Abstract
Background and aims
Grassland ecosystems play a vital role in soil carbon (C) sequestration and experience human disturbances that influence soil C storage. Nitrogen (N) enrichment elevates C assimilation and C return, and pervasive interactions between N and C inputs occur but have seldom been addressed simultaneously. This study evaluated the effects of N addition on the soil C pool under labile C input (glucose) and the underlying mechanisms.
Methods
In a full factorial designed temperate grassland field experiment treated with five different levels of N addition (0, 2.5, 5, 10 and 20 g N m−2 yr−1) and C addition (0, 25 and 50 g C m−2 yr−1) we tracked C pools in plant, litter and soil for 5 years.
Results
The soil C pool significantly increased with time, and was enhanced by increased N addition rates. Additionally, N enrichment (0–20 g N m−2 yr−1) caused a significant increase in C pools of fresh plants and litter. The positive response of the litter C pool to increasing N addition rates increased with time. The structural equation model revealed a trade-off between the fresh plant and soil C pools and a direct contribution of the litter C pool to the increase in the soil C pool under N input over the 5 years.
Conclusions
The intermediate rates of labile C input favored soil C storage by stimulating C transformation from plant to soil, but more labile C lessened the positive effects of continuous N addition in temperate grassland ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Grassland ecosystems cover approximately 40% of the terrestrial area on Earth and store one-third of the global carbon (C) (Bai and Cotrufo 2022; White et al. 2000). Globally, grasslands store considerably more C in soils than vegetation by 2.5–8.2 times, accounting for 579 Gg C in the upper 100 cm of the soil (White et al. 2000). Moreover, grasslands are highly vulnerable to human activities, e.g., a total of 150 Tg yr−1 anthropogenic nitrogen (N) is applied to the land surface due to increased fertilization and the production of N oxides from industry and vehicles (Bardgett et al. 2021; Schlesinger 2009). The input of N has been shown to significantly increase soil C storage in grasslands, which might strengthen the importance of this system as a C sink under increasing N input (Bai and Cotrufo 2022; Yue et al. 2016).
Enhanced plant N uptake caused by N addition increases leaf N contents and plant photosynthesis and ultimately stimulates plant growth in N-limited grasslands (Beier et al. 2009; Yue et al. 2016). Consequently, more C is assimilated into plant biomass and then increases labile C inputs, such as plant litter and roots exudates to soils (Fleischer et al. 2019; Xu et al. 2021). Several meta-analyses reported that the fresh plant C pool, litter C pool, and soil C pool significantly increased under N addition in temperate grassland ecosystems, especially at higher N addition rates (Hou et al. 2021; Song et al. 2019; Yue et al. 2016). However, opposite results have often been reported. For example, N addition simultaneously increased the plant C pool and recalcitrant plant C compounds, thereby slowing litter turnover without significant effects on soil C (Yang et al. 2019a). Likewise, a long-term field experiment found that N addition increased total plant inputs, yet this increase did not translate to greater soil C storage (Keller et al. 2022). In addition, responses of C pools such as soil C and litter C to N addition have been reported to differ with the experimental duration, in which the soil C pool weakly increased with an increasing duration, while the addition of N only accelerated early-stage decomposition of litter (Gill et al. 2022; Yue et al. 2016).
Since C and N cycles are highly coupled in terrestrial ecosystems, C pools will indeed exhibit positive or negative feedback to N enrichment and in return differentially regulate the effects of N enrichment on C storage (Hyvönen et al. 2007; Yue et al. 2016). For example, labile C input may be used as a source or energy to promote decomposition of litter C and thus increase soil C capture and storage (Fleischer et al. 2019; Yue et al. 2016). Alternatively, inputs of labile C may stimulate the mineralization of soil C, causing greater C loss in soils (Hicks et al. 2019; Yue et al. 2016). Therefore, the balances among plant C, litter C and soil C will determine whether and how C sequestration varies in response to the increasing N addition rates. However, due to limited literature, how soil C will be influenced by a future increase in plant-derived C combined with N enrichment is still poorly understood.
The main objective of this study was to investigate and quantify the coeffects of labile C and N addition on the soil C pool and the underlying mechanisms. Specifically, the following questions are addressed: (1) what is the contribution of N enrichment to plant C, litter C and soil C pools among years in grasslands? (2) does labile C input influence the effects of N enrichment on C pools in grasslands, and if so, how? Nitrogen addition increases plant productivity in N-limited ecosystems (Zhang et al. 2015), and some studies suggested that critical thresholds of plant productivity occur in response to increasing N inputs, including the area of this study (Groffman et al. 2006; Yang et al. 2023). Thus, we first hypothesized that continuous N addition could increase both fresh plant C and litter C pools and consequently soil C pool, sometimes with a threshold. In addition, labile C addition stimulates N immobilization under N treatments (Ning et al. 2021), and thus our second hypothesis is labile C inputs could lessen the positive effects of continuous N addition on C pools. To answer these questions, an experiment with five levels of N addition and three levels of labile C addition was conducted in a temperate grassland. Fresh plants, litter, and soils were sampled annually for five consecutive years, and the C pools, N pools and C:N ratios were examined. Variations in these indices and correlations between them and varying rates of N and C addition over five consecutive years were quantified, further providing insights into the causes and consequences of soil C pool changes in response to N enrichment in temperate grasslands.
Materials and methods
Study sites and experimental design
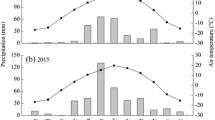
The trial was initiated in May 2014 at the Erguna Forest-Grassland Ecotone Ecosystem Research Station (50°12′19″ N, 119°30′28″ E) in Inner Mongolia, China. The experimental field was a native grassland that large grazers were excluded for several years (Luo et al. 2023). We conducted the study in a disturbance-suppressed grassland (no fire, no cattle, and no mowing). The region is characterized by a meadow steppe and chernozems, with a mean temperature of -2 °C and a mean annual precipitation of 336 mm. The initial values of soil C, soil N, and bulk density were 30.45 ± 0.28 g kg−1, 2.65 ± 0.02 g kg−1 and 1.12 ± 0.03 g cm−3, respectively. Weather data in the study area in 2014–2018, including the average maximum temperature, the average minimum temperature, and the annual precipitation, are shown in Table 1.
The split-plot experimental design consisted of 30 plots (each 10 m × 10 m in size) with five N concentrations in a randomized block design with six replicates per treatment (Fig. S1). To simulate N enrichment, N addition rates were 0, 2.5, 5.0, 10.0 and 20.0 g N m−2 year−1 and denoted as N0, N2.5, N5, N10 and N20, respectively. Within each plot, three subplots (3 m × 3 m) were arranged with C addition rates of 0, 25 and 50 g C m−2 year−1 and denoted as C0, C25 and C50, respectively (Fig. S1). Consequently, a total of 90 subplots were set up. N addition was applied as granular urea in early May annually. Moreover, glucose (used for C addition) was weighed, mixed with water, and sprinkled evenly on the soil surface using a sprayer. To provide a clear schedule of the experiment, a research flowchart is shown in Fig. S2.
Vegetation sampling and measurements
For each subplot, aboveground plant parts (by cutting all plants) and litter were collected within two 25 cm × 25 cm grids in August from 2014 to 2018 (denoted as D1-D5, respectively, to show the experimental durations). The plant species and litter were separately put into a paper bag, dried for 48 h at 80 °C and weighed to determine the biomass. The dried plants and litter were then ground and the concentrations of plant total C and plant total N were measured using a Vario MACRO cube analyzer (Elementar Analysensysteme Vario MACRO cube, Germany). Then, the C or N pool in plant/litter (g m−2) was calculated as follows: C or N concentration (g kg−1) × plant/litter biomass (kg m−2).
Soil sampling and measurements
Soil samples were collected in August from 2014 to 2018. In each subplot, five undisturbed topsoil samples (0–10 cm) were collected using a soil core (3-cm diameter) and composited into a single sample. After removal of plant residues and stones, each soil sample was homogenized, passed through a 2-mm sieve, and then air-dried to measure the concentrations of soil C and soil N using a Vario MACRO cube analyzer (Elementar Analysensysteme Vario MACRO cube, Germany). The C and N pools (0–10 cm) were calculated based on the following equation: C or N pool in soils (g m−2) = C or N concentration (g kg−1) × soil bulk density (1,120 kg m−3) × soil depth (0.1 m).
Statistical analyses
A three-way ANOVA was carried out to analyze the effects of different factors (C addition rate, N addition rate, and duration) and their interactions on the C/N pool of plants and soil. The results are shown in Table 2, in which the interactions without any significant effect were removed. Regression with the least squares method was used to determine the responses of plant and soil properties to the N addition rate and duration. Pearson correlations between different C pools were calculated. All analyses described above were performed and visualized in R (version 3.6.2).
To estimate the responses of C pools (including fresh plant C pool, litter C pool, and soil C pool) to N addition rates and their correlations under three levels of C addition over five successive years, structural equation models were established using AMOS (version 21.0.0) and SPSS 16.0 software. The maximum likelihood χ2 test, comparative fit index (CFI), and root mean square error of approximation (RMSEA) were used to assess the model fitness.
Results
Effects of N addition rate, duration, and C addition rate on fresh plant C and N pools
The fresh plant C pool was significantly influenced by the N addition rate, duration, C addition rate and the interaction between the N addition rate and duration as well as the interaction between the C addition rate and duration (Table 2). The plant C pool significantly increased with increasing N addition rates whether labile C was added or not (Fig. 1a), and the slopes of the linear models between the plant C pool and N addition rates were not significantly correlated with duration (Fig. 1a insert). In addition, the plant C pool was significantly decreased with the input of C at both addition rates (Fig. 1b insert) and only significantly increased with duration under the input of labile C (Fig. 1b). The plant N pool showed a similar trend to that of the plant C pool (Table 2 and Fig. S3).
Relationship between plant C pools and plant C:N ratio and N addition rates (a, c) and duration (b, d). Linear regression equations are labeled. The correlations between the slope of the linear models and duration are inserted in (a) and (c). D1-D5 indicate a duration of 1–5 years of N input, respectively. The comparison of the plant C pool and plant C:N ratio between different C addition rates is shown as inserted boxplots (Duncan’s test, P < 0.05) in (b) and (d), respectively. C0, C25, and C50 indicate inputs of 0, 25 and 50 g C m−2 year−1, and are labeled in red, green, and blue, respectively. The lowercase letters indicate significant differences between different C addition rates at the P < 0.05 level. *, ** and *** indicate the significance at P < 0.05, P < 0.01 and P < 0.001, respectively
The fresh plant C:N ratio was only significantly influenced by the N addition rate, duration, and the interaction between the N addition rate and duration (Table 2). In addition, the fresh plant C:N ratio significantly decreased with increasing N addition rates, which could be enhanced by duration (Fig. 1c), while C addition had little effect on the plant C:N ratio (Fig. 1d).
Effects of N addition rate, duration, and C addition rate on litter C and N pools
Likewise, the litter C pool was significantly influenced by the N addition rate, duration, C addition rate and the interaction between the N addition rate and duration (Table 2). Specifically, the litter C pool significantly increased with increasing N addition rates and duration at all rates of C addition (Fig. 2a and b). The slopes of the linear models between the litter C pool and N addition rate significantly increased with duration (Fig. 2a insert). In addition, although a downward trend in the litter C pool with C input was detected, there was no significant difference between different rates of C addition (Fig. 2b). The litter N pool showed a similar trend to that of the litter C pool (Table 2 and Fig. S4).
Litter C pool (a, b) and C:N ratio (c, d) across the samples. (a) Relationship between litter C pools and N addition rates. (b) The comparison of the litter C pool between different C addition rates is shown as boxplots (Duncan’s test, P < 0.05). The relationship between litter C:N ratio and N addition rate and duration is shown in (c) and (d), respectively. Linear regression models and equations are labeled. The correlations between the slope of the linear models and duration are inserted in (a) and (c). The comparison of the litter C:N ratio between different C addition rates is shown as inserted boxplots (Duncan’s test, P < 0.05) in (d). D1-D5 indicate a duration of 1–5 years of N addition, respectively. C0, C25, and C50 indicate inputs of 0, 25 and 50 g C m−2 year−1, and are labeled in red, green, and blue, respectively. The lowercase letters indicate significant differences between different C addition rates at the P < 0.05 level. *, ** and *** indicate the significance at P < 0.05, P < 0.01 and P < 0.001, respectively
The litter C:N ratio significantly decreased with increasing N addition rates but not with duration (Fig. 2c and d), and the slopes of the linear models between the litter C:N ratio and N addition rate were significantly correlated with duration in a quadratic regression model (Fig. 2c inserted plot). In addition, the litter C:N ratio increased with the addition of labile C, increasing significantly at the higher addition rate (Fig. 2d inserted plot).
Effects of N addition rate, duration, and C addition rate on soil C and N pools
Duration, C addition rate and the interaction between N addition rate and duration significantly influenced the soil C and N pools (Table 2). Specifically, the soil C pool significantly increased with increasing N addition rates at D1-D5 (Fig. 3a), and the slopes of these linear models were significantly correlated with N addition rates in a quadratic regression model (Fig. 3a inserted plot). The soil C pool was significantly the highest with the input of C at a lower addition rate (Fig. 3b). Similar results were detected in the soil N pool (Fig. S5).
Soil C pool (a and b) and C:N ratio (c and d) across the samples. (a) Relationship between soil C pools and duration. Linear regression models and equations are labeled. The correlations between the slope of the linear models and duration are inserted. (b) The comparison between different C addition rates is shown as boxplots (Duncan’s test, P < 0.05). The relationship between soil C:N ratio and N addition rates and duration is shown in (c) and (d), respectively. Linear regression models and equations are labeled. The comparison of the soil C:N ratio between different C addition rates is shown as inserted boxplots (Duncan’s test, P < 0.05) in (c). C0, C25, and C50 indicate inputs of 0, 25 and 50 g C m−2 year−1, and are labeled in red, green, and blue, respectively. D1-D5 indicate a duration of 1–5 years of N addition, respectively. The lowercase letters indicate significant differences between different C addition rates at the P < 0.05 level. *, ** and *** indicate the significance at P < 0.05, P < 0.01 and P < 0.001, respectively
However, the soil C:N ratio was not significantly influenced by C addition (Fig. 3c inserted plot), but was significantly decreased with increasing N addition rates (Fig. 3c) and significantly increased with duration (Fig. 3d).
Structural equation model
Significant correlations were detected between the pools of fresh plant, litter, and soil (Fig. 4). The structural equation model showed that N addition rates had significant and positive direct effects on both the fresh plant C pool and litter C pool at all C addition rates, but only significantly influenced the soil C pool in samples without labile C addition (Fig. 5). Labile C addition, especially at the higher addition rate (C50), enhanced and decreased the fresh plant C pool and litter C pool, respectively (Fig. 5). Duration had significant and positive direct effects on litter C and soil C at all rates of C input, but significantly and directly influenced fresh plant C only when labile C was added (Fig. 5).
Structural equation model for the effects of the N addition rate on C pools under three C addition rates. Goodness-of-fit statistics: chi-square = 0.0, df = 3, P = 1.0, comparative fit index (CFI) = 1.0, root mean square error of approximation (RMSEA) = 0.0. Numbers between variables are standardized partial regression coefficients. Forward slashes separate numbers under three C addition rates, including 0 (black), 25 (red), and 50 (blue) g m−2 year−1. *, ** and *** indicate the significance at P < 0.05, P < 0.01 and P < 0.001, respectively
In addition, the fresh plant C pool had a significant and positive direct effect on the litter C pool at all C addition rates, which further significantly and positively increased the soil C pool at the C0 and C25 rates but not at the C50 rate. Likewise, the fresh plant C pool had significant and negative direct effects on the soil C pool at the C0 and C25 rates (Fig. 5).
Discussion
Fresh plant and litter C pools increased with N enrichment, but responded differently with duration
In this study, first, partially as expected, experimental N addition caused a significant increase in the size of the C pool in both fresh plants and litter by 1.1–3.0 times and 1.1–3.9 times, respectively, when the effects of the experimental duration were not considered (Figs. 1a and 2a). N addition has been reported to promote plant growth and significantly increase plant biomass in temperate grasslands, which consequently contributes to enhanced plant C pools (Yang et al. 2022b; Yue et al. 2016). The size of plant C pools was further significantly accelerated by an increasing N addition rate, which is also common in most available studies (Lu et al. 2021; Yang et al. 2022b). However, neither plant C pool nor litter C pool had a threshold in response to the increasing N rates, which we believed it was, at least partially, contributed to the experimental duration. A linear relationship between the plant productivity and N addition rates was reported at the initial years of the experiment (Zhang et al. 2015), and the responses of plant productivity to the N addition rates always changed among different years, even after a few years of experimentation (Isbell et al. 2013; Wang et al. 2019; Yang et al. 2023). Thus we supposed that the thresholds of plant and litter C pools under N addition may occur after a period in addition to be influenced by climate. Moreover, the duration of N addition did not have a positive relationship with the effects of N addition rates on the C pool in fresh plants, although it substantially changed the linear relationships between them (Fig. 1a inserted plot). More surprisingly, the fresh plant C pool was substantially lower in D2-D4 than in D1 and D5. Plant biomass is highly dependent on precipitation in temperate grasslands (Hao et al. 2018; Hovenden et al. 2019). In our study, the annual precipitation was indeed lower in D2-D4, and we inferred that lower precipitation could, at least partially, attenuate the effects of N addition rates on plant biomass accumulation, and then on fresh plant biomass C. However, in contrast, the duration of N addition significantly enhanced the upward trends of the litter C pool with increasing N addition rates. The cumulative effect is likely because the grassland had never been mowed before our experiment, and changes in plant biomass in one year had little influence on the whole C pool in litter. Therefore, under consecutive years of N addition, the C pool in fresh plants may be a mirror of nutrient assimilation for the current year, while the C pool in litter may reflect the potential for nutrient release to soils.
Soil C pool sequestered over time in response to N enrichment
Regarding the soil C pool, a significant increase over time was observed in this study, independent of the N treatments (Fig. 3a). Temporal trends in soil C accumulation have been reported in aggrading grasslands where plants turn over annually (Pastore et al. 2021; Yang et al. 2019b). Likewise, N addition has often been reported to increase terrestrial soil C pools globally (Deng et al. 2020; Fleischer et al. 2019). However, unlike our first hypothesis, the soil C pool only significantly increased when the highest rate of N was added in the fifth year (Fig. 3a), suggesting that the effects of N addition on the soil C pool could not be achieved in a short-term treatment, even with a relatively high addition rate. Nevertheless, our results found that increasing N addition rates significantly enhanced the accumulation of the soil C pool over time, with a point of inflection (Fig. 3a inserted plot). For example, it took four years to significantly increase soil C by 10.6% in treatments without N addition, while a significant increase in soil C had occurred at 3 years of N input at any rate (Fig. 3a). Alternatively, increases in the soil C pool over time in the fifth year peaked at 24.4% with 10.0 m−2 yr−1 N addition, and then decreased to 23.1% when the N addition rate was 20.0 m−2 yr−1 (Fig. 3a). In this case, N addition indeed had a positive effect on soil C sequestration, as previously reported (Fleischer et al. 2019). However, it should be pointed out that we only examined the soil C pool at the surface layer, considering applications of C and N without tillage. Movements of nutrients and their effects on the soil C pool are also important and needed to be investigated in the future. In addition, because N pools increased more in fresh plant and litter in response to N addition rates, downward trends in the C:N ratio of both fresh plant and litter with increasing N addition rates were found, which were enhanced by the experimental duration (Figs. 1c, 2c, S3a, and S4a). Plants with a lower plant C:N ratio may decompose more easily and release more nutrients back to soils (Fleischer et al. 2019; Pastore et al. 2021; Yang et al. 2022b). Unlike plants, the soil C:N ratio significantly decreased with N addition rates but increased with duration (Fig. 3c-d). Litter decomposition increases as soil C:N ratio decreases (Blanco et al. 2023), further strengthens the importance of plant C pools under N addition in grasslands. Overall, the soil C pool accumulated more than the soil N pool over time in response to N enrichment.
Plant-soil C pool relationship and its underlying mechanisms in response to N enrichment under C addition
N addition rates had only a weak direct effect on the soil C pool in the structural equation model. Then, we explored the relationships between the C pools of plants and soil in response to consecutive years of different levels of N addition. In addition to a positive effect of the litter C pool on the soil C pool, there was a trade-off between the fresh plant C pool and the soil C pool in response to N addition rates. A trade-off between plant and soil C was also reported under elevated CO2, although positive responses of both were observed (Terrer et al. 2021). This trade-off seemed to be attributed to plant nutrient acquisition, in which plants mine the soil to increase their biomass when plant growth is severely limited by other nutrients (Jiang et al. 2020; Terrer et al. 2021). The study highlighted that the trade-off was a key point but always overlooked (Terrer et al. 2021). The same applies in our study, and the final fate of soil C also depends on whether litter C could make up for the consumption of soil C.
More importantly, the annual input of 25 g labile C m-2 significantly enhanced the soil C pool by 0.12 kg C m-2 (at a depth of 10 cm) across the samples (Fig. 3b), indicating that labile C may also benefit to soil C sequestration in response to N addition. Since N input stimulates C return driven by plant C pools (Fleischer et al. 2019; Huang et al. 2020) as discussed above, further investigations on the effects of labile C on the response of C pools of both fresh plants and litter were also conducted. First, a lower rate of labile C addition increased the direct and positive effects of N addition rates and duration on the fresh plant C pool, while those on the litter C pool showed the opposite pattern. The scenarios were contrary to our second hypothesis and suggested that a lower input of labile C, like plant-derived C return, would enhance the positive effects of continuous N addition on the fresh plant C pool on the one hand. Then, the trade-off of C pools between fresh plants and soil increased. On the other hand, the input of labile C stimulates the decomposition of litter C, thereby promoting the coupling between the C pools of soil and litter, i.e., transforming C from litter to soil. The input of labile C has often been reported to increase microbial growth and activity in soils, which could compete with plants for nutrients as well as decompose litter (Mehnaz et al. 2018; Ning et al. 2021). Our results suggested that the litter C pool occupied the central stage after calculating the sum of effects in the structural equation model, which was enhanced by a lower rate of labile C addition.
When the addition rate of labile C increased, the positive effect on the fresh plant C pool weakened, but the stimulation of litter decomposition remained. The results suggested that the importance of litter decomposition became stronger with a high rate of labile C addition, which could also be confirmed by the significantly changed litter C:N ratio at a higher C addition rate, although the litter C pool was not changed in this study. In this case, the litter C pool may be more active. However, there was no corresponding increase in the soil C pool, indicating that native soil C was also decomposed more with a high rate of C addition (Kundu et al. 2007). Furthermore, the contributions of the C pools of fresh plants and litter to the soil C pool disappeared, indicating that the relationships between them were uncoupled when more liable C was added. Although the root C pool was not examined in this study, meta-analyses demonstrated that additional N input significantly decreased root biomass in N-limited grasslands (Bai et al. 2021; Peng et al. 2017). In this case, plants should not invest more C to roots to acquire N when experimental N was added (Li et al. 2011; Peng et al. 2017; Yang et al. 2022a). Therefore, although N input maintains a positive effect on C sequestration in grassland (Fleischer et al. 2019), the outlook of soil carbon sequestration may be grim due to more C released by plants caused by continuous N input. At present, C loss in gaseous forms cannot be ignored and needs to be given more attention in the future.
Conclusion
The soil C pool significantly increased with duration, which was enhanced by N enrichment up to a limit. Likewise, the size of C pools in fresh plants and litter significantly increased under experimental N addition, both of which were enhanced by increasing N addition rates, but only the latter was enhanced by duration. There was a trade-off between the fresh plant C pool and soil C pool, and the litter C pool contributed to the increase in the soil C pool in response to the five-year continuous addition of different levels of N under labile C input. Moreover, an intermediate rate of labile C input favored soil C storage by stimulating the transformation of plant C to soil. However, when more labile C is added, the positive effects of continuous N addition on C pools are weakened in temperate grassland ecosystems.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Bai TS, Wang P, Ye CL, Hu SJ (2021) Form of nitrogen input dominates N effects on root growth and soil aggregation: A meta-analysis. Soil Biol Biochem 157. https://doi.org/10.1016/j.soilbio.2021.108251
Bai Y, Cotrufo MF (2022) Grassland soil carbon sequestration: Current understanding, challenges, and solutions. Science 377:603–608. https://doi.org/10.1126/science.abo2380
Bardgett RD, Bullock JM, Lavorel S, Manning P, Schaffner U, Ostle N, Chomel M, Durigan G, L Fry E, Johnson D (2021) Combatting global grassland degradation. Nat Rev Earth Environ 2:720-735.https://doi.org/10.1038/s43017-021-00207-2
Beier C, Emmett BA, Tietema A, Schmidt IK, Penuelas J, Lang EK, Duce P, De Angelis P, Gorissen A, Estiarte M, de Dato GD, Sowerby A, Kroel-Dulay G, Lellei-Kovacs E, Kull O, Mand P, Petersen H, Gjelstrup P, Spano D (2009) Carbon and nitrogen balances for six shrublands across Europe. Glob Biogeochem Cycles 23:Gb4008. https://doi.org/10.1029/2008gb003381
Blanco JA, Durán M, Luquin J, San Emeterio L, Yeste A, Canals RM (2023) Soil C/N ratios cause opposing effects in forests compared to grasslands on decomposition rates and stabilization factors in southern European ecosystems. Sci Total Environ 888:164118
Deng L, Huang C, Kim D, Shangguan Z, Wang K, Song X, Peng C (2020) Soil GHG fluxes are altered by N deposition: New data indicate lower N stimulation of the N2O flux and greater stimulation of the calculated C pools. Glob Change Biol 26. https://doi.org/10.1111/gcb.14970
Fleischer K, Dolman AJ, Molen MKVD, Rebel KT, Erisman JW, Wassen MJ, Pak B, Lu X, Rammig A, Wang Yi (2019) Nitrogen Deposition Maintains a Positive Effect on Terrestrial Carbon Sequestration in the 21st Century Despite Growing Phosphorus Limitation at Regional Scales. Glob Biogeochem Cycles 33. https://doi.org/10.1029/2018GB005952
Gill AL, Adler PB, Borer ET, Buyarski CR, Cleland EE, D’Antonio CM, Davies KF, Gruner DS, Harpole WS, Hofmockel KS, MacDougall AS, McCulley RL, Melbourne BA, Moore JL, Morgan JW, Risch AC, Schütz M, Seabloom EW, Wright JP, Yang LH, Hobbie SE (2022) Nitrogen increases early-stage and slows late-stage decomposition across diverse grasslands. J Ecol 110:1376–1389. https://doi.org/10.1111/1365-2745.13878
Groffman P, Baron J, Blett T, Gold A, Goodman I, Gunderson L, Levinson B, Palmer M, Paerl H, Peterson G, Poff N, Rejeski D, Reynolds J, Turner M, Weathers K, Wiens J (2006) Ecological thresholds: The key to successful environmental management or an important concept with no practical application? Ecosystems 9:1–13. https://doi.org/10.1007/s10021-003-0142-z
Hao T, Song L, Goulding K, Zhang F, Liu X (2018) Cumulative and partially recoverable impacts of nitrogen addition on a temperate steppe. Ecol Appl 28:237–248. https://doi.org/10.1002/eap.1647
Hicks LC, Meir P, Nottingham AT, Reay DS, Stott AW, Salinas N, Whitaker J (2019) Carbon and nitrogen inputs differentially affect priming of soil organic matter in tropical lowland and montane soils. Soil Biol Biochem 129:212–222. https://doi.org/10.1016/j.soilbio.2018.10.015
Hou SL, Hattenschwiler S, Yang JJ, Sistla S, Wei HW, Zhang ZW, Hu YY, Wang RZ, Cui SY, Lu XT, Han XG (2021) Increasing rates of long-term nitrogen deposition consistently increased litter decomposition in a semi-arid grassland. New Phytol 229:296–307. https://doi.org/10.1111/nph.16854
Hovenden MJ, Leuzinger S, Newton PCD, Fletcher A, Fatichi S, Lüscher A, Reich PB, Andresen LC, Beier C, Blumenthal DM (2019) Globally consistent influences of seasonal precipitation limit grassland biomass response to elevated CO2. Nat Plants 5:167–173
Huang X, Terrer C, Dijkstra FA, Hungate BA, Groenigen K (2020) New soil carbon sequestration with nitrogen enrichment: a meta-analysis. Plant Soil. https://doi.org/10.1007/s11104-020-04617-x
Hyvönen R, Ågren GI, Linder S, Persson T, Cotrufo MF, Ekblad A, Freeman M, Grelle A, Janssens IA, Jarvis PG (2007) The likely impact of elevated [CO2], nitrogen deposition, increased temperature and management on carbon sequestration in temperate and boreal forest ecosystems: a literature review. New Phytol 173(3):463–480. https://doi.org/10.1111/j.1469-8137.2007.01967.x
Isbell F, Reich PB, Tilman D, Hobbie SE, Polasky S, Binder S (2013) Nutrient enrichment, biodiversity loss, and consequent declines in ecosystem productivity. Proc Natl Acad Sci USA 110:11911–11916. https://doi.org/10.1073/pnas.1310880110
Jiang MK, Medlyn BE, Drake JE, Duursma RA, Anderson IC, Barton CVM, Boer MM, Carrillo Y, Castaneda-Gomez L, Collins L, Crous KY, De Kauwe MG, dos Santos BM, Emmerson KM, Facey SL, Gherlenda AN, Gimeno TE, Hasegawa S, Johnson SN, Kannaste A, Macdonald CA, Mahmud K, Moore BD, Nazaries L, Neilson EHJ, Nielsen UN, Niinemets U, Noh NJ, Ochoa-Hueso R, Pathare VS, Pendall E, Pihlblad J, Pineiro J, Powell JR, Power SA, Reich PB, Renchon AA, Riegler M, Rinnan R, Rymer PD, Salomon RL, Singh BK, Smith B, Tjoelker MG, Walker JKM, Wujeska-Klause A, Yang JY, Zaehle S, Ellsworth DS (2020) The fate of carbon in a mature forest under carbon dioxide enrichment. Nature 580: 227-+. https://doi.org/10.1038/s41586-020-2128-9
Keller AB, Borer ET, Collins SL, DeLancey LC, Fay PA, Hofmockel KS, Leakey ADB, Mayes MA, Seabloom EW, Walter CA, Wang Y, Zhao Q, Hobbie SE (2022) Soil carbon stocks in temperate grasslands differ strongly across sites but are insensitive to decade-long fertilization. Global Change Biol 28:1659–1677. https://doi.org/10.1111/gcb.15988
Kundu S, Bhattacharyya R, Prakash V, Ghosh BN, Gupta HS (2007) Carbon sequestration and relationship between carbon addition and storage under rainfed soybean-wheat rotation in a sandy loam soil of the Indian Himalayas. Soil till Res 92:87–95. https://doi.org/10.1016/j.still.2006.01.009
Li JZ, Lin S, Taube F, Pan QM, Dittert K (2011) Above and belowground net primary productivity of grassland influenced by supplemental water and nitrogen in Inner Mongolia. Plant Soil 340:253–264. https://doi.org/10.1007/s11104-010-0612-y
Lu XT, Hou SL, Reed S, Yin JX, Hu YY, Wei HW, Zhang ZW, Yang GJ, Liu ZY, Han XG (2021) Nitrogen Enrichment Reduces Nitrogen and Phosphorus Resorption Through Changes to Species Resorption and Plant Community Composition. Ecosystems 24:602–612. https://doi.org/10.1007/s10021-020-00537-0
Luo WT, Muraina TO, Griffin-Nolan RJ, Ma W, Song L, Fu W, Yu Q, Knapp AK, Wang ZW, Han XG, Collins SL (2023) Responses of a semiarid grassland to recurrent drought are linked to community functional composition. Ecology 104: e3920. https://doi.org/10.1002/ecy.3920
Mehnaz KR, Keitel C, Dijkstra FA (2018) Effects of carbon and phosphorus addition on microbial respiration, N 2 O emission, and gross nitrogen mineralization in a phosphorus-limited grassland soil. Biol Fertil Soils. https://doi.org/10.1007/s00374-018-1274-9
Ning Q, Httenschwiler S, Lü X, Kardol P, Han X (2021) Carbon limitation overrides acidification in mediating soil microbial activity to nitrogen enrichment in a temperate grassland. Global Change Biol. https://doi.org/10.1111/gcb.15819
Pastore MA, Hobbie SE, Reich PB (2021) Sensitivity of grassland carbon pools to plant diversity, elevated CO 2, and soil nitrogen addition over 19 years. Proc Natl Acad Sci USA 118:e2016965118. https://doi.org/10.1073/pnas.2016965118
Peng YF, Guo DL, Yang YH (2017) Global patterns of root dynamics under nitrogen enrichment. Glob Ecol Biogeogr 26:102–114. https://doi.org/10.1111/geb.12508
Schlesinger WH (2009) On the fate of anthropogenic nitrogen. Proc Natl Acad Sci U S A 106:203–208. https://doi.org/10.1073/pnas.0810193105
Song J, Wan S, Piao S, Knapp AK, Classen AT, Vicca S, Ciais P, Hovenden MJ, Leuzinger S, Beier C (2019) A meta-analysis of 1,119 manipulative experiments on terrestrial carbon-cycling responses to global change. Nat Ecol Evol 3:1309–1320. https://doi.org/10.1038/s41559-019-0958-3
Terrer C, Phillips RP, Hungate BA, Rosende J, Pett-Ridge J, Craig ME, van Groenigen KJ, Keenan TF, Sulman BN, Stocker BD, Reich PB, Pellegrini AFA, Pendall E, Zhang H, Evans RD, Carrillo Y, Fisher JB, Van Sundert K, Vicca S, Jackson RB (2021) A trade-off between plant and soil carbon storage under elevated CO2. Nature 591:599-+. https://doi.org/10.1038/s41586-021-03306-8
Wang J, Gao Y, Zhang Y, Yang J, Smith MD, Knapp AK, Eissenstat DM, Han X (2019) Asymmetry in above- and belowground productivity responses to N addition in a semi-arid temperate steppe. Glob Chang Biol 25:2958–2969. https://doi.org/10.1111/gcb.14719
White RP, Murray S, Rohweder M, Prince S, Thompson K (2000) Grassland ecosystems. World Resources Institute Washington, DC, USA
Xu CH, Xu X, Ju CH, Chen HYH, Wilsey BJ, Luo YQ, Fan W (2021) Long-term, amplified responses of soil organic carbon to nitrogen addition worldwide. Global Change Biol 27:1170–1180. https://doi.org/10.1038/s41559-019-0958-10.1111/gcb.15489
Yang GJ, Hautier Y, Zhang ZJ, Lu XT, Han XG (2022a) Decoupled responses of above- and below-ground stability of productivity to nitrogen addition at the local and larger spatial scale. Glob Change Biol 28:2711–2720. https://doi.org/10.1111/gcb.16090
Yang GJ, Stevens C, Zhang ZJ, Lu XT, Han XG (2023) Different nitrogen saturation thresholds for above-, below-, and total net primary productivity in a temperate steppe. Glob Change Biol 29:4586–4594. https://doi.org/10.1111/gcb.16803
Yang S, Liu WX, Guo LL, Wang CZ, Deng MF, Peng ZY, Liu LL (2022b) The changes in plant and soil C pools and their C:N stoichiometry control grassland N retention under elevated N inputs. Ecol Appl 32. https://doi.org/10.1002/eap.2517
Yang S, Liu WX, Qiao CL, Wang J, Deng MF, Zhang BB, Liu LL (2019a) The decline in plant biodiversity slows down soil carbon turnover under increasing nitrogen deposition in a temperate steppe. Funct Ecol 33:1362–1372. https://doi.org/10.1111/1365-2435.13338
Yang Y, Tilman D, Furey G, Lehman C (2019b) Soil carbon sequestration accelerated by restoration of grassland biodiversity. Nat Commun 10:1–7. https://doi.org/10.1038/s41467-019-08636-w
Yue K, Peng Y, Peng CH, Yang WQ, Peng X, Wu FZ (2016) Stimulation of terrestrial ecosystem carbon storage by nitrogen addition: a meta-analysis. Sci Rep-Uk 6. https://doi.org/10.1038/srep19895
Zhang YH, Feng JC, Isbell F, Lu XT, Han XG (2015) Productivity depends more on the rate than the frequency of N addition in a temperate grassland. Sci Rep-Uk 5:12558. https://doi.org/10.1038/srep12558
Acknowledgements
We gratefully appreciate all our colleagues who worked on this field trial at the Erguna Forest-Grassland Ecotone Ecosystem Research Station. This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA28090300), the Natural Science Foundation of China (41877108) and the Major Program of Institute of Applied Ecology, Chinese Academy of Sciences (IAEMP202201).
Funding
This work was financially supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDA28090300), the National Natural Science Foundation of China (41877108) and the Major Program of Institute of Applied Ecology, Chinese Academy of Sciences (IAEMP202201).
Author information
Authors and Affiliations
Contributions
Lijun Chen, Zhenhua Chen, and Nan Jiang contributed to the study conception and design. Material preparation and data collection were performed by Dongqi Jiang, Chenran Wu and Wenjing Huang. Data analysis were performed by Nan Jiang, Yulan Zhang and Zimeng Yao. The first draft of the manuscript was written by Nan Jiang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Feike A. Dijkstra.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jiang, N., Huang, W., Chen, Z. et al. More labile carbon inputs lessen the positive effects of nitrogen enrichment on soil carbon storage in a temperate grassland. Plant Soil (2023). https://doi.org/10.1007/s11104-023-06339-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-023-06339-2