Abstract
We examined chemical changes from leaf tissue to soil organic matter (SOM) to determine the persistence of plant chemistry into soil aggregate fractions. We characterized a slow (Dicranopteris linearis) and fast-decomposing species (Cheirodendron trigynum) and surface (O), and subsurface (A-horizon) SOM beneath each species using pyrolysis-gas chromatography/mass spectrometry (py-GC/MS), with and without derivatization. The live tissues of Dicranopteris had greater lignin content whereas Cheirodendron had a greater lipid, N-bearing, and polysaccharide component. Despite this difference in leaf chemistry, SOM chemistry was similar between soil aggregate fractions, but different between horizons. The O-horizon contained primarily lignin and polysaccharide biomarkers whereas the A-horizon contained polysaccharide, aromatic, and N-derived compounds, indicating considerable microbial processing of plant litter. The soils beneath Cheirodendron inherited a greater lipid signal composed of cutin and suberin biomarkers whereas the soils beneath Dicranopteris contained greater aromatic biomarker content, possibly derived from plant lignins. The soils beneath both species were more similar to root polysaccharides, lipids, and lignins than aboveground tissue. This study indicates that although plant-derived OM is processed vigorously, species-specific biomarkers and compound class differences persist into these soils and that differences in plant chemical properties may influence soil development even after considerable reworking of plant litter by microorganisms.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There are broad differences in decomposition rates between plant functional types including conifers, deciduous trees, and grasses (Cornelissen 1996; Cornwell and others 2008; Weedon and others 2009). Although it is clear that plant functional characteristics affect litter decomposition rates, it is less clear how these differences influence organic matter (OM) preservation in soils. Some studies hypothesize a broad similarity in soil chemistry across a range of conditions, including variable vegetation types, suggesting homogenization of plant inputs during decomposition and soil development (Gleixner and others 1999; Mahieu and others 1999; Grandy and Neff 2008). Other studies suggest that soils may inherit key aspects of plant tissue characteristics that then influence soil properties (Filley and others 2008; Vancampenhout and others 2010). These latter studies suggest a role for plant species in the control of soil organic matter (SOM) composition and highlight the potential for plant–soil feedbacks to occur via the formation and decomposition of SOM.
Plant chemical characteristics, including high concentrations of polyphenols and lignins, are known to slow litter decomposition (Melillo and others 1982) and are recognized as a key factor in plant–soil feedbacks including nutrient cycling (Hobbie and Vitousek 2000). It is, however, considerably less clear as to whether plant characteristics can result in distinct patterns of soil development and SOM composition and even whether plant chemical differences are inherited by and persist in soils. Most organic inputs to soils are plant derived, and the organic materials comprising humic fractions derive from modified polyphenolic compounds originally from plants (Sollins and others 2007) and therefore polyphenols dominate the OM composition of some O- and A-soil horizons (Buurman and others 2007; Nierop and Filley 2007). However, investigations using NMR (Sollins and others 2007), X-ray spectrometry (Lehmann and others 2007), and analyses of specific lignin monomers (Kiem and Kogel-Knabner 2003; Nierop and Filley 2007) suggest that plant-derived phenols make up a small proportion of the carbon stabilized in deeper soil horizons (Buurman and others 2007; Nierop and Filley 2007).
The accumulation of plant-derived compounds in soils is a complex process that can be controlled by mechanisms related to the initial litter chemical composition (for example, biochemical recalcitrance, lignin, or cutin composition), and/or by physical protection and interaction of minerals with the SOM (Six and others 2002; Kiem and Kogel-Knabner 2003). Plant-derived compounds that are difficult or inefficient to degrade are retained in the soils, and include lignin and aliphatic compounds, such as cutin (aboveground) and suberin (belowground). Prior study suggests that aliphatic compounds preserved in soils are plant derived (Nierop and others 2007; Filley and others 2008; Vancampenhout and others 2010). However, relatively undecomposed plant fragments can be preserved in soils through physical protection and mineral interactions which decrease SOM accessibility to soil decomposers (Six and others 2002). Minerals such as allophane and imogolite have considerable surface charge and large specific surface areas that form stable mineral–OM complexes (Buurman and others 2007; Mikutta and others 2009).
The islands of Hawaii provide an interesting case study in the role of plant composition on decomposition processes. These ecosystems include many vegetation types, including an exceptionally high abundance of ferns, which possess a range of unusual attributes including a slow decomposition rate and unusually high concentrations of leaf waxes (Amatangelo and Vitousek 2008, 2009). Components of leaf waxes have been found in fossilized fern cuticles illustrating their potential long-term persistence in some environments (Lyons and others 1995; Zodrow and Mastalerz 2002; Gupta and others 2007). These attributes have the potential to alter decomposition dynamics but relatively little is known about their influence on soil chemistry in these ecosystems.
Pyrolysis-gas chromatography/mass spectrometry (py-GC/MS) is a method of assessing the molecular biochemistry of OM comprising a diverse range of probable source molecules and compounds including lignins, polysaccharides, aromatics, lipids, and proteins, allowing the “fingerprinting” of an entire soil sample to illustrate the relative contribution of these classes to SOM. Recent study illustrates py-GC/MS can be generalized into categories similar to the 13C NMR molecular-mixing model (Baldock and others 2004). Kaal and others (2007) obtained high correlations between the two methods’ categorical grouping of lignin, lipid, and carbohydrate groups, although sometimes with large quantitative differences between the two methods, given their inherent limitations (Kaal and others 2007).
In this study, we explore how differences in plant tissue and root chemistry affect the chemical composition of soil aggregate fractions. We examine OM composition using categorical chemical groupings (lignin, lipid, and so on) similar to those proposed by Kaal and others (2007) and subsequently examine if variation in specific plant biomarkers (pyrolysis products) are retained in the soils that form beneath each species. The specific hypothesis we explore here is whether there will be a comparatively higher abundance of plant-derived molecules in SOM (polyphenols as well as aliphatic leaf waxes) under the slow-decomposing fern Dicranopteris linearis due to accumulation of chemically recalcitrant cell-wall components compared to the rapidly decomposing species, Cheirodendron trigynum. In contrast, we would expect that the comparatively more decomposable lignin/polyphenols will not persist in soils and therefore differences in plant lignin content would exert relatively little influence on the resulting soil biochemistry. In making both these predictions, we assume that the capacity to stabilize OM is similar across these broad compound classes, an assumption that we further test and discuss below.
Methods
Field Sampling
We sampled a wet tropical forest on the island of Hawaii in the Kohala Mountains. The site is at 1200 m asl within a cool wet rainforest (16°C and 2500 mm MAP) (Vitousek 2004). Two species of plants were chosen due to their widespread presence throughout the Hawaiian Islands (Kitayama and others 1995), and their large difference in organic chemistry (Raab and others, unpublished-a), nutrient concentrations, and decomposition rates (Allison and Vitousek 2004a; Amatangelo and Vitousek 2008). We sampled three field replicates of live leaves, root mats, and attached, recently senesced litter from C. trigynum, a native angiosperm tree and D. linearis, a thicket-forming non-polypod fern. In the case of Cheirodendron, the litter layer beneath the species contains some litter contributions from other forest species including the common Hawaiian tree Metrosideros polymorpha. For D. linearis, the dense thicket structure means that most of the surface litter under this species is derived from the fern—and fern mats can be relatively long-lived (decades) and so have been contributing to SOM formation for an extended period of time.
For both species, we sampled the soils beneath three replicates of each plant from two genetic soil horizons 0–3, 3–10 cm (O- and A-horizons, respectively) which have high OM contents (Table 1). These soils have been extensively characterized (Crews and others 1995; Vitousek and others 1997), but are briefly described here. The soil is classified as a Hydric Hydrudand, dominated by a non-crystalline mineralogy including allophane, imogolite, and ferrihydrite (Torn and others 1997, 2005). Sodium-extractable Fe3+ + Al3+ comprised 1.5 and 2.9% of the O and A-horizon, respectively (Torn and others 2005). Soil texture is difficult to determine in Andisols and may be approximated from 15-bar water content (2.5% × 15 bar water content) (Torn and others 2005). For the O and A-horizon, water contents were 69.9 and 33.2%, respectively, resulting in a texture of 43% clay, 51% silt, and 6% sand for the A-horizon (Torn and others 2005). Interaction of soil water with the high OM content of the O-horizon resulted in silt + clay estimates greater than 100% and should not to be used quantitatively (Torn and others 2005). Soil pH for the A-horizon was 3.9 (Mikutta and others 2009). Samples were frozen at 0°C and transported to the laboratory within 48 h of collection, on ice, and subsequently freeze-dried in bulk.
Soil Fractionation and C/N Measurements
Soils were sieved to 2 mm removing large rocks and roots and subsequently dry sieved to obtain four aggregate fractions: 850–590, 590–180, 180–53, and less than 53 μm. Samples were placed on the top sieve of the series of stacked sieves and shaken at moderate speed on a standard sieve shaker for 20 min (Grandy, personal comm.). To assure that samples were separated, individual fractions were shaken at 5-min intervals until the sample loss was less than 0.01 g per interval. Soil fractions were finely ball-ground, and C and N were measured using an EA 1110 CNS combustion analyzer (Thermo Electron Corporation, Waltham, Massachusetts).
Pyrolysis-Gas Chromatography/Mass Spectrometry and Methylation
Py-GC/MS is a method of assessing the molecular chemistry of OM including lignins, polysaccharides, aromatics, lipids, and proteins. Py-GC/MS uses thermal, non-oxidative fragmentation of large molecules into fragments that can be identified and traced (in some cases) back to particular plant or microbial sources (Hempfling and Schulten 1990; White and others 2004). Unlike NMR that observes functional groups, py-GC/MS enables the observation of a diverse range of chemical constituents in fragments large enough to identify probable source molecules (that is, lignins, aromatics, proteins, and so on). Although the method is generally only semi-quantitative without the use of internal or external standards, it provides a relatively comprehensive evaluation of SOM chemistry, although slightly underestimating polysaccharides and fatty acid methyl esters (Gauthier and others 2002; Grandy and others 2007; Stewart, unpublished). Use of differing GC columns can improve detection of select compounds. The addition of tetramethylammonium-hydroxide (TMAH) to the sample improves detection of acids (Hatcher and others 1995) and of lignin monomer derivatives of guaiacyl (G), syringyl (S)- and p-coumaryl-derived (P) aldehydes, carboxylic acids, and ketones, which may be lost in conventional py-GC/MS due to their polarity (Saiz Jimenez 1994; del Rio and others 1998).
Py-GC/MS and TMAH-py-GC/MS analyses were performed as described elsewhere (Stewart, unpublished). In brief, approximately 5–10 mg of sample was weighed into a pyrofoil (Pyrofoil F590, Japan Analytical Company, Tokyo, Japan) with and without preapplied 5 μl tetramethylammoniumhydroxide. The samples were incubated for 24 h before pyrolyzing to allow TMAH to absorb into the sample (Klingberg and others 2005), then pyrolyzed at 590°C within a Curie-point pyrolyzer (Pyromat; Brechbuhler Scientific Analytical Solutions, Houston, Texas). Pyrolysis products were transferred online to a gas chromatograph (ThermoQuest Trace GC; Thermo-Finnigan, San Jose, California) with an interface temperature of 250°C with a split-injection (split ratio 50:1, He carrier gas flow-rate 1.0 ml min−1). Separation of pyrolysis products was done on a BPX-5 column (60 m × 0.25 mm, film thickness 0.25 μm) using a temperature program of 40°C for 5 min, 5°C min−1 to 270°C followed by a jump (30°C min−1), to a final temperature of 300°C. The column outlet was coupled to a Thermo Polaris-Q ion trap mass spectrometer (Polaris Q; Thermo Finnigan, San Jose, California) operated at 70 eV in (electron ionization) EI mode. The transfer line was heated to 270°C, and source temperature held at 200°C. Live, litter, roots, O- and A-horizon samples were analyzed with py-GC/MS, but the A-horizon samples were not included with TMAH.
Data Analysis
Products were identified with reference spectra after de-convolution and identification based on the primary characteristic ions (for example, Buurman and others 2007) using AMDIS v 2.64 and National Institute of Standards and Technology 2002 mass spectral libraries. Each spectrum was inspected manually. All the identified components with a signal intensity greater than 0.5% of the spectra were selected using a Matlab script (version 7) to ensure data quality. Requiring a high signal-to-noise ratio for peak identification increased the probability of correct compound identification. Using this method, we identified more than 80% of the peaks in a given spectra. The data are quantitatively expressed as relative intensity with respect to total measured intensity (for example, Vancampenhout and others 2009). Relative intensity (RI) is expressed as
where x ij is the total integrated area for compound j in sample i, and ∑x i is the sum of all identified compounds.
Compound identification and classification were done according to probable source using the published literature (for example, Hempfling and Schulten 1990; Chiavari and Galletti 1992; Gutierrez and others 1995; Hatcher and others 1995; Galletti and others 1997; del Rio and others 1998, 2007; Moldoveanu 1998; Gleixner and others 1999, 2002; Chefetz and others 2002; Filley and others 2002; Gauthier and others 2002). During source classification, compounds were grouped into six source categories including lignin, polysaccharide, lipids, N-bearing, phenols, aromatic, and unidentified, based on the literature (Appendix 1, 2 in Supplemental Material). Classification included all the compounds identifiable by spectra. Of the compounds extracted, compounds which could not be assigned to a source category comprised fewer than 5% of the spectra. Summing relative intensities of py-GC/MS does not yield comparable results to wet-chemical analyses for whole plant content, but is useful, like the molecular mixing model with 13C-NMR data, to interpret general differences in bulk chemistry content (Kaal and others 2007; Baldock and others 2004). Evaluation of total sample chemistry by groups (lignin, lipid, polysaccharide, N-bearing) was performed on unmethylated data (Kaal and others 2007); due to the more complete chemical characterization that includes N-bearing compounds and polysaccharides (Raab and others, unpublished-b). Differences between soils were expressed as a proportion of the summed differences in compound classes (Appendix 1 in Supplemental Material). Methylated data were used to confirm the presence of lignin-derived acids and fatty-acids.
The origin of compound groups cannot be assigned plant versus microbial origin unequivocally except for lignins, but individual compounds can be biomarkers of plant (above and belowground) and more processed C sources. Lignin biomarkers are entirely of plant-origin (guaiacyl, syringyl, and p-hydroxyphenyl groups), as are odd numbered long-chained alkenes from plant waxes. Even chained fatty acid methyl esters can indicate cutin versus suberin sources. Smaller-chained lipids are primarily of microbial origin. Some polysaccharide biomarkers that are indicative of plant cellulose (Steinbeiss and others 2006) can be distinguished from those of more microbial processed products, although some compounds, may be derived from both sources (for example, Moldoveanu 1998). Some pyrolysis products have multiple origins, such as tannins and some types of lignins (Nierop and Filley 2007), whereas phenols may be derived from lignin or protein sources and have been separated into their own category (Appendix 1).
Statistics
The chemistry of the soil fractions was evaluated using principle component analysis (PCA) with a correlation matrix to account for differences in variance between chemical groups in SAS (SAS Institute, Cary, North Carolina). Results were orthogonally rotated using Proc FACTOR to correlate components with variables and consequently to provide the most interpretable results. We used categorical groupings based on probable origin and included lignin, lipid, phenol, polysaccharide, aromatic, N-bearing, and unidentified categories (Appendix 1). Soil C and N were analyzed using an ANOVA. To explain variation between angiosperm versus fern biomarkers, we performed a PCA analysis with a correlation matrix on each class of compounds present in the major chemical classes [lignin (13), polysaccharide (17), lipid (14), and aromatic (11)] (Appendix 1).
Results
Soil Fractions
The SOM chemistry was surprisingly similar across soil fractions although the O-horizon had a greater lignin content than the A-horizon (Table 2). Incorporating the six compound groups together into a PCA, soil fractions did not show a unique chemistry, although the three PCA axes combined explained 86% of the variability (Table 3). The O- and the A-horizons separated along PCA1, dominated by positive loading for aromatic (0.402) and polysaccharides (0.256) (A-horizon) and negative loadings for lignin (0.447, O-horizon) (Figure 1). PCA2 was dominated by negative loadings for N-bearing (−0.491) and positive loadings for lipid (0.663) and PCA3 was dominated by negative loading of polysaccharides (−0.544) and positive loading for phenol (0.638). Owing to the small differences in SOM chemistry between soil fractions, we focused the remaining analysis on the less than 53-μm fraction and compared it to aboveground tissue (live and litter) of the two species.
Chemical Groups Along the Continuum
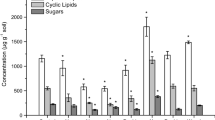
The two species’ live tissue had different lignin, polysaccharide, lipid, and N-bearing contents (Figure 2). Dicranopteris had a greater lignin-derived signal compared to Cheirodendron (Figure 2A) whereas Cheirodendron had a greater lipid (Figure 2D), N-bearing (Figure 2E), and polysaccharide component compared to Dicranopteris (Figure 2D). The low lignin and high lipid content of Cheirodendron as well as the high lignin content of Dicranopteris agreed with previously reported wet chemistry data of these two species (Allison and Vitousek, 2004b; Amatangelo and Vitousek 2008; Raab and others, unpublished-a).
Relative intensity (RI) of compound classes: lignin (A), polysaccharides (B), aromatic (C), lipid (D), N-bearing (E), and phenol (F) from unmethylated py-GC/MS chemical characterization of Cheirodendron and Dicranopteris over the decomposition sequence (live tissue to A-horizon). Units are relative intensity; error bars represent the standard error of the mean, n = 3.
The species differences in live tissue chemistry also were reflected in different decomposition trajectories from the live tissue into the soil (Figure 2). Lignin-derived compounds decreased from live tissue through litter and the A-horizon soil under the fern Dicranopteris (0.59–0.10 RA) (Figure 2A). Cheirodendron, however, had a maximum lignin-derived content in the O-horizon (0.35) that then decreased to the A-horizon (Figure 2A). Polysaccharides and aromatics increased from live tissue into the A-horizon (Figure 2B, E). The high lipid content of the live tissue of Cheirodendron decreased into the O-horizon and was only slightly less in the A-horizon compared to litter tissue, whereas the lipid content of the A-horizon soils under Dicranopteris was greater than that of the live tissue (Figure 2D).
The chemistry of A-horizon soils was different between the species although both soils contained similar compound classes compared to live tissues, (polysaccharides, aromatics, N-bearing compounds, and lipids). Aromatic compounds comprised more of the chemical signature of soil under Dicranopteris, whereas lipid and polysaccharide compound signatures were greater in soil under Cheirodendron (Figure 2C, D, B).
Compound-Specific Chemistry Within Groups
Lignin
There was clear separation in the PCA between the two species live tissue, the roots, and the A-horizon soils. A PCA analysis explained a combined 66% of the variability between the first three axes. The live tissue of both species separated along PCA1, which had positive loadings for guaiacyl structures (trans-isoeugenol 0.220, 4-propylguaiacol 0.220, and vinylguaiacol 0.449) and negative loading for some syringyl structures (syringol −0.073 and ethylphenol −0.097) (Figure 3; Table 4). PCA2 had positive loading for both guaiacyl and syringyl lignins (vanillic acid 0.395, and ethylsyringol 0.377) and negative loading for 4-eugenol (−0.243). PCA3 separated guaiacyl from syringyl lignins and had positive loading for syringol (0.415) and vanillic acid (0.415) and negative loading for acetosyringone (−0.325).
Lignin-derived chemical signatures of the live tissue and litter of two species reflected their different growth forms (Figure 3). Angiosperms, such as Cheirodendron, are known to contain a mix of guaiacyl (G) and syringyl (S) lignin compounds, whereas ferns (Dicranopteris) and non-woody plants contain G, or a mixture of G and p-hydroxyphenyl (P) structures (Ros and others 2007). Cheirodendron live tissue and litter were associated with loadings for syringyl compounds, whereas Dicranopteris was associated with loadings of biomarkers for ferns and non-woody plants, including 4-vinylphenol, a known pyrolysis product of p-coumaric acid and a large constituent of non-woody tissue (Del Rio and others 2007).
The O-horizon and roots of both species were associated with positive PCA2 loadings that included vanillin, syringaldehyde, and benzenediol suggesting a strong root component to the O-horizon soils. The A-horizon soils were associated with negative loadings for PCA1, indicative of syringyl lignin and slightly negative loading for PCA2. Although the compositions of both species’ A-horizon soils are different from either the live tissue, litter, or roots, their lignin composition appears to be distinct from one another (Figure 3).
Polysaccharides
A PCA analysis of polysaccharide-derived biomarkers explained a combined 73% (first three axes) of the variability that separated the two species live tissue, the roots, and the A-horizon soils (Figure 4; Table 5). The live tissues separated along PCA1 with positive loadings for maltol (0.169), and 2-cyclopenten-1-one, 3-ethyl-2-hydroxy (0.157), and the roots and soils separated along negative loading furfural (−0.112) and levoglucosenone (−0.105). PCA2 had positive loadings for 2,5-dimethyl-4-hydroxy-3(2H)-furanone (0.270), and negative loadings for furan, 3-methyl- (−0.258). Some 2-cyclopent-1-ones have been identified as pyrolysis products of lignin–cellulose (Gauthier and others 2003; Galletti and others 1997) and of purified cellulose in angiosperm leaves (Stewart, unpublished data) and may reflect greater cellulose content in Cheirodendron (Raab and others, unpublished-a).
Compound-specific differences in polysaccharides between these two species may reflect known differences in non-cellulose composition. The pectic polysaccharides of Cheirodendron have a predominant five-carbon base (arabinose), whereas the hemicellulose of the fern Dicranopteris is dominated by six carbon sugars (mannose and galactose) (Raab and others, unpublished-a). The greater 2-cyclopent-1-one content of live and litter tissue in Cheirodendron may reflect the greater proportion of 5-C sugars, although the py-GC/MS temperature of our run was not optimal for recovery of anhydrose sugar biomarkers (Moldoveanu 1998).
Roots of both species contained levoglucosan and 2(5H)-furanone, both plant cellulose biomarkers (Steinbeiss and others 2006). Levoglucosenone, another plant cellulose biomarker was present in the soils along with furfural, which can be derived from cellulose, lignin, or microbial sources (Gutierrez and others 1995; Steinbeiss and others 2006).
Both species’ O- and A-horizons had distinct polysaccharide chemistry and generally had negative loading for PCA1 and PCA2 (Figure 4; Table 2). In the A-horizon, non-specific polysaccharides were dominated by furfurals (2-furaldehyde and 5-methyl furaldehyde), and general polysaccharide biomarkers were also found in fungal biomass (Gutierrez and others 1995), and cellulose as well as 3-methyl furan, a polysaccharide commonly observed in soils (Saiz Jamaiz 1986) (Table 5).
Lipids
The two plant species were different in both lipid content and chemistry, with Cheirodendron having much greater lipid relative intensities in live tissue compared to Dicranopteris (Figure 2D). A PCA of the methylated fatty acids (TMAH-py-GC/MS) explained 60.5% of the variability (PCA1, 2 and 3 combined) (Figure 5; Table 6). Live tissue and roots separated along PCA1 with positive loadings associated with C20:0, C22:0 and C24:0 fatty acids (0.333, 0.333, and 0.305) and negative loading toward succinic acid, dimethyl ester (−0.84) and propanoic acid, methyl ester (−0.81). PCA2 had positive loading for tridecanoic acid, 12-methyl-, methyl ester (0.300) and negative loadings for hepta-2,4-dienoic acid, methyl ester (−0.233).
The live tissues and litter of both species as well as Dicranopteris O-horizon were indistinguishable in the PCA. Both species contained lipids derived from plant epicuticular waxes that were evident as fatty acid methyl esters (C16, C18:2). Although both C16 and C18 fatty acids may also be found in microbial sources (Jandl and others 2005), these fatty acids are likely to be of plant-derived origin. Despite the similarity in aboveground lipid composition in the PCA analysis, the roots of both species and the O-horizon of Cheirodendron separated belowground suberin-derived biomarkers C22 and C24 fatty acids (Naafs and others 2004; Jandl and others 2005) (Figure 5). Short-chained aliphatic compounds are generally of microbial origin, derived from fragments of microbial lipids or microbial degradation of longer chained lipids by microbes. The soils contained microbial lipid biomarkers, which were more fungal (alkenoic acid 18:1) (Rubino and others 2009) and bacterial (C15) (Naafs and van Bergen 2002).
However, with the unmethylated data (alkanes and alkenes), the PCA analyses separated the Cheirodendron live tissue from Dicranopteris tissue (Table 7) and explained 75% of the variability. The PCA1 had positive loading for short-chained alkanes and alkenes (C8:0 0.162) and negative loading for longer-chained alkanes (C29:0) whereas PCA2 had positive loading for the long-chained alkanes (C25:0–29:0) and negative values for mid-length alkanes (C16:0). The clear separation of Cheirodendron along positive PCA1 and PCA2 axes indicative of long-chained plant waxes (C25, C27, C29, and C31) (Chefetz and others 2002; Gupta and others 2007) supports wet chemical data that document a thick wax layer on the outside of Cheirodendron live tissue (Raab and others, unpublished-b).
Aromatic compounds
The PCA analysis of the aromatic biomarkers explained 86% of the variability in the first three axes and separated only the A-horizon soils and Cheirodendron O-horizon (Figure 6; Table 8). The A-horizon of Dicranopteris had positive PCA1 with positive loading from 1,3,5,7-cyclooctatetraene (0.334), and Cheirodendron had negative loading from 1,3,5-cycloheptatriene (−0.224). PCA2 had positive loading from 1,3,5-cycloheptatriene (0.427) and negative loading from 1,3,5,7-cyclooctatetraene (−0.199). The distinct separation between the soils beneath species in aromatic biomarkers is interesting, as these compounds indicate increasing decomposition either as a result of intense lignin modification or a component of fungal communities (Jelen and Wasowicz 1998).
The aromatic group consisted of ring structures primarily composed of benzenes and napthalenes that were unable to be classified into other categories (Appendix 1). Highly modified lignin phenols, or aromatic compounds are expected through a decomposition continuum (Stout and others 1988). However, these biomarkers are generally not specific to either a plant or microbial source classification (Hermosin and Saiz-Jimenez 1999; Buurman and others 2007) and might be a result of organic–soil minerals interaction during py-GC/MS (Faure and others 2006) although this is unlikely due to the extremely high OC content of these soils (Table 1).
N-Containing Compounds
Nitrogen-bearing compounds were greater in the live tissue of Cheirodendron compared to Dicranopteris, which is consistent with patterns (if not absolute concentrations differences) in foliar N content (Allison and Vitousek 2004b; Amatangelo and Vitousek 2009) (Table 2). In general, detection of N-bearing compounds will differ from bulk N determination in tissue and soils both because py-GC/MS detection does not address the stoichiometry of compounds (the intensity is based on compound detection rather than N concentration alone) and because py-GC/MS techniques often miss small compounds such as amino fragments that may be generated during the pyrolysis process. For these reasons, the GC/MS data can be interpreted across samples but should not be taken as a quantitative measure of N.
Leaf material was composed predominantly of indoles, pyrroles, and pyridines. Substituted pyrroles (Schulten and Schnitzer 1997) and 3-methylpyrrole (Bracewell and Robertson 1987) may be derived from the tetrapyrrole ring of chlorophyll whereas pyrroles (Chiavari and Galletti 1992) and indoles (Schulten and Schnitzer 1997) are primarily derived from amino acids.
Apart from chitin biomarkers, N-bearing compounds cannot be separated into microbial versus plant-derived compounds very well because of the ubiquitous nature of amino acids. Nitrogen-bearing compounds, doubled from the O- to the A-horizon under both species, comprised mostly pyrroles and pyridines. Pyridines have been found to be the most abundant pyrolysis product in commercially available chitin (Stankiewicz and others 1996). Pyridines also may derive from plant lignin decomposition with available ammonia (Schulten and Schnitzer 1997) or from other nitrogenous sources (amino acids, polypeptides, or chitin) (Buurman and others 2007). The increased N signal in the A-horizon may be due to the accumulation of decomposition products as well as decomposing organisms. The A-horizon in both soils had a large component of pyrazoles that have been found as pyrolysis products of chitin (Gleixner and others 1999).
Discussion
In this Hawaiian forest, soils inherited different proportions of compound classes from a fast-decomposing species, Cheirodendron, and a slow decomposing species Dicranopteris, and some specific plant-derived biomarkers persisted into the A-horizon of the soil. SOM chemical differences between the O- and the A-horizon followed a commonly observed pattern—the loss of lignin derived phenols (Kiem and Kogel-Knabner 2003; Nierop and others 2006) and increase in aromatic and N-bearing compounds. Depending on the species, however, we observed either an increase in polysaccharides (Dicranopteris) or lipids (Cheirodendron) from the O horizon to the A horizon (Gleixner and others 1999; Sollins and others 2007). The loss of lignin is consistent with the decomposition of plant material in tropical ecosystems and agrees with previous study on SOM composition of allophanic Andisols, which found a high contribution of polysaccharides and N-bearing compounds (Naafs and others 2004; Nierop and others 2005) but not plant-derived compounds to SOM chemistry.
We found surprisingly similar SOM chemistry between all aggregate sizes in contrast with others who observed chemical differences across soil aggregate fractions (Chefetz and others 2002; Poirier and others 2005; Grandy and Neff 2008). These Hawaiian soils have a much greater SOC content than soils of other aggregate studies and have high mineral–OM loadings (Mikutta and others 2009). Sorption to minerals effectively stabilizes organic material against oxidative degradation, but as minerals become more fully loaded with OM, C becomes more available to microbial attack (reviewed von Lutzow and others 2006) and more influenced by differences in SOC input sources and quantity (Hassink and others 1997; Stewart and others 2008). This could explain the similarity of size fraction chemistry observed here. In contrast to similarities across size classes in these soils, there were distinct patterns in SOM chemistry between the O and A horizon in these soils.
The inheritance of plant compounds into both O- and A-horizons is complex and varies across compound classes. Overall, plant lignins disappeared relatively quickly whereas plant-derived lipids were more strongly preserved in soils. In the case of lignin, the complexity of the lignin structure retards decomposition of aboveground tissue and indeed lignin content is often a good predictor of litter decomposition rates (Melillo and others 1989). Despite a much higher concentration of lignin in its tissue, the slow-decomposing species Dicranopteris, did not have greater lignin content in the A-horizon soil compared to Cheirodendron which suggests that despite the mat growth form of Dicranopteris, differences in microclimate (canopy and soil moisture) had minimal effects on decomposition between the two species.
This study suggests that plant lignins from a variety of species disappear quickly from these soils, and this finding is consistent with a number of other observations including studies that show that extracellular lignin degrading enzymes are prevalent in these Hawaiian soils (Allison and Vitousek 2004a; Amatangelo and Vitousek 2009) and a growing body of soil chemical study that finds lignin-derived compounds are rarely retained intact in soils with high decomposition rates (Gonzalez-Perez and others 2007; Nierop and others 2007). Although we did not observe intact lignin biomarkers in the soil, we did observe significantly more aromatic compounds that could be derived from lignins, under the fern. The largest difference between the two species’ A-horizon chemistry was in aromatic compounds; the presence of aromatic compounds, paralleled the greater concentration of lignins in aboveground plant tissue suggesting that although specific lignin biomarkers are not preserved in soils, the decomposition byproducts of the lignins may continue to influence SOM chemistry (Zech and others 1992).
In contrast to lignin dynamics, the greater lipid content of the soils underneath Cheirodendron suggests either preservation of plant-derived cutin and suberin and/or greater relative input combined with slower decomposition of these compounds compared to Dicranopteris. These results are intriguing in that, even in conditions that support rapid decomposition of OM, some plant-derived compounds persist (in a relatively undecomposed state) in soil horizons with turnover times of several decades (Torn and others 2005). These observations suggest that either these compounds are biologically recalcitrant or particularly prone to stabilization through mineral–OM association. Given that we observe relatively little differentiation in OM chemistry across soil aggregate fractions that typically are composed of a strong signal of microbial processing (Grandy and Neff 2008), it seems likely that these lipid constituents of ferns are somewhat or highly resistant to microbial degradation. In other settings as well, lipid-derived or alkyl-C are selectively preserved (Baldock and others 1997), and it has been suggested that this is due to the relatively high amount of energy necessary to decompose aliphatic bonds (Zech and others 1992; Baldock and others 2004; Nierop and others 2007). Also, lipids are hydrophobic, which can prevent access by degrading enzymes, but they can also adsorb on clay surfaces and intercalate existing mineral structure (von Lutzow and others 2006).
Although there is good evidence for biochemical mechanisms that explain the preservation of lipids in these soils, the possibility of physical (and preferential) stabilization of lipids cannot be ruled out. Allophanic Andic soils tend to have unusually high polysaccharide levels (Nierop and others 2005) and in alu-Andic soils, high lipid content (Gonzalez-Perez and others 2007; Nierop and others 2007), and are also well known for high surface area and sorption capacity (Torn and others 1997). In this regard, both chemical recalcitrance and mineral stabilization (to the degree these mechanisms can be separated) of lipids could play a role in the preservation of these compounds. Although the proximate mechanism responsible for lipid preservation is unclear, these findings, particularly when evaluated alongside those for plant lignins, suggest that lipids may represent one of the few plant-derived structural compounds that are likely to contribute to intermediate to slow-turnover time (decadal or longer) SOM fractions.
The slow C pool of these soils comprises between 80 and 90% of total soil and has a turnover from 15–40 years (O- and A-horizon, respectively) that may emerge from differential decompositions of chemical groups (Torn and others 2005). Previous incubations of a broad range of Hawaiian soils, including this site, found carbon turnover times in these soils were not significantly correlated with texture (15-bar water content), Fe3+, or Al3+ content, mineralogy or non-crystalline mineral content (Torn and others 2005). However, the turnover of litter pools at each site and the turnover times of the slow pool of SOC were positively correlated for the A-horizon and could be due to the inheritance of plant-derived recalcitrant compounds (Torn and others 2005). Amatangelo and others (unpublished) found that lipid compounds generally increased through litter decomposition, and we show that they can comprise a large proportion of plant-derived chemical SOM signature.
The observations from this study parallel a number of observations made in other soils; however, the measurements of chemical compounds from living tissue into soils offer several new insights as well. First, these results illustrate two mechanisms by which plant compositional variation may influence SOM chemistry. These mechanisms include the production and stabilization of secondary compounds related to relatively rapid lignin degradation as well as the direct stabilization of lipid-derived plant compounds by inherent recalcitrance, or onto mineral surfaces. Even across relatively small spatial scales within a forest (that is, under individual species), these differences in SOM chemistry are evident. There are a number of questions that emerge from these observations and include a fundamental one of whether the differences in SOM under differing plant species influence soil C or nutrient cycling on short-time scales. Although we cannot address that question here, this study suggests an interaction between factors that facilitate decomposition and factors that facilitate OM stabilization with variation in the relative importance of these two processes across compound classes. Because mineral–OM associations are one of the few mechanisms that can sequester terrestrial carbon into temperate or tropical soils for periods greater than a few decades, these associations represent an important repository of both carbon and nutrients in terrestrial ecosystems. The findings of this study suggest that plant composition and chemical diversity may play a role in the development of stabilized SOM. Further study including a broader range of species using both py-GC/MS and TMAH-py-GC/MS, a broader range of sites, as well as using compound-specific isotope analysis could clarify these results.
References
Allison SD, Vitousek PM. 2004a. Extracellular enzyme activities and carbon chemistry as drivers of tropical plant litter decomposition. Biotropica 36:285–96.
Allison SD, Vitousek PM. 2004b. Rapid nutrient cycling in leaf litter from invasive plants in Hawai’i. Oecologia 141:612–19.
Amatangelo KL, Raab TK, Stewart CE, Waldrop MP, Neff JC, Vitousek PM. Plant lignin controls microbial dynamics but not the decomposition trajectory across litter types of varying quality. Ecosystems (unpublished).
Amatangelo KL, Vitousek PM. 2008. Stoichiometry of ferns in Hawaii: implications for nutrient cycling. Oecologia 157:619–27.
Amatangelo KL, Vitousek PM. 2009. Contrasting predictors of fern versus angiosperm decomposition in a common garden. Biotropica 41:154–61.
Baldock JA, Masiello CA, Gelinas Y, Hedges JI. 2004. Cycling and composition of organic matter in terrestrial and marine ecosystems. Mar Chem 92:39–64.
Baldock JA, Oades JM, Nelson PN, Skene TM, Golchin A, Clarke P. 1997. Assessing the extent of decomposition of natural organic materials using solid-state C-13 NMR spectroscopy. Aust J Soil Res 35:1061–83.
Bracewell JM, Robertson GW. 1987. Characteristics of soil organic-matter in temperate soils by Curie-point pyrolysis-mass spectrometry. 2. The effect of drainage and illuviation in B-horizons. J Soil Sci 38:191–8.
Buurman P, Peterse F, Martin GA. 2007. Soil organic matter chemistry in allophanic soils: a pyrolysis-GC/MS study of a Costa Rican Andosol catena. Eur J Soil Sci 58:1330–47.
Chefetz B, Tarchitzky J, Deshmukh AP, Hatcher PG, Chen Y. 2002. Structural characterization of soil organic matter and humic acids in particle-size fractions of an agricultural soil. Soil Sci Soc Am J 66:129–41.
Chiavari G, Galletti GC. 1992. Pyrolysis-gas chromatography mass-spectrometry of amino-acids. J Anal Appl Pyrolysis 24:123–37.
Cornelissen JHC. 1996. An experimental comparison of leaf decomposition rates in a wide range of temperate plant species and types. J Ecol 84:573–82.
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M. 2008. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–71.
Crews TE, Kitayama K, Fownes JH, Riley RH, Herbert DA, Muellerdombois D, Vitousek PM. 1995. Changes in soil-phosphorus fractions and ecosystem dynamics across a long chronosequence in Hawaii. Ecology 76:1407–24.
del Rio JC, Gutierrez A, Rodriguez IM, Ibarra D, Martinez AT. 2007. Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FTIR. J Anal Appl Pyrolysis 79:39–46.
del Rio JC, McKinney DE, Knicker H, Nanny MA, Minard RD, Hatcher PG. 1998. Structural characterization of bio- and geo-macromolecules by off-line thermochemolysis with tetramethylammonium hydroxide. J Chromatogr A 823:433–48.
Faure P, Jeanneau L, Lannuzel F. 2006. Analysis of organic matter by flash pyrolysis-gas chromatography-mass spectrometry in the presence of Na-smectite: when clay minerals lead to identical molecular signature. Org Geochem 37:1900–12.
Filley TR, Boutton TW, Liao JD, Jastrow JD, Gamblin DE. 2008. Chemical changes to nonaggregated particulate soil organic matter following grassland-to-woodland transition in a subtropical savanna. J Geophys Res Biogeosci 113:G03009.
Filley TR, Cody GD, Goodell B, Jellison J, Noser C, Ostrofsky A. 2002. Lignin demethylation and polysaccharide decomposition in spruce sapwood degraded by brown rot fungi. Org Geochem 33:111–24.
Galletti GC, Bocchini P, Guadalix ME, Almendros G, Camarero S, Martinez AT. 1997. Pyrolysis products as markers in the chemical characterization of paperboards from waste paper and wheat straw pulps. Bioresour Technol 60:51–8.
Gauthier A, Derenne S, Dupont L, Guillon E, Largeau C, Dumonceau J, Aplincourt M. 2002. Characterization and comparison of two ligno-cellulosic substrates by C-13 CP/MAS NMR, XPS, conventional pyrolysis and thermochemolysis. Anal Bioanal Chem 373:830–8.
Gleixner G, Bol R, Balesdent J. 1999. Molecular insight into soil carbon turnover. Rapid Commun Mass Spectrom 13:1278–83.
Gleixner G, Poirier N, Bol R, Balesdent J. 2002. Molecular dynamics of organic matter in a cultivated soil. Org Geochem 33:357–66.
Gonzalez-Perez JA, Arbelo CD, Gonzalez-Vila FJ, Rodriguez AR, Almendros G, Armas CM, Polvillo O. 2007. Molecular features of organic matter in diagnostic horizons from andosols as seen by analytical pyrolysis. J Anal Appl Pyrolysis 80:369–82.
Grandy AS, Neff JC. 2008. Molecular C dynamics downstream: the biochemical decomposition sequence and its impact on soil organic matter structure and function. Sci Total Environ 404:297–307.
Grandy AS, Neff JC, Weintraub MN. 2007. Carbon structure and enzyme activities in alpine and forest ecosystems. Soil Biol Biochem 39:2701–11.
Gupta NS, Briggs DEG, Collinson ME, Evershed RP, Michels R, Jack KS, Pancost RD. 2007. Evidence for the in situ polymerisation of labile aliphatic organic compounds during the preservation of fossil leaves: implications for organic matter preservation. Org Geochem 38:499–522.
Gutierrez A, Martinez MJ, Almendros G, Gonzalezvila FJ, Martinez AT. 1995. Hyphal-sheath polysaccharides in fungal deterioration. Sci Total Environ 167:315–28.
Hassink J, Whitmore AP, Kubat J. 1997. Size and density fractionation of soil organic matter and the physical capacity of soils to protect organic matter. Eur J Agron 7:189–99.
Hatcher PG, Nanny MA, Minard RD, Dible SD, Carson DM. 1995. Comparison of two thermochemolytic methods for the analysis of lignin in decomposing gymnosperm wood: the CuO oxidation method and the method of thermochemolysis with tetramethylammonium hydroxide (TMAH). Org Geochem 23:881–8.
Hempfling R, Schulten HR. 1990. Chemical characterization of the organic-matter in forest soils by Curie-point pyrolysis-GC/MS and pyrolysis field-ionization mass-spectrometry. Org Geochem 15:131–45.
Hermosin B, Saiz-Jimenez C. 1999. Thermally assisted hydrolysis and methylation of milled beech leaf litter. J Anal Appl Pyrolysis 49:417–24.
Hobbie SE, Vitousek PM. 2000. Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–77.
Jandl G, Leinweber P, Schulten HR, Ekschmitt K. 2005. Contribution of primary organic matter to the fatty acid pool in agricultural soils. Soil Biol Biochem 37:1033–41.
Jelen H, Wasowicz E. 1998. Volatile fungal metabolites and their relation to the spoilage of agricultural commodities. Food Rev Int 14:391–426.
Kaal J, Baldock JA, Buurman P, Nierop KGJ, Pontevedra-Pombal X, Martinez-Cortizas A. 2007. Evaluating pyrolysis-GC/MS and C-13 CPMAS NMR in conjunction with a molecular mixing model of the Penido Vello peat deposit, NW Spain. Org Geochem 38:1097–111.
Kiem R, Kogel-Knabner I. 2003. Contribution of lignin and polysaccharides to the refractory carbon pool in C-depleted arable soils. Soil Biol Biochem 35:101–18.
Kitayama K, Muellerdombois D, Vitousek PM. 1995. Primary succession of Hawaiian montane rain-forest on a chronosequence of 8 lava flows. J Veg Sci 6:211–22.
Klingberg A, Odermatt J, Meier D. 2005. Influence of parameters on pyrolysis-GC/MS of lignin in the presence of tetramethylammonium hydroxide. J Anal Appl Pyrolysis 74:104–9.
Lehmann J, Kinyangi J, Solomon D. 2007. Organic matter stabilization in soil microaggregates: implications from spatial heterogeneity of organic carbon contents and carbon forms. Biogeochemistry 85:45–57.
Lyons PC, Orem WH, Mastalerz M, Zodrow EL, Viethredemann A, Bustin RM. 1995. C-13 NMR, micro-FTIR and fluorescence-spectra, and pyrolysis-gas chromatograms of coalified foliage of late Carboniferous medullosan seed ferns, Nova-Scotia, Canada—implications for coalification and chemotaxonomy. Int J Coal Geol 27:227–48.
Mahieu N, Powlson DS, Randall EW. 1999. Statistical analysis of published carbon-13 CPMAS NMR spectra of soil organic matter. Soil Sci Soc Am J 63:307–19.
Melillo JM, Aber JD, Linkins AE, Ricca A, Fry B, Nadelhoffer KJ. 1989. Carbon and nitrogen dynamics along the decay continuum: plant litter to soil organic matter. Plant Soil 115:189–98.
Melillo JM, Aber JD, Muratore JF. 1982. Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–6.
Mikutta R, Schaumann GE, Gildemeister D, Bonneville S, Kramer MG, Chorover J, Chadwick OA, Guggenberger G. 2009. Biogeochemistry of mineral-organic associations across a long-term mineralogical soil gradient (0.3–4100 kyr), Hawaiian Islands. Geochim Cosmochim Acta 73:2034–60.
Moldoveanu SC. 1998. Analytical pyrolysis of natural organic polymers. Techniques and instrumentation in analytical chemistry, Vol. 20Amsterdam: Elsevier.
Naafs DFW, van Bergen PF. 2002. A qualitative study on the chemical composition of ester-bound moieties in an acidic andosolic forest soil. Org Geochem 33:189–99.
Naafs DFW, van Bergen PF, Boogert SJ, de Leeuw JW. 2004. Solvent-extractable lipids in an acid andic forest soil; variations with depth and season. Soil Biol Biochem 36:297–308.
Nierop KGJ, Filley TR. 2007. Assessment of lignin and (poly-)phenol transformations in oak (Quercus robur) dominated soils by C-13-TMAH thermochemolysis. Org Geochem 38:551–65.
Nierop KGJ, Preston CM, Kaal J. 2005. Thermally assisted hydrolysis and methylation of purified tannins from plants. Anal Chem 77:5604–14.
Nierop KGJ, Preston CM, Verstraten JM. 2006. Linking the B ring hydroxylation pattern of condensed tannins to C, N and P mineralization. A case study using four tannins. Soil Biol Biochem 38:2794–802.
Nierop KGJ, Tonneijck FH, Jansen B, Verstraten JM. 2007. Organic matter in volcanic ash soils under forest and paramo along an Ecuadorian altitudinal transect. Soil Sci Soc Am J 71:1119–27.
Poirier N, Sohi SP, Gaunt JL, Mahieu N, Randall EW, Powlson DS, Evershed RP. 2005. The chemical composition of measurable soil organic matter pools. Org Geochem 36:1174–89.
Raab TK, Amatangelo KL, Vitousek PM. Biochemical diversity among Hawaiian ferns, tree ferns and angiosperms: implications for life-forms and nutrient retention. J Ecol (unpublished-a).
Raab TK, Stewart CE, Kramer MG, Neff JC, Amatangelo KL, Vitousek PM. The Litter continuum—comparison of three analytical methods in a Hawaiian forest. Geoderma (unpublished-b).
Ros LVG, Aznar-Asensio GJ, Hernandez JA, Bernal MA, Nunez-Flores MJL, Cuello J, Barcelo AR. 2007. Structural motifs of syringyl peroxidases are conserved during angiosperm evolution. J Agric Food Chem 55:4131–8.
Rubino M, Lubritto C, D’Onofrio A, Terrasi F, Kramer C, Gleixner G, Cotrufo MF. 2009. Isotopic evidences for microbiologically mediated and direct C input to soil compounds from three different leaf litters during their decomposition. Environ Chem Lett 7:85–95.
Saiz Jamaiz C. 1986. Chemical characterization of soil organic matter fractions by analytical pyrolysis-gas chromatography mass spectrometry. J Anal Appl Pyrolysis 9:99–119.
Saiz Jimenez C. 1994. Analytical pyrolysis of humic substances—pitfalls, limitations, and possible solutions. Environ Sci Technol 28:1773–80.
Schulten HR, Schnitzer M. 1997. The chemistry of soil organic nitrogen: a review. Biol Fertil Soils 26:1–15.
Six J, Conant RT, Paul EA, Paustian K. 2002. Stabilization mechanisms of soil organic matter: implications for C-saturation of soils. Plant Soil 241:155–76.
Sollins P, Swanston C, Kramer M. 2007. Stabilization and destabilization of soil organic matter—a new focus. Biogeochemistry 85:1–7.
Stankiewicz BA, van Bergen PF, Duncan IJ, Carter JF, Briggs DEG, Evershed RP. 1996. Recognition of chitin and proteins in invertebrate cuticles using analytical pyrolysis gas chromatography and pyrolysis gas chromatography mass spectrometry. Rapid Commun Mass Spectrom 10:1747–57.
Steinbeiss S, Schmidt CM, Heide K, Gleixner G. 2006. delta C-13 values of pyrolysis products from cellulose and lignin represent the isotope content of their precursors. J Anal Appl Pyrolysis 75:19–26.
Stewart C. Evaluation of plant biomarker contributions to soil organic matter using two methods of pyrolysis-gas chromatography-mass spectrometry. Plant Soil (unpublished).
Stewart CE, Plante AF, Paustian K, Conant RT, Six J. 2008. Soil carbon saturation: linking concept and measurable carbon pools. Soil Sci Soc Am J 72:379–92.
Stout SA, Boon JJ, Spackman W. 1988. Molecular aspects of the peatifcation and early coalifcation of angiosperm and gymnosperm woods. Geochim Cosmochim Acta 52:405–14.
Torn MS, Trumbore SE, Chadwick OA, Vitousek PM, Hendricks DM. 1997. Mineral control of soil organic carbon storage and turnover. Nature 389:170–3.
Torn MS, Vitousek PM, Trumbore SE. 2005. The influence of nutrient availability on soil organic matter turnover estimated by incubations and radiocarbon modeling. Ecosystems 8:352–72.
Vancampenhout K, De Vos B, Wouters K, Van Calster H, Swennen R, Buurman P, Deckers J. 2010. Determinants of soil organic matter chemistry in maritime temperate forest ecosystems. Soil Biol Biochem 42:220–33.
Vancampenhout K, Wouters K, De Vos B, Buurman P, Swennen R, Deckers J. 2009. Differences in chemical composition of soil organic matter in natural ecosystems from different climatic regions - a pyrolysis-GC/MS study. Soil Biol Biochem 41:568–79.
Vitousek PM. 2004. Nutrient cycling and limitation: Hawai’i as a model system. Princeton, NJ: Princeton University Press.
Vitousek PM, Chadwick OA, Crews TE, Fownes JH, Hendricks DM, Herbert D. 1997. Soil and ecosystem development across the Hawaiian Islands. GSA Today 7:1–7.
von Lutzow M, Kogel-Knabner I, Ekschmitt K, Matzner E, Guggenberger G, Marschner B, Flessa H. 2006. Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–45.
Weedon JT, Cornwell WK, Cornelissen JHC, Zanne AE, Wirth C, Coomes DA. 2009. Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12:45–56.
White DM, Garland DS, Ping CL, Michaelson G. 2004. Characterizing soil organic matter quality in arctic soil by cover type and depth. Cold Reg Sci Technol 38:63–73.
Zech W, Ziegler F, Kogelknabner I, Haumaier L. 1992. Humic substances distribution and transformation in forest soils. Sci Total Environ 118:155–74.
Zodrow EL, Mastalerz M. 2002. FTIR and py-GC-MS spectra of true-fern and seed-fern sphenopterids (Sydney Coalfield, Nova Scotia, Canada, Pennsylvanian). Int J Coal Geol 51:111–27.
Acknowledgments
The authors wish to thank Daniel Fernandez for assistance with the py-GC/MS instrument, as well as Cody Flagg for sample preparation. The authors also wish to thank Heraldo Farrington, Ted Raab, and Rebecca Funk for help with field sampling. The authors also acknowledge the constructive comments of four anonymous reviewers. This study was funded by the University of Colorado Chancellor’s Postdoctoral Fellowship, and by a National Science Foundation Grant (DEB-0515918).
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Contributions
CES conceived/designed study, collected and analyzed data, and wrote the manuscript; JCN, KLA, & PMV conceived/designed study and assisted with writing manuscript.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stewart, C.E., Neff, J.C., Amatangelo, K.L. et al. Vegetation Effects on Soil Organic Matter Chemistry of Aggregate Fractions in a Hawaiian Forest. Ecosystems 14, 382–397 (2011). https://doi.org/10.1007/s10021-011-9417-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-011-9417-y