Abstract
Experimental nitrogen (N) deposition generally inhibits decomposition and promotes carbon (C) accumulation in soils, but with substantial variation among studies. Differences in ecosystem properties could help explain this variability: N could have distinct effects on decomposition and soil C due to differences in vegetation characteristics (that is, root C inputs and chemistry) that influence microbial biomass or soil properties like pH that can affect organic matter stabilization. We used a 12-year N addition experiment to determine effects of sustained N addition on soil C pool sizes and cycling across different grassland, conifer and deciduous forest sites in Minnesota, USA, while controlling for soil type and climate. We conducted a year-long soil incubation, and fit one- and two-pool decay models to respiration data to identify C pool sizes and decay rates. Contrary to previous studies, we found no consistent effects of N on soil C across sites: soil C stocks, microbial respiration, soil C decay rates and pool sizes all showed no general response to N in these sandy soils. Nevertheless, microbial biomass, microbial respiration, and the root biomass C pool responses to N addition were highly correlated, suggesting that soil C responses were ultimately driven by fine root biomass C responses to N addition, which in turn affected microbial biomass. However, the inconsistent directional responses to N among sites with similar vegetation cover highlight that N addition effects can be site-specific and raise caution for broad extrapolation of results from individual systems to global models.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Highlights
-
Across forest and grassland sites, soil C did not respond to long-term N addition.
-
Soil C cycling ranged widely across sites despite similar soils and climate.
-
Soil respiration response to N followed root C and microbial biomass responses to N.
Introduction
Soils represent the largest global terrestrial pool of carbon (C) (Ciais and others 2013), such that even small shifts in soil C pools could have large implications for atmospheric concentrations of carbon dioxide (CO2). Increasing availability of nitrogen (N) in ecosystems can influence soil C pools, as N is intricately connected to primary production (Vitousek and Howarth 1991; LeBauer and Treseder 2008), soil microbial biomass (Treseder 2008), and decomposition (Berg 2014). With global anthropogenic inputs of biologically reactive N up 12-fold since 1860 (because of agricultural practices, fertilizer use, and fossil fuel combustion), and expected to continue to rise (Gruber and Galloway 2008), understanding whether N addition could lead to measurable changes in soil C in the future is important. Yet, how soil C cycling responds to N addition across sites with different vegetation cover (for example, deciduous forests, coniferous forests, and grasslands) remains poorly understood because vegetation cover type is often confounded with soil texture or climate. Here, we focus on determining variation in N effects on soil C dynamics across eight forest and grassland sites with the same soil and climate.
It is not well understood whether N has similar effects on soil C among sites with different dominant plant species. For example, soil C responses to N addition across temperate forests can be quite variable: a meta-analysis of 36 temperate-forest studies found an average 15% reduction in microbial respiration with N addition, but responses ranged from a 57% suppression to a 63% increase (Janssens and others 2010). An experiment across temperate forests further suggests that N could have distinct effects on soil C across sites with different dominant canopy species (Waldrop and others 2004). Furthermore, although meta-analyses have shown N addition to decrease microbial biomass (Treseder 2008) and respiration (Janssens and others 2010) on average, variation in microbial communities associated with different dominant plant species and fertility (Wardle 2004) could influence the magnitude of microbial responses to N addition (Leff and others 2015). Added N could also alter belowground C inputs, depending on the plant response. If net primary productivity (NPP), including belowground net primary productivity (BNPP), increases, then C inputs belowground would also increase (Adair and others 2009; Yue and others 2016) (assuming there is no offsetting increase in root longevity). However, if plants partition NPP away from BNPP in response to N addition, C inputs belowground would decrease (Janssens and others 2010). These potential changes in C allocation with added N could further differ depending on dominant vegetation cover types and physiologies (Liu and Greaver 2010). Other site properties, such as root biomass, root chemistry, and soil pH, could lead to distinct N addition effects not only on microbial respiration and total soil C, but on C pools with different mean residence times as well.
Soil C pools with different mean residence times should respond differently to N addition (Neff and others 2002; Reid and others 2012; Riggs and others 2015); their rates of cycling have unique controls, which could be influenced differently by N and vegetation cover type. The fast-cycling C pool (Cf) is controlled largely by litter chemistry (Cleveland and others 2014) and microbial physiology (Schimel and Schaeffer 2012), which are both sensitive to N addition and likely vary among sites with differing vegetation cover. N addition can increase root N concentrations (Knops and others 2007) and alleviate microbial nutrient limitation, resulting in increased microbial efficiency (Ågren and others 2001; Schimel and Weintraub 2003; Manzoni and others 2012). Indeed, decomposition of fast-cycling C has been shown to increase with N addition (Neff and others 2002; Riggs and others 2015). However, N addition can also lead to lower rates of decomposition by decreasing microbial biomass (Treseder 2008) or reducing oxidative extracellular enzyme activities (Jian and others 2016).
In contrast, slow-cycling C (Cs), controlled by physical and chemical protection (Jastrow and others 2006; Dungait and others 2012; Angst and others 2017), has been shown to decay more slowly with added N (Riggs and others 2015). N addition could decrease the decay rate of slow-cycling C (ks) by affecting the capacity for organic matter stabilization via cation bridging. Specifically, N-induced acidification (Bouwman and others 2002) could lead to leaching losses of base cations (Aber and others 1998), but also increase the solubility of polyvalent cations like Al3+ and Fe3+, which strongly bind organic matter to soil mineral surfaces protecting it from decomposition (Hobbie and others 2007). However, because ambient pH levels differ substantially by vegetation cover (Reich and others 2005; Mueller and others 2012), and sites with low cation exchange capacity can be more susceptible to acidification from N addition (Clark and others 2007), the type of site might influence whether added N induces strong acidification and thus increases organic matter stabilization.
Our objective was to assess how N addition affects soil C cycling across sites with differing vegetation cover. Other comparative studies that control for soil type, climate, and N deposition history are, to our knowledge, non-existent; this is an important gap in our ability to determine whether N addition influences soil C cycling similarly across sites with different vegetation cover. The diversity of sites and vegetation cover within the 22 km2 of the Cedar Creek Ecosystem Science Reserve in central Minnesota present a unique opportunity to test this, as plant species composition varies (largely because of variation in land use and disturbance history), whereas soil type, climate, and N deposition history are similar. Our specific hypotheses were as follows:
-
1.
N addition will decrease microbial respiration and increase soil C. We expect this will be driven by reduced microbial biomass and decreased rates of decay of both the fast and slow pools (kf and ks, respectively) with N addition. Alternatively, N addition could alleviate microbial N limitation and increase fast pool decay rates.
-
2.
N addition will have variable effects on soil C cycling among sites because of variation in ambient root biomass, root chemistry, and soil pH. We expect sites with high fine root C:N and lignin:N will show more positive effects of N on kf, and sites with low ambient pH will show more negative effects of N addition on kf and ks.
Methods
Study Site
Experimental plots were established in 1999 in eight sites of differing vegetation cover at the Cedar Creek Ecosystem Science Reserve in East Bethel, MN (latitude 45.40°N, longitude 93.20°W, elevation 270 m) (Hobbie 2005). From 1999 to 2011, average annual precipitation was 744 mm/year and mean annual temperature was 7.2 °C. The eight sites, all within 5 km of each other, included different canopy dominants: 2 pin oak stands (Quercus ellipsoidalis), 2 white pine stands (Pinus strobus) (one plantation, Pine 1, and one natural stand, Pine 2), 1 maple-basswood stand (Acer saccharum, Tilia americana, and Quercus ellipsoidalis), 1 clonal bigtooth aspen stand (Populus grandidentata) that had invaded an old field, and 2 abandoned agricultural fields now dominated by tallgrass prairie species (mix of C3 and C4; Old Fields 1 and 2) (Hobbie 2005, 2008). The sites are all on a sandy outwash plain (> 90% sand), and soils are classified as Udipsamments (Grigal and Homann 1994). At each site, 12 2.5 m by 2.5 m plots were set up and randomly assigned to either the N-fertilized treatment (receiving a total of 10 g N/m2/year as NH4NO3 applied in aqueous solution in three applications over the growing season) or control (receiving equal amounts of water instead). Given the size of the plots, changes in vegetation composition only occurred in the grassland sites (N-addition plots shifted from domination by Schizachyrium scoparium and other C3 and C4 grasses to domination by Elymus repens).
Soil Sampling and Characterization
Soil cores were taken in October 2011 after 12 years of treatment. Prior to taking soil cores, the organic horizon (minimal to non-existent) was removed if present. Five cores (2 cm diameter) were randomly collected per plot to 10 cm depth, combined and homogenized. Soils were transported to the laboratory on ice and stored in the refrigerator for no more than 48 h. Soils were passed through a 2-mm sieve, and fine roots were picked out and frozen. Soil not used immediately was air-dried for at least 48 h. In September 2018, two cores were taken at each plot to assess bulk density using a 5 cm diameter core to 10 cm diameter depth.
Given their role in C cycling, we quantified soil moisture, soil pH, soil %C, soil %N, soil C:N ratio, fine root biomass, microbial biomass C, microbial biomass N, and microbial biomass C:N ratio. Fresh soils were used to measure gravimetric soil moisture (105 °C). Soil pH was measured on air-dried soil using a 2:1 water-to-soil method (ThermoScientific Orion 420A pH meter, Waltham, MA, USA; Hendershot and others 1993). Total soil %C and %N were measured via dry combustion of air-dried soils (Costech ECS 4010 Elemental Analyzer, Valencia, CA, USA). Soil %C was converted to total soil C (g/m2) using plot-level bulk density data. The soils from these sites do not contain carbonates so total soil C is equivalent to total soil organic C. Microbial biomass was assessed using chloroform fumigation (Brookes and others 1985). Two aliquots of fresh soil (equivalent to 10 g dried soil) from each sample were extracted with 0.5 M K2SO4 immediately or after 72 h of chloroform fumigation in the dark. Extracts were immediately frozen and later measured for TOC/TN (Shimadzu TOC-V, Shimadzu Corporation, Kyoto, Japan). Microbial biomass C and N were determined by subtracting the non-fumigated sample from the fumigated sample. Results of chloroform fumigation are presented as chloroform-labile C and N, uncorrected for extraction efficiency. To determine fine root biomass, frozen roots were thawed, washed with DI water, dried at 60 °C for at least 48 h, and then weighed.
Fine Root Chemistry
Fine roots were analyzed for C and N concentration and C chemistry. Dried roots were ground on a Thomas Wiley Mill (Thomas Scientific, Swedesboro, NJ, USA) using a 0.85 mm catch screen (standard size 20) and analyzed for C chemistry using an Ankom 200 Fiber Analyzer (ANKOM Technology, Macedon, NY, USA) (% soluble cell contents, % hemicellulose and bound proteins, % cellulose, and % acid unhydrolyzable residue, lignin hereafter). Roots were further ground with a mortar and pestle and tested for %C and %N via combustion (Costech CN Elemental Analyzer, Costech Analytical Technologies Inc., Valencia, CA, USA) using Atropine as a standard. We tested two analytical replicates per sample and took their average.
Soil C Decomposition
We measured microbial respiration in long-term laboratory incubations to assess soil C decomposition. Within 48 h of soil collection, 50 g of fresh, root-free soil from each plot (n = 6 treatment and n = 6 control, from each of the eight sites) was weighed into a plastic cup and placed in a 1-l glass mason jar. Jars were covered with gas-permeable, low-density polyethylene film to avoid contamination and desiccation but to prevent O2 depletion and minimize CO2 build-up, and were stored in a dark room at 21 °C. Soil moisture was maintained throughout the incubation at 75% field capacity with routine re-wetting with DI water. Respiration was measured after a 24-h incubation period 16 times over 385 days (on days 1, 4, 7, 12, 19, 31, 38, 44, 54, 68, 84, 124, 171, 251, 341, and 384 after soil collection). Jars were flushed to release built-up CO2, capped, and headspace was then sampled using a syringe immediately and 24 h after capping. The 24-h CO2 efflux was determined by difference. Gas samples were analyzed using an infrared gas analyzer (LICOR LI-7000 CO2 Analyzer, Lincoln, NE, USA). Cumulative respiration (mg C/g soil C and mg C/g soil) was determined using daily respiration at each sample point, accounting for days in between respiration sampling (that is, by multiplying the average rates at t1 and t2 by the number of days between t1 and t2, following the methods of Riggs and others 2015).
Daily respiration rates (Crate) were fit to both one-pool and two-pool decay models. For the one-pool model (Eq. 1), Ct is the size of the entire C pool at time t and k is the rate of decay for the C pool. In contrast, in the two-pool model (Eq. 2), Cf is the size of the fast pool and kf is its decay rate. The second, slow pool, which is the total C pool less the size of the fast pool (Cf), decays at rate ks.
Maximum likelihood estimation (MLE) was used to determine model parameters for C pools and decay rates at the plot (that is, jar) level (bbmle package in R). One- and two-pool models were assessed using Akaike Information Criterion (AIC) values, corrected for small sample size (AICc). Two-pool models were the better fit for 72% of samples (69/96) (difference in AICc ≥ 2), and one- and two-pool models were essentially indistinguishable (difference in AICc < 2) for 25% of samples (24/96). Therefore, all results reported hereafter are from the two-pool models. Finally, because there are multiple parameter sets that could fit each model, we tested the possibility of “parameter equifinality” where different combinations of parameters result in similarly good models (Beven 2006). We found no evidence for equifinality (results not shown).
Statistical Analysis
Because we were interested in assessing the effects of N addition and site on various elements of C cycling, we developed linear statistical models to test their main and interactive effects on soil C stocks, cumulative respiration, and decay constants and pool sizes. Additionally, we developed statistical models for microbial respiration, decay rates, and pool sizes that incorporated soil and microbial characteristics along with N treatment and site as explanatory variables. For these models, we included site-level averages of ambient soil and microbial characteristics, N treatment, site, N treatment by site interaction, and each of the N treatment by soil and microbial interactions. We only included soil and microbial characteristics that were not highly correlated (r < 0.8, see Table S1), which led us to include soil %C, soil C:N, microbial biomass C, and fine root biomass C (g root C/g soil or g root C/m2) as explanatory variables in the models. Soil pH and %C were highly correlated (r = − 0.85), which is why pH was left out. Finally, we used a log response ratio approach to test the relationships between responses of different variables to N addition at each site. We calculated response ratios as ln(treatment)—ln(control) from the six ambient and six +N plots. In all cases, data were checked to ensure model assumptions of normality and equal variance were met, and were natural log-transformed as needed to achieve model assumptions. All data analysis was done in R (version 3.0.2, The R Foundation for Statistical Computing).
Results
N Effects on Cumulative Microbial Respiration and Soil C Stocks Across Sites
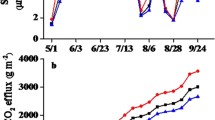
Contrary to our hypotheses, N addition did not lead to general reductions in microbial respiration or increases in soil C stocks (or C concentration) across sites. There was wide site-to-site variation in microbial respiration per g soil C, but N addition had no effect across sites (ANOVA, Site P < 0.0001; +N P = 0.6845; Site*N P = 0.2047, Figure 1 and Table 2). Respiration per g soil also differed among sites, and the effect of N addition depended on site (ANOVA Site P < 0.0001; Site*N P = 0.0204, Figure 1): in most cases, N addition did not change cumulative respiration per g soil; however, N addition decreased cumulative respiration per g soil in Old Field 2 and increased it in Pine 1. Site identity influenced cumulative respiration even after including additional soil and microbial characteristics in the model (P < 0.0001 for both per g soil C and per g soil; Table S2). There was also no effect of added N on soil C stocks (P > 0.3), and although soil C stocks differed substantially by site (P < 0.0001; Table 2 and Figure S1), there was no interaction of added N*Site (P > 0.2; Table 2). Soil %C also did not respond to N addition (P > 0.1, Tables 1 and 2).
Cumulative microbial respiration by site and N treatment. Cumulative respiration over the duration of the incubation expressed A per gram soil C, and B per gram soil. Respiration per gram soil C did not differ by N treatment (N Treatment P = 0.6845; N*Site interaction P = 0.2047), but did differ significantly between sites (P < 0.0001). The response of respiration per gram soil to N addition depended on site (N*Site interaction P = 0.0204), and overall differed significantly among sites (P < 0.0001). Original data are shown, although statistics were run using natural log-transformed data to validate model assumptions.
N Effects on Fast- and Slow-Cycling C Across Sites
We also did not find support for our hypotheses that long-term N addition would decrease C cycling in both the fast and slow pools. Across sites, neither the decay rate of the fast pool (kf) nor that of the slow pool (ks) responded to long-term N addition (ANOVA, P = 0.9482 and P = 0.8648, respectively, Figure 2 and Table 2). Pool sizes also did not generally change (ANOVA, Cf P = 0.5888 and Cs P = 0.1229, Figure 2 and Table 2). However, there was a marginal Site*N addition interaction for Cf (ANOVA, P = 0.0775), where although most sites had no effect of N addition, Old Field 2 had a smaller fast pool and Pine 1 had a larger fast pool with N addition. There was no Site*N addition interaction effect for the slow pool, Cs (P = 0.5089, Table 2).
Carbon pools and decay rates by site and N treatment. A Fast pool decay rate (kf); B slow pool decay rate (ks); C fast pool size (Cf); D slow pool size (Cs). All carbon cycling metrics shown differed among sites (kf P < 0.0001, ks P < 0.0001, Cf P = 0.0020, Cs P < 0.0001), but not with N addition (P > 0.1 for all). Original data are shown for ease of interpretation; however, kf, ks, and Cs were natural log-transformed for statistical analysis to meet model assumptions.
As expected, both fast- and slow-cycling C decay rates and pool sizes differed substantially by site (ANOVA, kf Site P < 0.0001; ks Site P < 0.0001; Cf Site P = 0.0020; Cs Site P < 0.0001; Figure 2 and Table 2). However, although there were some patterns in C cycling by vegetation cover, there was as much variation within as across sites with different vegetation cover (Figure 2). In kf and ks models that included soil and microbial characteristics, site identity still had a significant effect after accounting for other soil parameters (P = 0.0043 and P < 0.0001, respectively, Table S2). In contrast, in Cf and Cs models that included soil and microbial characteristics, site identity no longer mattered, and instead soil %C was the key driver for Cs such that higher soil %C meant a larger slow pool (P < 0.0001, R2 = 0.8086; Table S2) and Cf was not explained at all by site parameters (Table S2).
Effects of N Addition on Microbial, Root, and Soil Characteristics Across Sites
Effects of N addition on the hypothesized drivers of soil C cycling—microbial biomass, root characteristics, and soil pH—varied across sites. N addition influenced microbial C and N in some cases, but the direction of the effect depended on site (ANOVA Site*N P = 0.0002 and P = 0.0005, respectively; Tables 1 and 2): the two grassland sites and one of the oak stands (Oak 1) had lower microbial C and N with N addition, whereas the aspen and maple sites had higher microbial C with N addition (Table 1). Nitrogen addition also affected fine root C and chemistry, but not consistently across sites. Fine root C:N ratio was lower with added N across sites, but driven mostly by the two grassland sites (ANOVA, +N P = 0.0002, Site*N P = 0.0240, Tables 1 and 2). The proportion of root soluble cell contents, the most labile C, increased with N addition across all sites, but most notably in the grasslands where they went up by almost one third (ANOVA, +N P = 0.0116, Tables 1 and 2, Figure S2). Concentrations of other C compounds in roots (hemicellulose and bound proteins, cellulose, and lignin) did not change with N addition, and there were no Site*N addition interactions (ANOVA, P > 0.1 Tables 1 and 2, Figure S2). Fine root lignin:N was generally lower with added N (ANOVA, P < 0.0001, Table 2). Soil pH was consistently lower with N addition, as expected, but the magnitude of the effect differed by site, with the two grassland sites showing larger effects (ANOVA, +N P < 0.0001, Site*N P = 0.0199, Tables 1 and 2).
Site characteristics related to soil C cycling also differed substantially across sites, in some cases even under similar vegetation types (Tables 1 and 2). Microbial biomass C varied more than twofold and fine root C also differed more than fourfold. Fine root C:N, the proportions of soluble cell contents, hemicellulose and bound proteins, and lignin (but not cellulose) in roots also differed across sites (ANOVA, P < 0.0001, P < 0.0001, P < 0.0001, P = 0.0293, P = 0.4520, respectively, Tables 1 and 2, Figure S2). However, fine root lignin:N did not differ between sites (ANOVA, P = 0.2152, Table 2). Average site-level soil pH ranged from 5.1 to 5.7. In a principle component analysis of all site soil, root, and microbial characteristics, the two grassland sites clustered and the forested sites mostly clustered together, although the pine sites, particularly Pine 1, stood out as being different from the other forested sites (Figure S3).
Can Site-to-Site Variation in Microbial and Root Responses to N Addition Predict C Cycling Response to N Addition?
Given substantial site-to-site variation in key site parameters, C cycling, and their responses to N, we were interested to see if the response of hypothesized drivers of C cycling to N addition could explain variation in C cycling responses to N addition.
We had hypothesized that microbial respiration and soil C stocks would change in response to reductions in microbial biomass from soil acidification and reduced root C inputs. Accordingly, the responses of soil pH and microbial biomass C to N addition were positively related (See Effects of N Addition on Microbial, Root, and Soil Characteristics Across Sites), although the relationship was marginally significant (P = 0.0529). Despite no consistent effects of N addition on either root C or microbial biomass C (Effects of N Addition on Microbial, Root, and Soil Characteristics Across Sites), microbial biomass C response to N addition tracked closely with root C responses to N addition (Figure 3c; R2 = 0.4497, P = 0.0411). Microbial biomass C response to N addition was also related to the fine root C:N response to N addition, where sites with more of a reduction in the root C:N with N addition also had a greater reduction in microbial biomass C (R2 = 0.4181, P = 0.0494). Furthermore, responses of microbial respiration per g soil C to N addition also closely followed microbial biomass C and root C responses to N addition (Figure 3, microbial biomass C R2 = 0.7052, P = 0.0056; root C R2= 0.5333, P = 0.0240), although not root C:N response (P > 0.2). There were no other significant relationships between other root chemistry responses to N addition (% soluble cell contents, % hemicellulose and bound proteins, % cellulose, % lignin, and root lignin:N) and microbial biomass, respiration, or decay rate responses to N addition (results not shown). The relationships between microbial respiration response to N addition and microbial biomass C and root C responses were similar when respiration was expressed per g soil as well (Figure S4). In contrast, the soil C stock response to N addition did not relate to the microbial biomass response, root C response, or soil pH response to N addition (P > 0.5 for all).
Comparison of microbial respiration per gram soil C, microbial biomass C, and fine root C responses to N addition. Responses to N are compared between: A Microbial respiration and microbial biomass C (y = 0.3898*x + 0.0160; R2= 0.7052, P = 0.0056); B microbial respiration and root biomass C (y = 0.2921*x − 0.0820; R2= 0.5333, P = 0.0240); and C Microbial biomass C and fine root biomass C (y = 0.6077*x − 0.2190; R2= 0.4497, P = 0.0411). Response ratios (RR) were calculated as ln(treatment)—ln (control). Site-level response ratios are calculated from the six ambient and six +N plots (with the exception of Old Field 1 where microbial biomass C n = 5).
Although we had expected changes in respiration and soil C stocks to be associated with decreases in decay rates of both fast- and slow-cycling C, respiration responses to N addition were mostly related to responses in the fast pool, Cf (Figure S5; per g soil C P = 0.0215; per g soil P = 0040), as well as to responses of root C and microbial biomass to N addition (Figure S4). Interestingly, microbial respiration response to N addition tracked very closely to the slow pool decay rate response to N addition (per g soil C R2 = 0.8191, P = 0.0012; per g soil R2 = 0.7998, P = 0.0017; Figure S6), but not to fast pool decay rate response to N addition (P > 0.5 when expressed both per g soil C and per g soil). Soil C stock response to N addition was not related to responses of microbial respiration, decay rates, or pool sizes to N addition (P > 0.1 for all).
Discussion
Contrary to our predictions that long-term N addition would reduce respiration rates and result in greater soil C stocks, we found no overall effect of 12 years of N addition on soil C stocks, cumulative microbial respiration, or fast and slow pool decay rates or pool sizes in these sandy soils. We had further expected sites to differ in the magnitude of their responses to N addition, based on vegetation cover-induced differences in key characteristics related to C cycling. Although C cycling responses to N addition did differ across sites, those responses were not necessarily consistent among similar vegetation cover types (for example, N addition led to less cumulative respiration in only one of the two grassland sites and more cumulative respiration in only one of the two pine-dominated sites). Here, we explore possible explanations for the lack of an effect of N addition across sites, suggest potential site-specific differences that could have led to different responses to N addition, and discuss the mechanistic framework that emerges from the relationships we found between respiration, microbial biomass, and root C responses to N addition.
Soil Characteristics May Contribute to the Small or Absent Effect of N Addition
It is possible that slow-cycling C dynamics at Cedar Creek are negligibly responsive to N addition because the sandy soils are less likely to demonstrate increased chemical protection of organic matter with N-induced acidification. Even though N addition reduced pH by 0.1 to 0.6 units, corresponding to a one- to fourfold increase in acidity, the sandy soils (> 90%, Grigal and others 1974) have low surface area and charge, and therefore a low potential for chemical stabilization of organic matter. Despite the high sand content, the slow-cycling pools at Cedar Creek are as large or larger than those measured using similar methods in grassland soils in Nebraska, Iowa, and Colorado, in soils ranging from 71.3 to 87.5% sand, and in the Entisol, Mollisol, and Aridisol orders (Riggs and others 2015). Thus, the slow-cycling pools at Cedar Creek are not unusually low considering the range of grassland soils in the USA, even though the mechanisms of stabilization are likely limited. Indeed, N-induced acidification may not have increased cation availability for organic matter bridging in a meaningful way given the soil’s low clay content, which is the source of polyvalent cations Al3+ and Fe3+. This could partially explain the lack of Cs and microbial respiration response since soil acidification has been shown to be the primary control of N-induced reductions in microbial respiration (Chen and others 2015). Although a prior study in a grassland experiment at Cedar Creek also found no effect of N addition on microbial respiration (Riggs and others 2015), other high-sand sites have demonstrated a reduction in microbial respiration with N addition (Zak and others 2016). Those sites had about 5 percentage points more silt/clay than Cedar Creek soils (~ 15% silt+clay compared to < 10%), which could have been enough to result in substantial increases in occluded particulate organic matter under N addition (Zak and others 2016).
Cedar Creek soils generally lack an organic horizon (Grigal and others 1974), including at sites used in this study, which could also help explain the lack of a N addition effect. The reported positive effect of N addition on soil C is most common in the organic horizon, and often not observed in mineral soil (Liu and Greaver 2010; Frey and others 2014; Maaroufi and others 2015). It is possible that mechanisms that lead to lower decomposition in the organic horizon with N addition are less prominent or not present in mineral soil. Specifically, in some temperate forests, N addition inhibits oxidative enzyme activity and lignin degradation (Zak and others 2008) and can also decrease the abundance of lignolytic fungi on wood and other high-lignin substrates (Entwistle and others 2018). A recent meta-analysis across ecosystem types also found reduced oxidative enzyme activities with N addition (Jian and others 2016). Similarly, prior work in the same Cedar Creek experiment used here showed negative effects of N addition on late-stage leaf litter decomposition (that is, higher asymptotes) in a subset of these sites (Hobbie and others 2012). It is possible, however, that N inhibition of lignin-degrading enzymes, lignolytic fungi, and late-stage litter decomposition is just more important in the organic horizon (Zak and others 2008), or even in mineral soils that have a well-developed organic horizon (Zak and others 2016).
Overall, because a positive effect of N addition on soil C does not appear consistently in mineral soil (Liu and Greaver 2010), as confirmed in this study, and could be less pronounced in sandy soil, it is worth using caution in extrapolating observed positive mean effects of N addition on soil C from certain systems (Janssens and others 2010) or organic soils (Liu and Greaver 2010) to global C models, and in understanding future carbon-climate feedbacks (Heimann and Reichstein 2008). Models that assume N addition increases soil C in all soils could overestimate C storage enhancement under increased N deposition, since much of soil C is below the organic horizon (Jobbágy and Jackson 2000).
Site-Specific Differences in N Responses
Despite controlling for climate, soil type, and N deposition history, and despite no main effects of N addition on soil cycling responses across all sites, we did find substantial differences in how soil C cycling responded to N addition—across sites, and even between sites with similar vegetation cover. This result highlights how seemingly similar landscapes can behave differently. Land use history may have played an important role here. Several of the sites in this study were previously cultivated likely contributing to their lower soil C content (McLauchlan and others 2006)—the two old fields, the aspen site, and likely Pine 1 (it is a plantation and, given local history, was likely an abandoned old field before that). Of all the characteristics we included in models, soil %C explained much of the variation in decay rates and pool sizes, which might be expected since C decay metrics were analyzed per gram soil. However that relationship may mask other important factors, as soil %C was highly positively correlated with soil %N, highly negatively correlated with pH, and moderately positively correlated with soil C:N, and microbial biomass C and N, although, as noted above, pH responses here are less relevant for mineral stabilization processes given the sandy soils, pH has been linked to microbial community composition (Rousk and others 2010) and microbial activity (Whittinghill and Hobbie 2011). Yet, given the strong correlation with %C, we cannot further disentangle the mechanisms behind the relationships with C cycling.
Coupled Root C and Microbial Biomass Responses to N Addition as Potential Drivers of Respiration Responses
The lack of a consistent N addition effect on cumulative microbial respiration was likely due to a minimal and inconsistent microbial biomass response to N. Reductions in microbial respiration (Riggs and Hobbie 2016) and total soil CO2 flux (Treseder 2008) with N addition have been shown to be associated with reductions in microbial biomass. However, unlike previous studies (Treseder 2008; Lu and others 2011; Liu and others 2015), here N addition generally had no effect, but did tend to reduce microbial biomass in some sites (the two grassland sites and one of the oak stands), and increase it in others (the aspen and maple sites).
Yet although microbial respiration did not respond to N addition in our study overall, sites with N-induced reductions in microbial respiration tended to have lower microbial biomass C with N addition, as well as lower root C in response to added N. Thus, we observed the expected coupling of responses of roots and microbes to N addition across sites. There were also some site-specific fine root chemistry responses to N addition (lower fine root C:N and increased % soluble cell contents most notably in the grassland sites). And, sites that responded to N addition with more of a reduction in fine root C:N also had more of a reduction in microbial biomass. However, these effects on root chemistry did not translate to differences in cumulative respiration, or decay rates of the fast or slow pool. It therefore appears that the site-specific N addition effects on microbial biomass may relate most closely to root C responses to N addition.
The question for further investigation, then, is what explains site variation in root C response to N addition (and hence microbial biomass C response). Our results also beg the question of why N addition did not consistently reduce microbial biomass C across our sites. Although declines in pH can inhibit microbial abundance and alter community composition (Rousk and others 2010), we observed only a marginally significant relationship between microbial biomass response to N addition and pH response. Instead, microbial biomass response to N addition was explained largely by the root C response to N addition.
Study Duration and Potential Shift in Responses to N Addition Over Time
An earlier study in this experiment (Keeler and others 2008) found a site*N addition interaction (P = 0.0093) for labile soil C decomposition rate responses to N addition after 5 years of treatment, with slightly lower labile decay rates with N addition in Field 1 and Oak 1, and a trend toward slightly higher decay rate in Pine 2 (from Figure 1b, Keeler and others 2008). The two studies sampled to different depths (20 cm in Keeler and others, 10 cm in the present study). Nevertheless, the decay rates of the fast pool were correlated between the two time points (P = 0.0192, R2 = 0.5343). However, unlike Keeler and others (2008), we found no N or site*N addition effects. There was no relationship between the responses of the decay rates to N addition between the two studies (P > 0.2), as there was no consistent shift in the response ratios: compared to the earlier study, sites showed shifts in magnitude and direction of response in a seemingly idiosyncratic fashion. This could be due to a change in the response of the drivers of respiration to N addition, or how respiration responds to those drivers. However, with the data available, we cannot say.
Conclusion
We found no evidence for soil C accumulation with long-term N addition across eight forested and grassland sites where climate, soil type, and N deposition history were similar Across sites, 12 years of N addition led to no general change in soil C stocks, microbial respiration per gram soil C, fast- and slow-cycling C pools or decay rates. This unexpected lack of effect occurred despite N-induced declines in pH across sites, and changes in microbial biomass C and N and decreased root lignin:N and C:N in some sites. Nevertheless, the site-to-site variability in microbial biomass response to N addition tracked closely with microbial respiration and root C responses to N addition, highlighting the connections between these processes. Additional questions remain regarding why root C—and then microbial biomass—responded positively in some sites and negatively in others. The sandy, nutrient-poor Cedar Creek soils could have contributed to the general lack of response, given little opportunity for organic matter stabilization on mineral surfaces and a low likelihood that N-induced acidification would increase availability of polyvalent cations that could facilitate organic matter bridging. However, our work indicates that prior findings that mineral soil C content tends not to respond to N addition hold for these sandy soils (Liu and Greaver 2010). Overall, the results reported here contradict the often-cited inhibitory effect of N addition on microbial respiration (Treseder 2008; Janssens and others 2010), and related build-up of stored soil C (Liu and Greaver 2010; Yue and others 2016), and suggest that modeling efforts that assume that N addition leads to lower microbial respiration or greater soil C content across soil types and horizons could overestimate future C storage under increasing N deposition.
References
Aber J, McDowell W, Nadelhoffer K, Magill A, Berntson G, Kamakea M, McNulty S, Currie W, Rustad L, Fernandez I. 1998. Nitrogen saturation in temperate forest ecosystems. BioScience 48:921–34
Adair E, Reich PB, Hobbie SE, Knops JMH. 2009. Interactive effects of time, CO2, N, and Diversity on total belowground carbon allocation and ecosystem carbon storage in a grassland community. Ecosystems 12:1037–52.
Angst G, Mueller KE, Kögel-Knabner I, Freeman KH, Mueller CW. 2017. Aggregation controls the stability of lignin and lipids in clay-sized particulate and mineral associated organic matter. Biogeochemistry 132:307–24.
Ågren G, Bosatta E, Magill A. 2001. Combining theory and experiment to understand effects of inorganic nitrogen on litter decomposition. Oecologia 128:94–8.
Berg B. 2014. Decomposition patterns for foliar litter—A theory for influencing factors. Soil Biol Biochem 78:222–32
Beven K. 2006. A manifesto for the equifinality thesis. J Hydrol 320:18–36
Bouwman AF, Van Vuuren DP, Derwent RG. 2002. A global analysis of acidification and eutrophication of terrestrial ecosystems. Water Air Soil Pollut 141:349–82.
Brookes PC, Landman A, Pruden G. 1985. Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–42.
Chen D, Li J, Lan Z, Hu S, Bai Y. 2015. Soil acidification exerts a greater control on soil respiration than soil nitrogen availability in grasslands subjected to long-term nitrogen enrichment. Funct Ecol 30:658–69.
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chhabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton P. 2013. Carbon and other biogeochemical cycles. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley PM (eds) Climate change 2013: the physical science basis. contribution of working group i to the fifth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA.
Clark CM, Cleland EE, Collins SL, Fargione JE, Gough L, Gross KL, Pennings SC, Suding KN, Grace JB. 2007. Environmental and plant community determinants of species loss following nitrogen enrichment. Ecol Lett 10:596–607.
Cleveland CC, Reed SC, Keller AB, Nemergut DR, O’Neill SP, Ostertag R, Vitousek PM. 2014. Litter quality versus soil microbial community controls over decomposition: a quantitative analysis. Oecologia 174:283–94.
Dungait JAJ, Hopkins DW, Gregory AS, Whitmore AP. 2012. Soil organic matter turnover is governed by accessibility not recalcitrance. Global Change Biol 18:1781–96.
Entwistle EM, Zak DR, Argiroff WA. 2018. Anthropogenic N deposition increases soil C storage by reducing the relative abundance of lignolytic fungi. Ecol Monogr 88:1–20
Frey SD, Ollinger S, Nadelhoffer K, Bowden R, Brzostek E, Burton A, Caldwell BA, Crow S, Goodale CL, Grandy AS, Finzi A, Kramer MG, Lajtha K, LeMoine J, Martin M, McDowell WH, Minocha R, Sadowsky JJ, Templer PH, Wickings K. 2014. Chronic nitrogen additions suppress decomposition and sequester soil carbon in temperate forests. Biogeochemistry 121:305–16.
Grigal DF, Chamberlain LM, Finney HR, Wroblewski DV, Gross ER. 1974. Soils of the cedar creek natural history area. Miscellaneous report 123. University of Minnesota, Agricultural Experiment Station, Saint Paul, Minnesota, USA
Grigal DF, Homann PS. 1994. Nitrogen mineralization, groundwater dynamics, and forest growth on a Minnesota outwash landscape. Biogeochemistry 27:171–85.
Gruber N, Galloway JN. 2008. An earth-system perspective of the global nitrogen cycle. Nature 451:293–6.
Heimann M, Reichstein M. 2008. Terrestrial ecosystem carbon dynamics and climate feedbacks. Nature 451:289–92.
Hendershot WH, Lalande H, Duquette M. 1993. Soil reaction and exchangeable acidity. In: Carter MR, Ed. Soil sampling and methods of analysis. Boca Raton: Lewis Publishers.
Hobbie S. 2008. Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–44.
Hobbie SE, Eddy WC, Buyarski CR, Adair EC, Ogdahl ML, Weisenhorn P. 2012. Response of decomposing litter and its microbial community to multiple forms of nitrogen enrichment. Ecol Monogr 82:389–405.
Hobbie SE, Ogdahl M, Chorover J, Chadwick OA, Oleksyn J, Zytkowiak R, Reich PB. 2007. Tree species effects on soil organic matter dynamics: the role of soil cation composition. Ecosystems 10:999–1018.
Hobbie SE. 2005. Contrasting effects of substrate and fertilizer nitrogen on the early stages of litter decomposition. Ecosystems 8:644–56.
Janssens IA, Dieleman W, Luyssaert S, Subke J-A, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze E-D, Tang J, Law BE. 2010. Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–22.
Jastrow JD, Amonette JE, Bailey VL. 2006. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim Change 80:5–23.
Jian S, Li J, Chen J, Wang G, Mayes MA, Dzantor KE, Hui D, Luo Y. 2016. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilization: a meta-analysis. Soil Biol Biochem 101:32–43.
Jobbágy EG, Jackson RB. 2000. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–36.
Keeler BL, Hobbie SE, Kellogg LE. 2008. Effects of long-term nitrogen addition on microbial enzyme activity in eight forested and grassland sites: implications for litter and soil organic matter decomposition. Ecosystems 12:1–15.
Knops JMH, Naeem S, Reich PB. 2007. The impact of elevated CO2, increased nitrogen availability and biodiversity on plant tissue quality and decomposition. Global Change Biol 13:1960–71.
LeBauer DS, Treseder KK. 2008. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–9.
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N. 2015. Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. Proc Natl Acad Sci 112:10967–72.
Liu L, Greaver TL. 2010. A global perspective on belowground carbon dynamics under nitrogen enrichment. Ecol Lett 13:819–28.
Liu L, Wang X, Lajeunesse MJ, Miao G, Piao S, Wan S, Wu Y, Wang Z, Yang S, Li P, Deng M. 2015. A cross-biome synthesis of soil respiration and its determinants under simulated precipitation changes. Global Change Biol 22:1394–405.
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B. 2011. Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–44.
Maaroufi NI, Nordin A, Hasselquist NJ, Bach LH, Palmqvist K, Gundale MJ. 2015. Anthropogenic nitrogen deposition enhances carbon sequestration in boreal soils. Global Change Biol 21:3169–80.
Manzoni S, Taylor P, Richter A, Porporato A, Ågren G. 2012. Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91.
McLauchlan KK, Hobbie SE, Post WM. 2006. Conversion from agriculture to grassland builds soil organic matter on decadal timescales. Ecol Appl 16:143–53.
Mueller KE, Eissenstat DM, Hobbie SE, Oleksyn J, Jagodzinski AM, Reich PB, Chadwick OA, Chorover J. 2012. Tree species effects on coupled cycles of carbon, nitrogen, and acidity in mineral soils at a common garden experiment. Biogeochemistry 111:601–14.
Neff JC, Townsend AR, Gleixner G, Lehman SJ, Turnbull J, Bowman WD. 2002. Variable effects of nitrogen additions on the stability and turnover of soil carbon. Nature 419:915–17.
Reich PB, Oleksyn J, Modrzyński J, Mrozinski P, Hobbie SE, Eissenstat DM, Chorover J, Chadwick OA, Hale CM, Tjoelker MG. 2005. Linking litter calcium, earthworms and soil properties: a common garden test with 14 tree species. Ecol Lett 8:811–18.
Reid JP, Adair EC, Hobbie SE, Reich PB. 2012. Biodiversity, nitrogen deposition, and CO2 affect grassland soil carbon cycling but not storage. Ecosystems 15:580–90.
Riggs CE, Hobbie SE, Bach EM, Hofmockel KS, Kazanski CE. 2015. Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125:203–19.
Riggs CE, Hobbie SE. 2016. Soil biology and biochemistry. Soil Biol Biochem 99:54–65.
Rousk J, Baath E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N. 2010. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–51.
Schimel JP, Schaeffer SM. 2012. Microbial control over carbon cycling in soil. Front Microbiol 3:348.
Schimel JP, Weintraub MN. 2003. The implications of exoenzyme activity on microbial carbon and nitrogen limitation in soil: a theoretical model. Soil Biol Biochem 35:549–63.
Treseder KK. 2008. Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–20.
Vitousek PM, Howarth RW. 1991. Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115.
Waldrop MP, Zak DR, Sinsabaugh RL, Gallo M, Lauber C. 2004. Nitrogen deposition modifies soil carbon storage through changes in microbial enzymatic activity. Ecol Appl 14:1172–7.
Wardle DA. 2004. Ecological linkages between aboveground and belowground biota. Science 304:1629–33.
Whittinghill KA, Hobbie SE. 2011. Effects of pH and calcium on soil organic matter dynamics in Alaskan tundra. Biogeochemistry 111:569–81.
Yue K, Peng Y, Peng C, Yang W, Peng X, Wu F. 2016. Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: a meta-analysis. Sci Rep 6:19895
Zak DR, Freedman ZB, Upchurch RA, Steffens M, Kögel-Knabner I. 2016. Anthropogenic N deposition increases soil organic matter accumulation without altering its biochemical composition. Global Change Biol 23:933–44.
Zak DR, Holmes WE, Burton AJ, Pregitzer KS, Talhelm AF. 2008. Simulated atmospheric NO3-deposition increases soil organic matter by slowing decomposition. Ecol Appl 18:2016–27.
Acknowledgements
This work was supported by the Cedar Creek Long-Term Ecological Research program (National Science Foundation (NSF) DEB-0080302) and by an NSF CAREER award (DEB-0347103). CEK was personally supported by a University of Minnesota College of Biological Sciences Graduate Excellence Fellowship and a NSF Graduate Research Fellowship (00039202). The authors would like to further acknowledge Chris Buyarski for assistance with experiment maintenance, Jake Grossman for help in the laboratory, and Allison Gill for help with fieldwork. Authors also thank Dave Tilman, Nancy Johnson, Jessica Gutknecht, Lauren Cline, Melissa Pastore, Craig See, Chris Walter, and Rachel King for helpful comments on manuscript drafts.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
CEK and SEH conceived study, CEK performed the research and analyzed the data, CEK, CER, PBR, and SEH all contributed to interpretation and framing, CEK wrote the first paper version, CEK, CER, PBR, and SEH all contributed to paper revisions.
Data available at
http://www.cedarcreek.umn.edu/research/data/experiment?e145.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kazanski, C.E., Riggs, C.E., Reich, P.B. et al. Long-Term Nitrogen Addition Does Not Increase Soil Carbon Storage or Cycling Across Eight Temperate Forest and Grassland Sites on a Sandy Outwash Plain. Ecosystems 22, 1592–1605 (2019). https://doi.org/10.1007/s10021-019-00357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-019-00357-x