Abstract
Background and aims
Ecosystems are expected to experience simultaneous environmental changes. This study examines the interactive effects of atmospheric CO2 and plant community composition on grassland ecosystem functioning after a severe drought.
Methods
Monocultures of the grass Dactylis glomerata were compared to a four-species grassland community under ambient and elevated CO2, with or without drought. Greenhouse gas fluxes, C and N pools in plants and soil were measured over a 55-day, post-rewetting period for all mesocosms.
Results
Experimental drought reduced aboveground biomass production, but increased soil inorganic N and dissolved organic C (DOC) across CO2 and community composition treatments. Following rewetting, droughted mesocosms had lower ecosystem respiration and higher N2O emissions. After 55 days, negative drought effects persisted on above- and belowground C stocks and root N stocks. Elevated CO2 reduced the magnitude of drought effects on ecosystem respiration, N2O fluxes and plant C:N ratios but increased drought-induced changes to soil DOC. The four-species mixture buffered ecosystem respiration from drought effects, but showed higher drought-induced increases in soil inorganic N shortly after rewetting.
Conclusions
Elevated CO2 mitigates the effects of extreme drought on multiple grassland functions. In contrast, grassland composition appears to have mainly additive effects with drought and elevated CO2 in our simple sown systems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Agricultural grasslands and rotational leys cover large land areas and make a significant contribution to global food security (O’Mara 2012; Finn et al. 2013). These low-diversity, perennial systems are managed for the production of high grass yields, and can increase soil organic matter stocks and mitigate greenhouse gas emissions within temperate farming systems (Persson et al. 2008; Peeters 2009). However, intensively-managed agricultural grasslands also have the potential for high carbon (C) and nitrogen (N) losses (Soussana and Lemaire 2014), and face the challenge of sustainable forage production and resource use against a background of fluctuating environmental conditions. This is of particular importance since climate change projections suggest an increase in the frequency and magnitude of extreme weather events such as drought, as well as changes in the mean values of atmospheric carbon dioxide (CO2), temperature and rainfall (Easterling et al. 2000; IPCC 2013). Understanding how climate extremes interact with global change drivers is therefore critical for determining grassland responses to future climatic conditions (Roy et al. 2016).
Shifts in both mean climatic conditions and climatic variability can have dramatic effects on grassland ecosystem structure and function (e.g. Fay et al. 2003; Smith 2011; Cantarel et al. 2013; Reyer et al. 2013). For example, rising levels of atmospheric CO2 are typically associated with reduced stomatal conductance and increased photosynthesis, which may have positive effects on plant growth depending on nitrogen supply (Long et al. 2004; Ainsworth and Rogers 2007; Franks et al. 2013). Elevated CO2 can also promote soil biological activity and modify microbial community composition via increases in plant litter inputs, root exudates or efficiency of water use by plants, which reduces soil water loss through transpiration (Hungate et al. 2003; Drigo et al. 2010; Niboyet et al. 2010; van Groenigen et al. 2011). Improved plant water relations, and subsequent increases in soil moisture availability, have the potential to maintain photosynthesis in dry periods and mitigate drought effects on grassland functioning (Morgan et al. 2011; Roy et al. 2016). Indeed, drought-induced reductions in soil moisture generally decrease plant biomass production and soil microbial activities via a combination of direct and indirect effects (van der Molen et al. 2011). However, the net outcome of the combination of negative drought effects and positive CO2 effects on plants is more difficult to predict in situations where increases in leaf area under elevated CO2 limit soil water savings at the ecosystem scale, or where droughts are severe and limit photosynthetic carbon gain (Naudts et al. 2011; Franks et al. 2013). Moreover, CO2-induced changes in soil resource availability, microbial activities and/or microbial community structure have the potential to influence greenhouse gas emissions (respiratory CO2, N2O) from soil during drying-rewetting events (Borken and Matzner 2009; Dijikstra et al. 2010; Frank et al. 2015). To date, knowledge on the impacts of elevated CO2 on ecosystem C and N fluxes following a severe drought-rewetting event remains limited.
Variation in grassland ecosystem responses to changing environmental conditions is often attributed to differences in plant community composition (Niklaus and Korner 2004; Suding et al. 2008; Mariotte et al. 2013; Isbell et al. 2015). Patterns of plant biomass response to climate change can be mediated by plant species richness, differences in species abundance or plant functional traits (Hooper et al. 2005; Polley et al. 2013; Volaire et al. 2014), with cascading effects on soil processes and plant-soil interactions (Orwin et al. 2010; Bloor and Bardgett 2012). Growing evidence suggests that communities which are more diverse in species or functional groups may be more stable under a range of conditions due to temporal and/or functional complementarity and shifts in species interactions (Loreau and de Mazancourt, 2013; Isbell et al. 2015). In theory, complementarity in resource-use arising from niche differentiation and/or facilitation in diverse, multi-species communities could reinforce the benefits of improved plant water-use efficiency under elevated CO2, and promote post-drought recovery (De Boeck et al. 2006). In practice, the importance of community composition for ecosystem stability and post-drought performance under current and future CO2 climates has rarely been investigated.
In this study, grassland monocultures (Dactylis glomerata) and mixtures (Dactylis glomerata, Lolium perenne, Festuca arundinacea, Trifolium repens) were used to examine drought responses and ecosystem properties related to C and N cycling (greenhouse gas fluxes: CO2, N2O, CH4; C and N pools in plants and soil) after a severe drought event. The overall goal of this multifactorial experiment was to determine the interactive effects of drought, atmospheric CO2 and plant community composition on ecosystem functioning in model agricultural grasslands. We focused on short-term drought recovery as rewetting events can promote substantial shifts in plant-soil interactions (Schimel et al. 2007; Bloor and Bardgett 2012). Three main hypotheses were addressed: i) severe drought reduces plant biomass production and promotes a build-up of soil nutrients; ii) elevated atmospheric CO2 interacts with drought and enhances drought recovery in both plant and soil processes, i.e. less negative drought effects in high CO2 versus low CO2 treatments; iii) plant community composition interacts with drought and the positive effects of elevated CO2 on grassland drought recovery.
Material and methods
Experimental design and growing conditions
Experimental mesocosms were established in ambient and elevated atmospheric CO2 treatments under glasshouse conditions at the University of Paris XI (Orsay, France). Drought treatments (control, drought) and plant community treatments (monoculture, four-species mixture) were crossed with CO2 treatments (ambient, elevated) in order to investigate the interactive effects of CO2, severe drought and plant community composition. This resulted in two CO2 treatments x two composition treatments x two drought treatments x six replicates = 48 pots.
Soil used in the experiment was sandy loam topsoil collected at two depths (pHH2O of 6.58, 0.09% N, 0.11% C for the 0–20 cm soil layer; pHH2O of 7.56, 0.05% N, 0.06% C for the 20–40 cm layer) in the locality of the CEREEP-Foljuif experimental station, France in June 2012. Deep PVC pots (20 × 15 × 40 cm) were filled with 10.5 l of sieved topsoil (10 mm mesh size) in two layers (20–40 cm soil followed by 0–20 cm soil). Pots were assigned to one of twelve naturally-lit growth chambers (aluminium frame and clear plastic walls, 160 × 90 × 100 cm high) set up inside a large glasshouse. Each chamber had its own airflow supplied by a pipe system and was ventilated with ambient air taken from outside the glasshouse; half of these growth chambers were enriched in CO2. Elevated atmospheric CO2 concentrations in enriched chambers were adjusted using flowmeters and injection of pure CO2 resulting in a differential of 239 ± 2.6 ppm compared with ambient chambers. Chambers were arranged in six pairs or blocks (one ambient chamber next to one elevated CO2 chamber) to avoid possible positional effects in the glasshouse. CO2 enrichment was operational from 22nd October 2012 (i.e. one week prior to sowing) and CO2 concentrations were monitored at the start of each morning throughout the experiment (i.e. until 25 June 2013) using a portable carbon dioxide analyser (M170 Measurement Indicator, Vaisala, Helsinki, Finland); these measurements indicated an average CO2 concentration of 463 ± 2 ppm and 702 ± 3.5 ppm in the ambient and elevated CO2 chambers respectively. No temperature difference was observed between the ambient and elevated CO2 chambers (maximum daily temperatures ranged between 12.0 and 35.8 throughout the experimental period). Maximum daily PAR values recorded during the study ranged between 85 and 1050 μmol s−1 m−2.
Plant community composition and drought treatments were applied in a complete factorial arrangement within each growth chamber (one pot per treatment combination per chamber). Low-diversity ‘model’ systems were established using native forage species; unlike semi-natural grasslands, intensively-managed agricultural systems have simple, synthesized plant communities with species selected for their capacity for resource acquisition and fodder production (Peeters 2009). On the 30th October 2012, seeds of Dactylis glomerata were sown into half of the pots at a density of 2000 seeds m−2. The remaining pots were sown to the same total seed density using four perennial species (Dactylis glomerata, Lolium perenne, Festuca arundinacea and Trifolium repens) in a 1:1:1:1 mixture and a random spatial arrangement. All seeds for the experiment were obtained from Agri Obtentions, Clermont Ferrand, France. Immediately prior to sowing, all mesocosms were fertilised using NPK 14–7-14, Multicote 12 (Haifa, Israel) slow-release granules (20 g m−2, equivalent to 30 kg N ha−1). Seedlings in all pots were left to grow in the experimental treatments and maintained close to field capacity by regular watering. On 7th February 2013, above-ground vegetation in each mesocosm was clipped to five cm above the soil surface to promote tillering and vegetation establishment. Plants were then left to re-grow.
Severe drought was applied to half of the experimental mesocosms within each growth chamber by stopping irrigation for three weeks (10 April to 2 May 2013). Soil moisture content was monitored twice-weekly during drought using an SM200 probe coupled to a HH2 moisture meter (Delta-T Devices, Cambridge, England). At the end of drought manipulation, above-ground vegetation in each mesocosm was clipped to 5 cm above the soil surface. Harvested material was oven-dried (60 °C, 48 h) and weighed to assess impacts of drought on biomass production. Droughted mesocosms received 500 ml water on the 2nd May to simulate rewetting (equivalent to 16.6 mm rainfall). Droughted and control mesocosms were then watered regularly and left to regrow until final harvest.
Soil sampling and soil measurements following drought
Soil cores (1.6 cm diameter, 10 cm deep) were taken from each mesocosm at the end of drought (2 May) and at two dates post-rewetting (7 May, 12 June). Dissolved organic carbon (DOC) was extracted from a sub-sample of freshly sieved soil (2 mm) by shaking 6 g of soil with 30 ml deionised water on an orbital shaker (16 h, 250 rpm). Samples were centrifuged and the supernatant was then filtered through a 0.45 μm membrane and stored at −20 °C prior to analysis. Values of DOC were obtained using a Shimadzu TOC-5050 analyzer. Soil mineral N was extracted from a sub-sample of freshly sieved soil by shaking 6 g of soil with 12 ml 1 M KCl for 2 h on an orbital shaker. The KCl extracts were filtered through Whatman glass fibre filters (grade GF/C: 1.2 μm) and filtrates were stored at −20 °C prior to analysis. Values for ammonium and nitrate were obtained by autoanalyzer procedures (Skalar Inc., Breda, Netherlands). Total soil mineral N was calculated as the sum of soil ammonium and soil nitrate contents. Additional soil samples were oven-dried (105 °C, 24 h) to determine gravimetric moisture content.
Gas flux measurements following drought
Measurements of ecosystem respiration (CO2), nitrous oxide (N2O) and methane (CH4) fluxes were made on five dates post-rewetting (7 May, 15 May, 22 May, 29 May, 12 June). Flux measurements were made using opaque PVC chambers (25 × 20 × 50 cm) each fitted with a rubber septa and a small fan. Each pot was sealed into a PVC board to facilitate flux measurements (Fig. S1), and chambers were placed over the mesocosms with their base in a water-filled groove, providing a gas-tight seal. Air samples were drawn from the chamber into 25 ml syringes at 5, 30 and 60 min after installation of the chamber, and injected into pre-evacuated 12 mL vials (Labco Ltd., UK). Gas samples were analysed using gas chromatography (Varian CP-3800 gas chromatograph equipped with an electron capture detector, Agilent technologies, USA), and gas fluxes were calculated based on the rate of changing gas concentrations in the chamber headspace using a linear regression of concentration versus time (Schrier-Uijl et al., 2010). Fluxes for N2O and CH4 were only recorded if significant regressions were obtained (P < 0.05, R2 ≥ 0.7). Regressions of CO2 concentration versus time were always highly significant and yielded an R2 ≥ 0.95 in all cases.
In conjunction with flux measurements we recorded soil temperature (EcoScan Temp 5 probe, Eutech Instruments, Singapore) and volumetric soil moisture for each pot (SM200 probe coupled to a HH2 moisture meter; Delta-T Devices, Cambridge, England).
Plant and soil sampling at final harvest
On 24–25 June 2013, all plants were harvested and separated into root and shoot material per pot. Roots were washed and all plant material was oven-dried (60 °C, 48 h) to obtain dry mass values for the roots and shoot material. Total C and N content in aboveground biomass and root samples were determined for 5 mg of finely ground material (Brinkmann ball grinder, Retsch, MM200) using an elemental combustion analyzer (Flash EA 1112 CNS analyzer, ThermoFinnigan, Milan, Italy). Total C and N in soil collected in the 0–10 cm soil layer at final harvest were also measured for dried, finely-ground sub-samples of soil using an elemental combustion analyser (EA-IRMS, Sira 10, VG Isogas, Middlewich, UK).
Data analyses
Treatment effects were assessed for each measurement date using a 3-way, fully-factorial split-plot analysis of variance and PROC MIXED in SAS 9.3 (SAS Institute, Cary, NC). Growth chambers were considered as a random factor, with CO2 treatment as a fixed whole-plot factor, and both plant community composition and drought treatments as fixed sub-plot factors within growth chambers. Where necessary, data were transformed prior to analysis to conform to assumptions of normality and homogeneity of variances. We further examined possible confounding effects of soil moisture on gas fluxes using 3-way split-plot analysis of covariance with soil moisture as a covariable; treatment effects were qualitatively the same with/without soil moisture included in the analyses.
Results
Drought effects on soil moisture and plant biomass
Drought manipulation resulted in an 87% decrease in gravimetric soil moisture content on average in droughted mesocosms at the end of drought (Fig. 1, F1,30 = 1767.6, p < 0.001). Drought treatment showed no interactions with either CO2 or community composition treatment on soil moisture at the end of drought (p > 0.05). However, elevated CO2 had a positive effect on soil moisture content across drought and community composition treatments (+21% on average, F1,5 = 7.63, p = 0.04). Moreover, soil moisture content was lower in the four-species mixture compared to the Dactylis monocultures across drought and CO2 treatments (−19% on average, F1,30 = 6.60, p = 0.015).
Effects of drought on aboveground plant biomass (> 5 cm) and gravimetric soil moisture (0–10 cm soil layer) under interactive CO2 and species composition treatments. Values are at the end of experimental drought (prior to rewetting). Treatment codes are given by: amb, ambient CO2; +CO2, elevated CO2; Dac, Dactylis monoculture, Mix, mixed grassland community. Means and SEs are presented (n = 6)
At the end of the experimental drought period, aboveground biomass (> 5 cm) ranged from 176.7 to 843.3 g m−2 across mesocosms (mean 481.0 ± 24.5 g m−2, Fig. 1). In general, drought had a negative effect on biomass production (−46% on average, F1,30 = 370.15, p < 0.001). In contrast, elevated CO2 had a positive effect on biomass (+29% on average, F1,5 = 21.39, p = 0.006), although the magnitude of increase varied depending on drought (Drought x CO2 interaction, F1,30 = 5.23, p = 0.03; Fig. 1). Positive effects of elevated CO2 on biomass were slightly higher in the droughted mesocosms (+29.0% versus +28.4% for droughted and control mesocosms respectively). Community composition did not have a significant effect on biomass at the end of drought, and showed no interaction with either drought or CO2 (p > 0.05).
Post-drought soil sampling: DOC and mineral N
At the end of drought, immediately prior to rewetting, droughted mesocosms showed a 68% increase in DOC on average (Fig. 2, F1,30 = 833.25, p < 0.001). Drought-induced increases in DOC were higher under elevated CO2 compared to ambient CO2 (significant Drought x CO2 interaction, Table S1), with increases of +78% versus +59% for DOC in elevated CO2 and ambient mesocosms respectively. Drought-induced increases in DOC were also apparent five days after rewetting, although the effect size was much smaller (+11% on average, F1,30 = 9.26, p = 0.005; Fig. 2). Five days after rewetting, the drought treatment showed no interactions with either CO2 or community composition treatment. Forty-one days after rewetting, drought-induced increases in DOC were only apparent for mesocosms grown under elevated CO2 (+20%; significant Drought x CO2 interaction, Table S1). Moreover, DOC was significantly higher in Dactylis monocultures compared to the four-species mixtures (+11% on average, F1,30 = 4.62, p = 0.040; Fig. 2).
Effects of drought on dissolved organic carbon (DOC) and total soil mineral nitrogen content in the 0–10 cm soil layer under interactive CO2 and species composition treatments. Values are provided immediately following experimental drought (A) and during drought recovery (two dates post-rewetting: 5 days, B; 41 days, C). Values are provided immediately following experimental drought and during drought recovery (two dates post-rewetting). Treatment codes are given by: amb, ambient CO2; +CO2, elevated CO2; Dac, Dactylis monoculture, Mix, mixed grassland community. Means and SEs are presented (n = 6)
Unlike DOC, soil inorganic N only showed a significant response to drought five days after rewetting (Table S1, Fig. 2). At this date, drought had a significant positive effect on soil inorganic N (+167% on average, F1,30 = 40.75, p < 0.001), and drought-induced increases in inorganic N were higher in the four-species mixtures (significant Drought x Composition interaction, +65% versus +391% for inorganic N in monocultures and four-species mixtures respectively). Soil inorganic N did not respond to CO2 at any measurement date (Table S1, Fig. 2).
Greenhouse gas fluxes during drought recovery
Ecosystem respiration (CO2 emissions) showed a decrease in the droughted mesocosms at the start of the drought recovery period (−46% on average, F1,30 = 501.54, p < 0.001; Fig. 3). Drought-induced reductions in CO2 emissions recorded five days after rewetting were smaller under elevated CO2 compared to ambient mesocosms (−6% on average, significant Drought x CO2 interaction, Table 1). Moreover, drought-induced reductions in CO2 emissions were significantly smaller in the four-species mixtures compared to Dactylis monocultures (−9% on average, significant Drought x Composition interaction, Table 1). Thirteen days after rewetting, ecosystem respiration remained lower in the droughted mesocosms but the drought-induced decrease in ecosystem respiration was less pronounced (−20% on average, F1,30 = 52.09, p < 0.001; Fig. 3).
Drought recovery in ecosystem respiration was faster under elevated CO2 (significant Drought x CO2 interaction, Table 1). Twenty days after rewetting, there was no difference in CO2 emissions from droughted- and non-droughted mesocosms in the high CO2 treatment while ecosystem respiration remained lower in the droughted- compared to non-droughted mesocosms under ambient CO2 (+21% on average, Fig. 3). From day 27 onwards, both drought and elevated CO2 were associated with an increase in respiration rates, but drought-induced increases in CO2 emissions were greater in the ambient CO2 treatment (+21% on average, Table 1, Fig. 3). Furthermore, drought-induced increases in ecosystem respiration were greater in the Dactylis monocultures compared to the four-species mixtures (+18% on average, Table 1, Fig. 3).
During the post-drought experimental period, N2O fluxes were negligible in control, non-droughted mesocosms (range of −0.27 to 0.34 mg N m−2 d−1, Fig. 3). Post-drought N2O emissions responded strongly to drought, and rewetting was associated with a peak in N2O emissions across all CO2 and community composition treatments (N2O values 119 times greater on average in droughted mesoscosms, Table 1, Fig. 3). Drought-induced increases in N2O emissions were still apparent 20 days after rewetting (8.5 times greater in droughted mesocosms, Fig. 3). Thirteen days after rewetting, the magnitude of drought-induced increases in N2O was four times smaller under elevated CO2 compared to ambient CO2 (Drought x CO2 interaction, F1,30 = 11.09, p = 0.002, Fig. 3). From day 27 onwards, no significant treatment effects were detected on N2O emissions. Unlike response patterns observed for CO2 fluxes, community composition had no significant effect on N2O fluxes at any time (Table 1).
Methane fluxes ranged from −1.60 to 0.88 mg C m−2 d−1 across treatments during the study (Fig. 3) but did not show any response to either drought, CO2 or community composition at any date during the study (Table S2).
Total C and N stocks in plant and soil compartments at final harvest
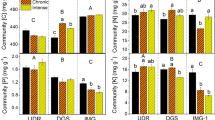
At final harvest, the mass of C in both aboveground vegetation and roots responded strongly to all experimental treatments (Fig. 4). Plant carbon pools in both shoots and roots decreased with drought (−40% on average, Table 2) but increased under elevated CO2 (+27% on average, Table 2). In addition, plant C was higher in Dactylis monocultures compared to the four-species mixtures (+13% on average, Table 2, Fig. 4). Unlike plant C, responses of plant N pools to experimental treatments varied in shoots and roots. Mass of N in aboveground vegetation responded to plant community composition alone, and was greater in Dactylis monocultures (+12% on average, F1,30 = 9.52, p = 0.004; Fig. 4). Mass of N in roots responded only to drought, with lower N pools in the droughted mesocosms (−25% on average, F1,30 = 22.1, p < 0.001; Fig. 4). Above- and below-ground plant C and N pools showed no interactions between drought, CO2 or community composition at final harvest (Table 2).
Effects of interactive drought, elevated CO2 and species composition treatments on carbon and nitrogen in total above-ground vegetation and plant roots 55 days after rewetting. Treatment codes are given by: amb, ambient CO2; +CO2, elevated CO2; Dac, Dactylis monoculture, Mix, mixed grassland community. Means and SEs are presented (n = 6)
In general, C:N ratios of above- and belowground plant biomass decreased in response to drought (−26.9%, F1,30 = 115.06, p < 0.001 and −18.9%, F1,30 = 30.75, p < 0.001 for shoot C:N and root C:N respectively). However, both shoot and root C:N ratios showed interactions between drought and CO2 (F1,30 = 5.18, p = 0.03 and F1,30 = 4.58, p = 0.04 for shoot C:N and root C:N respectively). Shoot C:N showed greater drought-induced decreases under ambient compared to elevated CO2 across all community composition treatments (−35% versus −19.8% for ambient and elevated CO2 respectively, Fig. 4). Root C:N decreased in response to drought under ambient CO2 (−26.5% on average), but showed no significant drought response under elevated CO2 across community composition treatments (Fig. 4). Drought responses of shoot C:N and root C:N did not interact with community composition (p > 0.05). Soil C, soil N and soil C:N showed no response to drought, CO2 or community composition at final harvest (p > 0.05 for all treatments, Fig. S2).
Discussion
The accurate assessment of ecosystem responses to global atmospheric change requires a new generation of multifactor-experiments addressing the complex interplay of driver variables and a broad range of above- and belowground response measurements (Beier et al., 2012; Kayler et al. 2015). Impacts of global change on C and N cycling and fluxes of greenhouse gases are of particular interest due to potential feedbacks to global warming (Lashof et al. 1997; Blankinship et al. 2010), but the interactions between atmospheric CO2 levels and extreme climate events such as drought on grassland function remain poorly understood. The present study addresses this knowledge gap by examining the effects of elevated atmospheric CO2 on post-drought (rewetting) responses for two model grassland communities, representative of sown, agricultural grasslands. These disturbed, ploughed systems may be more vulnerable to drought in the establishment phase due to modified soil food webs and plant-soil feedbacks (Shennan 2008).
Ecosystem function after a severe drought event
In line with predictions, we found that severe drought was associated with a significant reduction in soil moisture and plant biomass, but an increase in soil DOC and inorganic N content. Drought-induced changes in ecosystem respiration and N2O fluxes observed shortly after rewetting (decreased CO2 and increased N2O emissions) did not persist for longer than four weeks across CO2 and community composition treatments. Drought-induced increases in soil C and N substrates have previously been reported in grassland experiments (Bloor and Bardgett 2012), and can be linked to asynchrony in plant and soil processes during drought (modified source/sink relationships), as well as to greater mortality of microorganisms and fine roots during soil drying (White et al. 2004). Improved soil resource availability in combination with anaerobic conditions following rewetting has also been commonly shown to trigger short-lived bursts of N2O emissions in grasslands (Hartmann and Niklaus 2012). Unlike CO2 and N2O, CH4 fluxes showed no significant response to rewetting, consistent with the mixed effects of drought on CH4 oxidation documented in natural and agricultural soils elsewhere (Kim et al. 2012).
Within the plant system, changes in plant physiology and reductions in photosynthesis during drought have carry-over effects on plant carbohydrate reserves and subsequent plant performance (van der Molen et al. 2011). In agricultural grasslands, drought-induced changes in plant nutrient content may also impact livestock performance via changes in forage quality (Dumont et al. 2015). At the end of our study (55 days after the end of drought), drought had negative effects on above- and below-ground C stocks but improved forage quality (decreased C:N for aboveground biomass) across CO2 and community composition treatments. Our data corroborate previous grassland studies which indicate that water deficits alleviate N limitation and have a positive effect on forage quality (reviewed by Dumont et al. 2015); variation in the magnitude of drought effects on plant C:N may vary across studies depending on drought intensity, shifts in botanical composition and/or plant phenological stage as well as drought-induced changes in soil N availability. Although drought-induced reductions in grassland biomass typically disappear within a year (Mirzaei et al. 2008; Hoover et al. 2014; Zwicke et al. 2015; Isbell et al. 2015), episodic decreases in plant C stocks have the potential to cause reductions in longer-term soil C sequestration and affect the annual C balance (Wu et al. 2011).
Interactions between elevated atmospheric CO2 and drought
Plants commonly show increases in resource-use efficiency under CO2 enrichment that have the potential to stimulate plant growth (Korner 2000). Given that elevated CO2 may alleviate drought stress in plants via improved plant water relations (decreased stomatal conductance, increased in plant water use efficiency) and an improved capacity to extract water from the soil (increased allocation of C to root growth and osmotic adjustment) (Wullschleger et al. 2002), we hypothesized that elevated atmospheric CO2 would offset the negative effects of a severe drought on plant biomass. Our results were generally consistent with this hypothesis; elevated CO2 had a positive effect on aboveground plant biomass even under droughted conditions, and plant biomass showed a significant Drought x CO2 interaction. However, the magnitude of observed non-additive effects was very small (<1% biomass difference), suggesting that CO2-induced increases in plant biomass were not modified by drought to a biologically-meaningful degree. The marginal effect size of interactions between CO2 and drought on biomass in this study confirms results obtained for crop species under drought and elevated CO2 (Baker et al. 1997), and implies that the degree of CO2-induced changes to plant water-use efficiency may be relatively insensitive to rainfall regime.
Unlike plant biomass, we found convincing evidence for faster drought recovery of CO2 and N2O fluxes under CO2 enrichment. Elevated CO2 reduced the magnitude of drought effects on both ecosystem respiration and N2O emissions measured after rewetting. Consequently, these C and N fluxes in droughted mesocosms showed a faster rate of return to control levels under CO2 enrichment over the course of the post-rewetting period. Observed buffering effects of elevated CO2 on respiratory CO2 fluxes during the post-rewetting period agree with response patterns of established plant communities to combined warming, severe drought and CO2 enrichment (Albert et al. 2011; Roy et al. 2016). However, to our knowledge, this is the first study to report CO2-induced reductions in post-drought N2O emissions. Increased plant demand for N under elevated CO2 and/or shifts in plant-microbial competition for N may explain the smaller peak in N2O emissions after rewetting (Dijikstra et al. 2010; Bloor and Bardgett 2012). Our results suggest that the magnitude of N2O emissions may be reduced under future climates with both increased atmospheric CO2 and greater frequency of drought, adding to the increasing number of studies that show dampened responses of N2O efflux to multiple global changes (Brown et al. 2012).
Changes in plant C:N have significant implications for litter decomposition rates, nutrient cycling and plant-soil feedbacks (Wardle et al. 2004). In the present study, increases in plant C:N under elevated CO2 partly compensated for drought-induced decreases in aboveground C:N and entirely compensated for drought-induced decreases in belowground C:N (significant Drought x CO2 interactions). It is notable that aboveground C:N in droughted mesocosms under elevated CO2 was identical to that recorded in undroughted, ambient conditions across community composition treatments. Elevated CO2 typically reduces plant N concentration (and increases plant C:N) due to an increase in non-structural carbohydrates and/or N limitation linked to changes in plant-soil feedbacks and soil N availability (Korner 2000). Our findings suggest that elevated CO2 counteracts short-term drought-induced increases in soil N availability, reducing the magnitude of drought effects on plant C:N and thus promoting the stability of forage quality in a changing environment.
Although elevated CO2 generally buffered ecosystem properties from drought effects in our study, we did also find that elevated CO2 enhanced soil DOC measured throughout the post-rewetting period. Increases in labile C substrates exacerbate the risks of C leaching losses from soil, and may drive changes in microbial community structure and function (Dijikstra et al. 2010; De Deyn et al. 2011).
Plant community composition and future climate change
Plant community composition is considered to play a key role for ecosystem function via species-specific differences in resource-use, plant growth rates and litter inputs which modify biotic interactions and biogeochemical cycling (Hooper et al. 2005; Orwin et al. 2010; Finn et al. 2013). Numerous studies also suggest that the resident plant community may strongly influence the stability of above- and belowground processes to drought (Wardle et al. 2000; Rivest et al. 2014; Isbell et al. 2015; but see Kreyling et al. 2008). Our results provide partial support for this idea; the droughted, four-species mesocosms showed a greater short-term increase in soil inorganic N after rewetting and faster recovery (and stabilisation) of ecosystem respiration rates compared to the Dactylis glomerata monocultures. However, we found no “community composition x drought” interactions for N2O fluxes, plant biomass or plant C: N ratios. The increase in soil fertility observed in mixtures is consistent with a greater pulse of N mineralization following rewetting, and may reflect greater drought sensitivity of the soil microbial community present in this treatment (Schimel et al. 2007; Orwin et al. 2016). Unlike the mixed plant community, Dactylis monocultures showed overcompensation in ecosystem respiration during the post-rewetting period; such post-drought respiration pulses may be driven by soil moisture content as well as substrate availability (Borken and Matzner 2009), promoting transient shifts in ecosystem function from C sinks to C sources (Hoover et al. 2016).
Limited interactions between community composition and drought suggest that the speed and pattern of drought recovery in ecosystem function are relatively constant across our low-diversity study systems. Moreover, plant community composition did not modify the effects of elevated CO2 on grassland drought recovery as expected. Additive effects of community composition, CO2 and drought on ecosystem properties may be promoted by idiosyncratic species responses to experimental treatments, driven by variation in plant traits and interspecific competition (Dijikstra et al. 2010; Miranda-Apodaca et al. 2015). The prevalence of interactions between abiotic conditions and plant community composition could also depend on the age of the grassland system, as time since agricultural disturbance (i.e. ploughing and resowing) is known to modify plant-soil interactions (Herzberger et al. 2014). Of course the importance of plant community composition for ecosystem function is contingent on sampling effects (Huston 1997), and we do not rule out the possibility that composition x drought and/or CO2 interactions may be greater with different species combinations. Further work is needed to examine the importance of plant community composition on drought recovery under future climates for a broader range of sown grassland communities, and to compare the influence of component species on plant and soil properties in newly-sown and well-established grasslands.
References
Ainsworth EA, Rogers A (2007) The response of photosynthesis and stomatal conductance to rising [CO2]: mechanisms and environmental interactions. Plant Cell Environ 30:258–270
Albert KR, Ro-Poulsen H, Mikkelsen TN et al (2011) Interactive effects of elevated CO2, warming and drought on photosynthesis of Deschampsia flexuosa in a temperate heath ecosystem. J Exp Bot 62:4253–4266
Baker JT, Allen LH, Boote KJ, Pickering NB (1997) Rice responses to drought under carbon dioxide enrichment. Growth and yield. Glob Chang Biol 3:119–128
Beier C, Beierkuhnlein C, Wohlgemuth T et al (2012) Precipitation manipulation experiments - challenges and recommendations for the future. Ecol Lett 15:899–911
Blankinship JC, Brown JR, Dijikstra P, Hungate BA (2010) Effects of interactive global changes on methane uptake in an annual grassland. J Geophys Res 115:G02008. doi:10.1029/2009JG001097
Bloor JMG, Bardgett RD (2012) Stability of above-ground and below-ground processes to extreme drought in model grassland ecosystems: Interactions with plant species diversity and soil nitrogen availability. Perspect Plant Ecol 14:193–204
Borken W, Matzner E (2009) Reappraisal of drying and wetting effects on C and N mineralization and fluxes in soils. Glob Chang Biol 15:808–824
Brown JR, Blankinship JC, Niboyet A et al (2012) Effects of multiple global change treatments on soil N2O fluxes. Biogeochemistry 109:85–100
Cantarel AAM, Bloor JMG, Soussana JF (2013) Four years of simulated climate change reduces aboveground productivity and alters functional diversity in a grassland ecosystem. J Veg Sci 24:113–126
De Boeck HJ, Lemmens CMHM, Bossuyt H et al (2006) How do climate warming and plant species richness affect water use in experimental grasslands? Plant Soil 288:249–261
De Deyn GB, Quirk H, Oakley S et al (2011) Rapid transfer of photosynthetic carbon through the plant-soil system in differently managed grasslands. Biogeosciences 8:1131–1139
Dijkstra FA, Morgan JA, LeCain DR, Follet RF (2010) Microbially mediated CH4 consumption and N2O emission is affected by elevated CO2, soil water content and composition of semi-arid grassland species. Plant Soil 329:269–281
Drigo B, Pijl AS, Duyts H et al (2010) Shifting carbon flow from roots into associated microbial communities in response to elevated atmospheric CO2. PNAS 107:10938–10942
Dumont B, Andueza D, Niderkorn V et al (2015) A meta-analysis of climate change effects on forage quality in grasslands: specificities of mountain and Mediterranean areas. Grass Forage Sci 70:239–254
Easterling DR, Meehl GA, Parmesan C et al (2000) Climate extremes: observations, modeling and impacts. Science 289:2068–2074
Fay PA, Carlisle JD, Knapp AK, Blair JM, Collins SL (2003) Productivity responses to altered rainfall patterns in a C4-dominated grassland. Oecologia 137:245–251
Finn JA, Kirwan L, Connolly J et al (2013) Ecosystem function enhanced by combining four functional types of plant species in intensively managed grassland mixtures: a 3-year continental-scale field experiment. J Appl Ecol 50:365–375
Frank D, Reichstein M, Bahn M et al (2015) Effects of climate extremes on the teresstrial carbon cycle: concepts, processes and potential future impacts. Glob Chang Biol 21:2861–2880
Franks PJ, Adams MA, Amthor JS et al (2013) Sensitivity of plants to changing atmospheric CO2 concentration: from the geological past to the next century. New Phytol 197:1077–1094
Hartmann AA, Niklaus PA (2012) Effects of simulated drought and nitrogen fertilizer on plant productivity and nitrous oxide (N2O) emissions of two pastures. Plant Soil 361(1–2):411–426
Herzberger AJ, Meiners SJ, Towey JB et al (2014) Plant–microbe interactions change along a tallgrass prairie restoration chronosequence. Restor Ecol 23:220–227
Hooper DU, Chapin FS, Ewel JJ et al (2005) Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecol Monogr 75:3–35
Hoover DL, Knapp AK, Smith MD (2014) Resistance and resilience of a grassland ecosystem to climate extremes. Ecology 95:2646–2656
Hoover DL, Knapp AK, Smith MD (2016) The immediate and prolonged effects of climate extremes on soil respiration in a mesic grassland. J Geophys Res Biogeosci:121. doi:10.1002/2015JG003256
Hungate BA, Dukes JS, Shaw MR, Luo Y, Field CB (2003) Nitrogen and climate change. Science 302:1512–1513
Huston MA (1997) Hidden treatments in ecological experiments: re-evaluating the ecosystem function of biodiversity. Oecologia 110:449–460
IPCC (2013) Summary for Policymakers. In: Stocker TF, Qin D, Plattner GK et al (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, UK
Isbell F, Craven D, Connolly J et al (2015) Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nature 526:574–577
Kayler ZE, De Boeck HJ, Fatichi S et al (2015) Experiments to confront the environmental extremes of climate change. Front Ecol Environ 13:219–225
Kim DG, Vargas R, Bond-Lamberty B, Turetsky MR (2012) Effects of soil rewetting and thawing on soil gas fluxes: a review of current literature and suggestions for future research. Biogeosciences 9:2459–2483
Korner C (2000) Biosphere responses to CO2 enrichment. Ecol Appl 10:1590–1619
Kreyling J, Wenigmann M, Beierkuhnlein C, Jentsch A (2008) Effects of extreme weather events on plant productivity and tissue die-back are modified by community composition. Ecosystems 11:752–763
Lashof DA, DeAngelo BJ, Saleska SR, Harte J (1997) Terrestrial ecosystem feedbacks to global climate change. Annu Rev Energy Environ 22:75–118
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants FACE the future. Annu Rev Plant Biol 55:591–628
Loreau M, de Mazancourt C (2013) Biodiversity and ecosystem stability: a synthesis of underlying mechanisms. Ecol Lett 16:106–115
Mariotte P, Vandenberghe C, Kardol P et al (2013) Subordinate plant species enhance community resistance against drought in semi-natural grasslands. J Ecol 101:763–773
Miranda-Apodaca J, Perez-Lopez U, Lacuesta M et al (2015) The type of competition modulates the ecophysiological response of grassland species to elevated CO2 and drought. Plant Biol 17:298–310
Mirzaei H, Kreyling J, Hussain MZ et al (2008) A single drought event of 100-year recurrence enhances subsequent carbon uptake and changes carbon allocation in experimental grassland communities. J Plant Nutr Soil Sci 171:681–689
Morgan JA, LeCain DR, Pendall E et al (2011) C4 grasses prosper as carbon dioxide eliminates desiccation in warmed semi-arid grassland. Nature 476:202–205
Naudts K, Van den Berge J, Janssens IA, Nijs I, Ceulemans R (2011) Does an extreme drought event alter the response of grassland communities to a changing climate? Environ Exp Bot 70:151–157
Niboyet A, Barthes L, Hungate BA et al (2010) Responses of soil nitrogen cycling to the interactive effects of elevated CO2 and inorganic N supply. Plant Soil 327:35–47
Niklaus PA, Korner C (2004) Synthesis of a six year study of calcareous grassland responses to in situ CO2 enrichment. Ecol Monogr 74:491–511
O’Mara FP (2012) The role of grasslands in food security and climate change. Ann Bot 110:1263–1270
Orwin KH, Buckland SM, Johnson D et al (2010) Linkages of plant traits to soil properties and the functioning of temperate grassland. J Ecol 98:1074–1083
Orwin KH, Dickie IA, Wood JR et al (2016) Soil microbial community structure explains the resistance of respiration to a dry-rewet cycle but not soil functioning under static conditions. Funct Ecol 30:1430–1439
Peeters A (2009) Importance, evolution, environmental impact and future challenges of grasslands and grassland-based systems in Europe. Grassl Sci 55:113–125
Persson T, Bergkvist G, Kätterer T (2008) Long-term effects of crop rotations with and without perennial leys on soil carbon stocks and grain yields of winter wheat. Nutr Cycl Agroecosyst 81:193–202
Polley HW, Isbell FI, Wilsey BJ (2013) Plant functional traits improve diversity-based predictions of temporal stability of grassland productivity. Oikos 122:1275–1282
Reyer CPO, Leuzinger S, Rammig A et al (2013) A plant’s perspective of extremes: terrestrial plant responses to changing climatic variability. Glob Chang Biol 19:75–89
Rivest D, Paquette A, Shipley B et al (2014) Tree communities rapidly alter soil microbial resistance and resilience to drought. Funct Ecol 29:570–578
Roy J, Picon-Cochard C, Augusti A et al (2016) Elevated CO2 maintains grassland net carbon uptake under a future heat and drought extreme. PNAS 113:6224–6229
Schimel J, Balser TC, Wallenstein M (2007) Microbial stress-response physiology and its implications for ecosystem function. Ecology 88:1386–1394
Schrier-Uijl AP, Kroon PS, Hensen A et al (2010) Comparison of chamber and eddy covariance-based CO2 and CH4 emission estimates in a heterogeneous grass ecosystem on peat. Agric For Meteorol 150:825–831
Shennan C (2008) Biotic interactions, ecological knowledge and agriculture. Philos Trans R Soc B 363:717–739
Smith MD (2011) The ecological role of climate extremes: current understanding and future prospects. J Ecol 99:651–655
Soussana JF, Lemaire G (2014) Coupling carbon and nitrogen cycles for environmentally sustainable intensification of grasslands and crop-livestock systems. Agric Ecosyst Environ 190:9–17
Suding KN, Lavorel S, Chapin FS III et al (2008) Scaling environmental change through the community level: a trait-based response-and-effect framework for plants. Glob Chang Biol 14:1125–1140
Van der Molen MK, Dolman AJ, Ciais P et al (2011) Drought and ecosystem carbon cycling. Agric For Meteorol 151:765–773
van Groenigen KJ, Osenberg CW, Hungate BA (2011) Increased soil emissions of potent greenhouse gases under increased atmospheric CO2. Nature 475:214–216
Volaire F, Barkaoui K, Norton M (2014) Designing resilient and sustainable grasslands for a drier future: adaptive strategies, functional traits and biotic interactions. Eur J Agron 52:81–89
Wardle DA, Bardgett RD, Klironomos JN et al (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wardle DA, Bonner KI, Barker GM (2000) Stability of ecosystem properties in response to aboveground functional group richness and composition. Oikos 89:11–23
White CS, Moore DI, Craig JA (2004) Regional-scale drought increases potential soil fertility in semiarid grasslands. Biol Fertil Soils 40:73–78
Wu Z, Dijkstra P, Koch GW et al (2011) Responses of terrestrial ecosystems to temperature and precipitation change: a meta-analysis of experimental manipulation. Glob Chang Biol 17:927–942
Wullschleger SD, Tschaplinski TJ, Norby RJ (2002) Plant water relations at elevated CO2 - implications for water-limited environments. Plant Cell Environ 25:319–331
Zwicke M, Picon-Cochard C, Morvan-Bertrand A et al (2015) What functional strategies drive drought survival and recovery of perennial species from upland grassland? Ann Bot 116:1001–1015
Acknowledgements
This study was supported by the ‘Agence Nationale de la Recherche’ (PULSE project ANR-2010-CEPL-010-04). The authors thank the CEREEP-Ecotron Ile De France (UMS 3194, CNRS-ENS, Foljuif experimental station) for providing soil, and are grateful to D. Sévéré and S. Fontaine for help setting up and monitoring the experiment. We thank L. Conte, M. Coornaert, W. Daniel, M. Guérin, S. Lireux, F. Marmonier, and S-L. Redon for assistance with data collection and harvests. Thanks also to Katja Klumpp for helpful comments on a previous version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 820 kb)
Rights and permissions
About this article
Cite this article
Niboyet, A., Bardoux, G., Barot, S. et al. Elevated CO2 mediates the short-term drought recovery of ecosystem function in low-diversity grassland systems. Plant Soil 420, 289–302 (2017). https://doi.org/10.1007/s11104-017-3377-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3377-8