external oblique aponeurosis was divided. The necessary space for the mesh was created laterally along the inguinal ligament from the pubic tubercle towards the anterior superior iliac spine, and then between the external oblique aponeurosis and the conjoint tendon to exhibit the rectus muscle aponeurosis. The pubic bone was cleared, allowing the easy dissection of the cord. The cremasteric muscle and the three nerves were preserved. Dissection of the sac in relation to the type of hernia, indirect hernia sacs were excised and large direct sacs were inverted with absorbable sutures. In case of a direct component, with weak fascia transversalis, a posterior reinforcement by placing nonabsorbable sutures from the transverse fascia to the inguinal ligament. In group I, 11 × 9 cm Parietex® progrip mesh lay over the inguinal floor. It overlapped the pubic tubercle minimally by 1 cm. The process of fixation was achieved by applying pressure on the mesh, starting distally on the pubic bone, then medially onto the internal oblique structures. The cranial part of the mesh was fixed under the external oblique aponeurosis by digital manipulation and the spermatic cord passed through the existing slit. The size of the foramen was adapted to the diameter of the cord and the surrounding anatomical structures. After that, the mesh was pushed down towards the inguinal ligament and the lateral part was then allowed to fall onto the deep aspect of the divided external oblique aponeurosis. Care was taken to avoid folding the mesh. In group II, a sheet of polypropylene® (PP) mesh cut to shape and placed over the inguinal floor so it overlapped the pubic tubercle by at least 1 cm medially. Fixed with a running polypropylene 2/0 suture to the inguinal ligament and interrupted sutures to the conjoint area. A slit were cut for the spermatic cord and the tails secured back together around the cord with permanent sutures, as described by Lichtenstein [20]. Finally, the external oblique aponeurosis and the skin closed. Operation details were gathered immediately after the surgery was completed. Operative time (from skin incision until suturing of the cutis) and mesh fixation time were recorded in minutes.
The follow-up
After discharge, the patient was seen at the outpatient clinic by the surgeon with an initial post-operative visit at the 7th post-operative day, then at the 1, 3 and 6 months. As regard to the testicular volume and perfusion evaluation was done using U/S and color Doppler 3 months post-operative and compared to pre-operative values.
Statistical analysis of the data
Data were fed to the computer and analyzed using IBM SPSS software package version 20.0. Qualitative data were described using number and percent. Quantitative data were described using mean and standard deviation median, minimum and maximum. Comparison between different groups regarding categorical variables was tested using Chi-square test. When more than 20% of the cells have expected count less than 5, correction for Chi square was conducted using Fisher’s exact test or Monte Carlo correction. The distributions of quantitative variables were tested for normality using Kolmogorov–Smirnov test, Shapiro–Wilk test and D’Agostino test, also Histogram and QQ plot were used for vision test. If it reveals normal data distribution, parametric tests were applied. If the data were abnormally distributed, non-parametric tests were used. For abnormally distributed data, comparison between two independent populations were done using Mann–Whitney test while Kruskal–Wallis test was used to compare between different groups. To compare between two periods, Wilcoxon signed-rank test was used. Correlations between ordinal variables were assessed using Spearman’s coefficient.
Results
Patients in the two groups had similar demographic data with no statistical significant difference between them as regard to age, BMI, associated co-morbidities, ASA score, hernia type and location as illustrated in Table 1. The mean operative time was significantly shorter in group I (35.90 ± 15.92 min) than in group II (51.25 ± 12.55 min) with p value < 0.001. This was a reflection of the significantly shorter mesh fixation time in group I versus group II (3.11 ± 2.20 vs 12.05 ± 3.09 min) with (p < 0.001) Table 2.
9 patients (22.5%) experienced postoperative complications in group I compared to only 4 patients (10%) in group II, however, there was no statistical significant difference between them (p = 0.091) as illustrated in Table 2. Epididymo-orchitis diagnosed clinically and confirmed by U/S observed in two patients (5%) in group I, whereas none of group II patients suffered from it.
Two patients in group I needed hospital re-admission during the 1st postoperative month. The first patient was due to severe gastritis resulting from excessive use of analgesics for pain, while the second patient was due to epididymo-orchitis. The later patient complained of severe testicular pain on the 5th postoperative day, fever associated with scrotal swelling and swelling at the operated hernia side. On examination, scrotal tenderness was obvious, associated with scrotal edema and seroma at the wound site. U/s was done and it revealed hematoma at an internal ring about 3 × 3 cm, bilateral inflammation of the cord, and bilateral epididymitis with bilateral hydrocele and air foci in the scrotum. Both testes showed normal echo velocity with normal size average about 15 cc in both testes. Normal vascularity of both testes with the peak systolic is about 15 cm/s and the end diastolic volume is about 7.5 cm/s. Bilateral mild to moderate amount of turbid collection with low level of internal echos in both hemi-scrotums and bilateral grade II varicocele. The patient responded to conservative management in the form of bed rest, scrotal elevation and analgesia until fever and local inflammation had subsided in conjunction with antibiotics in the form of (ceftriaxone 250 mg IM plus levofloxacin 500 mg orally once daily for 10 days). Follow-up U/S after 1 and 3 months reveled normal finding except from mild hydrocele and grad II varicocele.
No case of testicular atrophy occurred in either group. In both groups, there was a significant postoperative decrease in testicular volume accompanied by a significant increase in RI as compared to their pre-operative values (Table 3). However, on comparing the two groups, patients in group I showed higher values of decrease in testicular volume accompanied by more increase in RI values postoperatively compared to group II patients (Table 4), but these values did not reach a significant value (p = 0.107, p = 0.136).
In group I, the pre-operative Doppler reported six patients (15%) with varicocele classified as grade I (5%), grade II (5%), grade III (5%) and two patients (5%) with minimal hydrocele. The post-operative Doppler reported 30 patients (75%) developed varicocele classified as 35% grade I, 30% grade II and 10% having grade III. It also reported 24 patients (60%) with minimal hydrocele and two patients (5%) with mild hydrocele. Comparing the pre- and post-operative Doppler finding in group I, there was a statistical significant difference between the pre- and post-operative finding with p < 0.001 as illustrated in Table 4. In group II, the pre-operative Doppler reported six patients (15%) with grade I varicocele. No cases of hydrocele were reported. The post-operative Doppler reported ten patients (25%) with varicocele classified as 15% grade I, and 10% grade II. Six patients (15%) reported having minimal hydrocele. There was no significant difference between the pre- and post-operative Doppler findings in group II Table 5. Comparing the pre- and post-operative Doppler findings between group I and group II, there was a significant increase in the number of postoperative varicocele and hydrocele in group I compared to group II. These findings are illustrated in Table 5. No patient in either groups developed recurrence during the follow-up period from 6 to 24 months.
Discussion
The ideal outcome in inguinal hernia surgery is to provide a repair that is free from recurrence, pain, infection, with minimal scarring and with improvement in patient’s quality of life. Presently, this outcome has yet to be definitively achieved after more than 100 years of hernia surgery. Countless studies have been done in attempts to improve outcomes, and the procedure has evolved greatly, especially over the last few decades.
Just when most experts were thinking that the problem of recurrence had been all but eliminated for primary inguinal herniorrhaphy because of the tension-free mesh concept, along comes the published manuscript by Shin and colleagues incriminating the mesh fibrotic reaction as a cause of infertility [2]. They reported on 14 patients with infertility secondary to obstructive azoospermia after hernia repair with implantation of polypropylene meshes [2]. All patients underwent surgical exploration with intra-operative vasography. The vasogram determined the site of the obstruction in the inguinal region, and the surgical exploration identified the cause of the obstruction to be the mesh [2]. As in the previously published study, the inflammatory reaction and the subsequent development of scar tissue surrounding the mesh may result in the compression of the testicular arteries or obstruction of the veins [9]. In addition, it is known that over time, the mesh contracts, shrink and fold. Its area is reduced to 60% of its original size or even to 10% in the case of mesh plugs and this contraction may cause congestion in the pampiniform plexus [12]. Moreover, the sharp edges of the mesh can erode the spermatic cord [21].
The protection given to the elements of the spermatic cord by the cremaster muscle has been cited as one advantage of the Lichtenstein procedure in which the prosthesis is not in contact with vessels and nerves [22]. However, cremasteric muscle preservation or division was not proven to be a sole agent in preventing or inducing fibrosis that causes diminution of blood flow. Technical issues should be considered. The presence of cremasteric muscle may help to prevent vas injury caused by direct erosion of the sharp edge of the mesh into the spermatic cord. However, it will not prevent the congestion of the vein or artery caused by the mesh contraction or the effect of compression by the scar tissue or shrinkage of the mesh over time.
Mechanical factors must be taken into consideration. It is important to make sure during the repair that the deep ring is not too tight. This technical factor which is an operator dependent can cause constriction of the spermatic cord which can result in congestion, ischemic orchitis and even a testicular atrophy. In our study, all surgeries was done under general anesthesia using laryngeal mask anesthesia (LMA) and spontaneous breathing without muscle relaxant, where we can ask the anesthesiologist at a certain time to make the patient strain to examine the muscle repair, accurate sac reduction and avoid excessive prosthesis constriction on the spermatic cord.
Our prospective study intended to evaluate the effect of mesh implantation and perimesh fibrosis on testicular flow using two different types of mesh material and mode of fixation. The assessment was done using gray-scale- and color Doppler sonography to evaluate testicular arterial impedance, perfusion, and venous flow. In our study, we found that the mesh repair itself had a significant effect on the testicular volume and its blood flow regardless of the type of mesh material or the mode of fixation. In both groups, the testicular volume decreased significantly postoperatively, while the RI significantly increased. The change in testicular volume in both groups remains within normal ranges with a mean of 13.10 ± 2.50 in group I and 14.74 ± 3.41 in group II and with no cases of testicular atrophy. Testicular volume is an important marker for testicular atrophy following corrective surgical operations on patients with inguinal hernias [23, 24]. Testes considered to be atrophied when it measures less than 2 cm in size [6]. Testicular atrophy was reported in the published literature as a consequence of ischemic orchitis that could occur in patients who underwent operations with or without prostheses [25].
Blood flow in the testicle can be represented by vascular resistance or resistive index (RI) (RI = systolic peak velocity − end diastolic peak velocity/systolic peak velocity). Pinggera et al. found a relationship between the RI and the sperm count. In patients with low sperm count, the RI was significantly increased (> 0.6) compared with patients with normal sperm counts. This relationship suggests that the intratesticular RI can be used as a means to quantify testicular perfusion and, indirectly, assess gonadal function. Hence, testicular perfusion is related to testicular function [15]. However, this was not enough to judge the patient’s fertility or answer the following question. Twenty-six years old newly married patient received parietex mesh. Post-operative he complained from pain during intercourse. He was concerned about his fertility so he had a semen analysis. The report came back with decrease in both count and motility. The pre-operative Doppler of this patient showed normal testicular volume, contra-lateral side subclinical indirect inguinal hernia with grade III varicocele. Post-operative Doppler showed bilateral grade III varicocele and minimal hydrocele on the repaired side. His RI post-operative was (0.73). Semen analysis was not performed pre-operative. The patient came with a question (is there a relation between the surgery and his compliant??). A question that was hard to answer in the absence of a pre-operative semen analysis.
The effect of mesh repair on sperm count and motility in the literature yielded conflicting results. A prospective randomized trail using both Doppler and spermiogram to assess testicular function between Lichtenstein mesh repair and mesh plug technique found that although the RI was elevated postoperatively in both groups, there was no significant alteration in terms of testicular volume and spermiogram [13]. Peters et al. in a randomized control trail found a decrease in the sperm motility with lightweight prolene mesh that was significant in case of bilateral repair [26].
An experimental study compared coated polyester and prolene mesh, concluded that the polyester-based mesh induced a more intense foreign body reaction and was exposed to significantly more shrinkage than the covered polypropylene mesh [27]. This more intense inflammatory reaction might explain the significant increase in development of postoperative varicocele and hydrocele in group I (polyester mesh) than in group II (prolene mesh). This might support the theory of more intense fibrosis and shrinkage with polyester-based mesh. Le Blanc et al. [28], and Peiper et al. [29], have noted testicular venous congestion after mesh implantation in animal studies. The scar tissue formed after mesh implantation and mesh contraction may cause congestion in the pampiniform plexus as mentioned before. In another experimental study, venous congestion in the testicular blood flow was seen after polypropylene mesh implantation. This typical inflammatory reaction was not seen after Shouldice (non mesh) herniorrhaphy [30]. This congestion may explain the post-operative varicocele in our results. In addition, the obstruction of the venous return or lymphatic drainage can result in an increase in the pressure in the testicle, hence the vascular resistance [19, 21]. This proposed mechanism has been suggested in patients with a hydrocele who also have an elevated RI.
In our study, the advantage of self-fixing mesh clearly emerged with respect to operative time which was significantly shorter in the progrip group (PG) with a median of [30] compared to the median of (47.5) in the standard prolene group. The lack of requirement for sutures to secure the mesh reduced the operative time by more than 15 min. The time needed for mesh fixation in group I, reached 1 min and 20-s (1.20 min) with a median of (2.0) compared to a median of [10] in group II. However, the self-fixing mesh did not add benefits as regard to post-operative pain, need of analgesia, return to work and overall patient satisfaction.
Conclusion
Mesh implantations has an effect on testicular size and blood flow by decreasing the testicular size and increasing the RI. This effect was more obvious in the parietex progrip than with the standard prolene mesh. Although there is an indirect relation between RI and the sperm count, testicular blood flow alone is not enough to judge fertility.
References
Yamaguchi K, Ishikawa T, Nakano Y, Kondo Y, Shiotani M, Fujisawa M (2008) Rapidly progressing, late-onset obstructive azoospermia linked to herniorrhaphy with mesh. Fertil Steril 90(5):e5–e7
Shin D, Lipshultz LI, Goldstein M, Barme GA, Fuchs EF, Nagler HM et al (2005) Herniorrhaphy with polypropylene mesh causing inguinal vasal obstruction: a preventable cause of obstructive azoospermia. Ann Surg 241(4):553–558
Ramadan SU, Gokharman D, Tuncbilek I, Ozer H, Kosar P, Kacar M et al (2009) Does the presence of a mesh have an effect on the testicular blood flow after surgical repair of indirect inguinal hernia? J Clin Ultrasound 37(2):78–81
Klinge U, Klosterhalfen B, Muller M, Schumpelick V (1999) Foreign body reaction to meshes used for the repair of abdominal wall hernias. Eur J Surg 165(7):665–673
Klosterhalfen B, Klinge U, Hermanns B, Schumpelick V (2000) Pathology of traditional surgical nets for hernia repair after long-term implantation in humans. Der Chirurg 71(1):43–51
Tekatli H, Schouten N, van Dalen T, Burgmans I, Smakman N (2012) Mechanism, assessment, and incidence of male infertility after inguinal hernia surgery: a review of the preclinical and clinical literature. Am J Surg 204(4):503–509
Uzzo RG, Lemack GE, Morrissey KP, Goldstein M (1999) The effects of mesh bioprosthesis on the spermatic cord structures: a preliminary report in a canine model. J Urol 161(4):1344–1349
Peiper C, Junge K, Klinge U, Strehlau E, Krones C, Ottinger A et al (2005) The influence of inguinal mesh repair on the spermatic cord: a pilot study in the rabbit. J Invest Surg 18(5):273–278
Ridgway PF, Shah J, Darzi AW (2002) Male genital tract injuries after contemporary inguinal hernia repair. BJU Int 90(3):272–276
Fitzgibbons RJ Jr (2005) Can we be sure polypropylene mesh causes infertility? Ann Surg 241(4):559–561
Hallen M, Sandblom G, Nordin P, Gunnarsson U, Kvist U, Westerdahl J (2011) Male infertility after mesh hernia repair: a prospective study. Surgery 149(2):179–184
Aydede H, Erhan Y, Sakarya A, Kara E, Ilkgul O, Can M (2003) Effect of mesh and its localisation on testicular flow and spermatogenesis in patients with groin hernia. Acta Chir Belg 103(6):607–610
Sucullu I, Filiz AI, Sen B, Ozdemir Y, Yucel E, Sinan H et al (2010) The effects of inguinal hernia repair on testicular function in young adults: a prospective randomized study. Hernia 14(2):165–169
Valenti G, Baldassarre E, Torino G (2006) Vas deferens obstruction due to fibrosis after plug hernioplasty. Am Surg 72(2):137–138
Pinggera GM, Mitterberger M, Bartsch G, Strasser H, Gradl J, Aigner F et al (2008) Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int 101(6):722–726
Chastan P (2006) Tension free open inguinal hernia repair using an innovative self gripping semi-resorbable mesh. J Minim Access Surg 2(3):139–143
Chastan P (2009) Tension-free open hernia repair using an innovative self-gripping semi-resorbable mesh. Hernia 13(2):137–142
Mihmanli I, Kantarci F, Kulaksizoglu H, Gurses B, Ogut G, Unluer E et al (2004) Testicular size and vascular resistance before and after hydrocelectomy. AJR Am J Roentgenol 183(5):1379–1385
Celik AS, Memmi N, Celebi F, Guzey D, Celik A, Kaplan R et al (2009) Impact of slit and nonslit mesh technique on testicular perfusion and volume in the early and late postoperative period of the totally extraperitoneal preperitoneal technique in patients with inguinal hernia. Am J Surg 198(2):287–291
Lichtenstein IL, Shulman AG, Amid PK, Montllor MM (1989) The tension-free hernioplasty. Am J Surg 157(2):188–193
Beddy P, Ridgway PF, Geoghegan T, Peirce C, Govender P, Keane FB et al (2006) Inguinal hernia repair protects testicular function: a prospective study of open and laparoscopic herniorraphy. J Am Coll Surg 203(1):17–23
Bendavid R (2004) The unified theory of hernia formation. Hernia 8(3):171–176
Akbulut G, Serteser M, Yucel A, Degirmenci B, Yilmaz S, Polat C et al (2003) Can laparoscopic hernia repair alter function and volume of testis? Randomized clinical trial. Surg Laparosc Endosc Percutan Tech 13(6):377–381
Lima Neto EV, Goldenberg A, Juca MJ (2007) Prospective study on the effects of a polypropylene prosthesis on testicular volume and arterial flow in patients undergoing surgical correction for inguinal hernia. Acta Cir Bras 22(4):266–271
Wantz GE (1993) Testicular atrophy as a risk of inguinal hernioplasty. II Giornale di Chir 14(4–5):205–206
Peeters E, Spiessens C, Oyen R, De Wever L, Vanderschueren D, Penninckx F et al (2010) Laparoscopic inguinal hernia repair in men with lightweight meshes may significantly impair sperm motility: a randomized controlled trial. Ann Surg 252(2):240–246
Zinther NB, Wara P, Friis-Andersen H (2010) Shrinkage of intraperitoneal onlay mesh in sheep: coated polyester mesh versus covered polypropylene mesh. Hernia 14(6):611–615
LeBlanc KA, Booth WV, Whitaker JM, Baker D (1998) In vivo study of meshes implanted over the inguinal ring and external iliac vessels in uncastrated pigs. Surg Endosc 12(3):247–251
Peiper C, Junge K, Klinge U, Strehlau E, Ottinger A, Schumpelick V (2006) Is there a risk of infertility after inguinal mesh repair? Experimental studies in the pig and the rabbit. Hernia 10(1):7–12
Goldenberg A, Paula JF (2005) Effects of the polypropylene mesh implanted through inguinotomy in the spermatic funiculus, epididim and testis of dogs. Acta Cir Bras 20(6):461–467
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Heba El Komy, Ahmed El-Gendi, Wael Abdel-salam, Mohamed Elseidy, Elsaid Elkayal declare that they have no conflict of interest.
Research involving human participants and/or animals
The ethics committee and review board in our institute approved the study and treatment protocol.
Informed consent
Informed consent was obtained from all patients who agreed to participate in the study.
Rights and permissions
About this article
Cite this article
El-Komy, H., El-Gendi, A., Abdel-salam, W. et al. Self-fixing parietex progrip versus the standard sutured prolene mesh in tension-free repair of inguinal hernia: effect on testicular volume and testicular blood flow. Updates Surg 70, 513–520 (2018). https://doi.org/10.1007/s13304-018-0554-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-018-0554-0