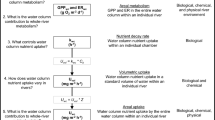
Abstract
Nutrient cycling and energy flow in ecosystems are tightly linked through the metabolic processes of organisms. Greater uptake of inorganic nutrients is expected to be associated with higher rates of metabolism [gross primary production (GPP) and respiration (R)], due to assimilatory demand of both autotrophs and heterotrophs. However, relationships between uptake and metabolism should vary with the relative contribution of autochthonous and allochthonous sources of organic matter. To investigate the relationship between metabolism and nutrient uptake, we used whole-stream and benthic chamber methods to measure rates of nitrate–nitrogen (NO3–N) uptake and metabolism in four headwater streams chosen to span a range of light availability and therefore differing rates of GPP and contributions of autochthonous carbon. We coupled whole-stream metabolism with measures of NO3–N uptake conducted repeatedly over the same stream reach during both day and night, as well as incubating benthic sediments under both light and dark conditions. NO3–N uptake was generally greater in daylight compared to dark conditions, and although day-night differences in whole-stream uptake were not significant, light–dark differences in benthic chambers were significant at three of the four sites. Estimates of N demand indicated that assimilation by photoautotrophs could account for the majority of NO3–N uptake at the two sites with relatively open canopies. Contrary to expectations, photoautotrophs contributed substantially to NO3–N uptake even at the two closed-canopy sites, which had low values of GPP/R and relied heavily on allochthonous carbon to fuel R.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
INTRODUCTION
Elemental cycling and energy flow are fundamental ecosystem processes that are tightly linked from the level of the stoichiometry of individual metabolic reactions (Nealson and Stahl 1997; Hedin and others 1998) to broad linkages at the level of the whole ecosystem (Redfield 1958; Reiners 1986; Elser and others 1996). These links exist because all organisms need both energy and elements for maintenance and growth. Autotrophs generally obtain nutrients from inorganic sources, whereas heterotrophs may obtain nutrients from inorganic forms as well as the organic carbon compounds sequestered as an energy source (Beck and others 1991). For both autotrophs and heterotrophs, low nutrient availability can limit rates of metabolism and, conversely, rates of metabolism ultimately determine rates of nutrient uptake, transformation, and release (Grimm 1987; Vitousek and Howarth 1991; Mulholland and others 1997; Schlesinger 1997).
Ecosystem studies of streams historically have capitalized on the downstream flow of water to assess either energy flow (Odum 1956; Fisher and Likens 1973; Minshall and others 1983; Meyer and Edwards 1990) or nutrient retention (Webster and Patten 1979; Newbold and others 1981; Triska and others 1989; Webster and Meyer 1997) and more recently, both concurrently (Mulholland and others 1997; Hall and Tank 2003; Webster and others 2003). Longitudinal resolution of dissolved oxygen (DO) concentrations over diel temporal scales have been coupled with measures of atmospheric exchange to provide insightful measures of whole-system metabolism (Odum 1956; Grimm and Fisher 1984; Marzolf and others 1994; Young and Huryn 1999; Mulholland and others 2001). Similarly, a set of techniques to quantify nutrient cycling in streams has been developed based on the longitudinal transport of materials participating in elemental cycling (Newbold and others 1981; Stream Solute Workshop 1990). The conceptual basis for these techniques was the recognition that advective flow results in downstream displacement of cycling activities (Webster and Patten 1979), stretching nutrient cycles into spirals oriented parallel to the direction of flow (sensu Elwood and others 1983).
Uptake of inorganic nutrients from the water column is a central component of nutrient retention in streams, and is the aspect of nutrient spiraling most often quantified. Common metrics used to describe uptake include: (a) uptake length (S w), the average distance traveled by an element in dissolved form, (b) uptake rate (U), the area specific uptake of an element, and (c) uptake velocity (v f) (Stream Solute Workshop 1990). Uptake velocity has increasingly been used for inter-site comparisons because it accounts for differences in water depth and velocity and, thus, emphasizes biological influence on solute transport and uptake relative to nutrient concentration (Davis and Minshall 1999; Bernhardt and others 2002; Hall and others 2002; Valett and others 2002; Hall and Tank 2003). For linking stream ecosystem metabolism and nutrient uptake, area specific uptake rate is an appropriate measure because both U and metabolism are typically expressed in units of mass of the element of interest per area per time, enabling the stoichiometric link between these processes to be emphasized.
Beyond theoretical interests in nutrient cycling, there is a growing concern about how ecosystems may respond to increased anthropogenic inputs of nutrients, particularly nitrogen (Aber and others 1989; Vitousek and others 1997). Understanding mechanisms and controls of nitrate–nitrogen (NO3–N) uptake and retention in aquatic ecosystems is central to this concern. More than 15 years ago, Smith and others (1987) reported increased NO3–N concentrations as the most evident change in stream and river quality in the U.S. Vitousek and others (1997) pointed out that the mechanisms and rates at which ecosystems retain nitrogen remain an area of significant uncertainty. Recently, several studies have suggested that headwater streams are active sites of nitrogen uptake and transformation and can influence catchment exports (Alexander and others 2000; Peterson and others 2001a; Mulholland 2004; Bernhardt and others 2005). At the reach scale, the metabolic activity of stream biota has been shown to be an important control on nitrogen uptake (Hall and Tank 2003; Webster and others 2003).
In general, nutrient uptake is expected to increase with increasing rates of metabolism due to assimilatory demand, but this relationship varies with the degree of dominance of autotrophic production and the sources of organic matter fueling heterotrophic production. Streams with open canopies can support substantial benthic algal production (Minshall 1978; Fisher and others 1982; Hill and others 2001; Mulholland and others 2001), and under these conditions, dissolved inorganic nutrient uptake is likely to be dominated by photoautotrophs. Although high rates of heterotrophic production may be supported by this autochthonous carbon, heterotrophic demand for dissolved inorganic nutrients should be relatively low because of the high quality of this organic matter (that is, low C:N ratio) (Romani and others 1998). If heterotrophic nutrient demand is met by organic substrates, uptake of inorganic nutrients would not be expected to be tightly linked to rates of heterotrophic respiration. On the other hand, nutrient uptake should relate directly to rates of gross primary production (GPP) based on the stoichiometry of biomass being produced and relate indirectly to autotrophic respiration through its relationship with GPP. A tight coupling between GPP and nitrate uptake is also expected because photoautotrophs typically reduce nitrate using reductant generated through photosynthesis (Huppe and Turpin 1994).
In well-shaded streams, in-stream photoautotrophic activity and, presumably, autotrophic assimilatory demand, are low at most times of the year. The combined effects of stream channel shading and litter deposition result in the dominance of terrestrial carbon inputs in forest stream organic matter budgets (Webster and Meyer 1997). Heterotrophic bacteria and fungi utilize these allochthonous inputs, but because terrestrial detritus generally has high carbon to nutrient ratios, heterotrophic production requires inorganic nutrient sources to supplement nutrients obtained from organic substrates (Suberkropp 1998; Gulis and Suberkropp 2003). For a given heterotrophic growth efficiency (del Giorgio and Cole 1998), greater rates of respiration are associated with greater production, and therefore are expected to be associated with higher nutrient demand in allochthonous-based ecosystems.
Across stream ecosystems, linkages between nutrient cycling and metabolism should vary predictably with the relative contributions of allochthonous and autochthonous organic matter supply. Because whole-system respiration (R) includes both autotrophic and heterotrophic activity, nutrient uptake should relate robustly to R despite variation in the sources of organic matter. At the same time, nutrient uptake should relate to GPP, but the influence of GPP may be evident only when autochthonous production is a significant energy flow pathway. To test these hypotheses, we investigated the relationship between metabolism and nutrient uptake in four headwater streams chosen to provide contrasts in canopy cover and related differences in the magnitude of GPP and the balance between allochthonous and autochthonous carbon supply. To assess the relative importance of photoautotrophic and heterotrophic processes, we conducted day and night measurements of metabolism and nutrient uptake. Reach scale NO3–N uptake was quantified using multiple short-term solute additions concurrent with whole-stream metabolism measurements. Benthic chamber measurements of uptake and metabolism allowed for the exclusion of deeper sediments and associated heterotrophs, therefore providing a contrast in the degree of autotrophy compared to whole-system measurements. We predicted that (1) autotrophic N demand would appear as greater NO3–N uptake during day compared to night, (2) the magnitude of difference between day and night uptake would increase with increasing autochthonous contributions (as measured by GPP/R ratio), (3) GPP and uptake would be closely related in ecosystems with substantial autochthonous production, and (4) ecosystem R would be a good predictor of NO3–N uptake.
METHODS
Study Sites
Four study streams were chosen to have similar discharges, but to vary in light availability and therefore presumably vary in rates of GPP and the relative importance of photoautotrophy. All four streams have been sites of previous research addressing stream metabolism and nutrient spiraling (Valett and others 1996; Mulholland and others 1997; Baker and others 2000; Fellows and others 2001) and site characteristics are summarized in Table 1. The study was conducted in summer to increase the relative importance of autotrophic processes: the period of highest algal production at the open canopy sites and farthest in time from the last autumnal leaf fall. All measurements were conducted during a three week period July–August, 1999.
Two sites with low canopy cover were located in high-elevation montane settings in New Mexico, USA. Rio Calaveras (RC) is a first-order stream that flows through a meadow where a sparse stream-side canopy provides little shading, and therefore the stream supports a substantial periphyton community (Peterson and others 2001b, c). Gallina Creek (GC) is a second-order stream that drains a forested catchment in north central New Mexico dominated by spruce and pine. The riparian canopy is moderately open and benthic periphyton is relatively abundant (Fellows and others 2001).
Two sites with closed canopies were located in southeastern deciduous forests of North Carolina and Tennessee, USA. The East Fork of Walker Branch (EFWB) is a first-order stream on the U.S. Department of Energy’s Oak Ridge National Environmental Research Park, Tennessee. EFWB is shaded by a relatively dense canopy of second-growth deciduous forest dominated by oak and hickory (Mulholland 1992). Benthic periphyton is sparse throughout most of the year with a short bloom in early spring before canopy development (P. Mulholland, unpublished data). Hugh White Creek (HWC) is a second-order stream at Coweeta Hydrologic Laboratory, North Carolina that drains a granitic catchment containing a mixture of hardwood species with a dense evergreen understory of rhododendron that reduces light to approximately 1% of incident levels (Webster and others 1997).
Solute Additions
Multiple solute additions were conducted at each site during both day and night to provide replicate measures of NO3–N uptake. The additions were conducted over a relatively short period of time (24–36 h) to avoid changes such as increased discharge that might result from a summer thunderstorm or other precipitation. Solute additions were used to quantify NO3–N uptake and characterize stream hydrologic conditions following methods described in the Stream Solute Workshop (1990). At each site, four to six stations were established along the study reach. A fluid metering pump introduced a solution of conservative tracer (Cl− as NaCl) and NO3–N (as NaNO3) into surface water at a constant rate. Introduction 10–20 m upstream of the study reach ensured complete mixing of solute and stream water. Water samples (n = 2–3) taken at each station characterized background conditions prior to each injection. Samples were filtered using glass fiber filters (Whatman GFF, 0.7 μm pore size) either during collection or within 12–24 h. Samples were frozen until analyzed for Cl− and NO3–N using ion-chromatography (Dionex DX-100, Sunnyvale, CA, USA).
Conservative tracer concentrations at the downstream station were monitored using specific conductance (YSI 30 meter, YSI Inc., Yellow Springs, OH, USA). Solute was injected for 0.75–2.5 h until approximately constant specific conductance was established at the most downstream sampling station (that is, plateau conditions, sensu Triska and others 1989). Plateau samples (n = 2–5) were taken at each station before turning off the injection pump. Sufficient time (greater than 2 reach travel times) occurred between injections to clear tracer from the study reach.
Time to plateau at RC and GC was relatively short (less than 1 h) and a total of six injections (three day and three night) were conducted within a period of 24 h at each of these sites. Time to plateau at EFWB and HWC was more than 2 h. Four injections over 36 h (two day and two night) were conducted at each of these sites. NO3–N concentrations during the solute additions were elevated relative to background by a factor of 2 (300 μg L−1), 15 (220 μg L−1), 9 (250 μg L−1), and 5 (130 μg L−1) at RC, GC, HWC, and EFWB, respectively.
Conservative tracer concentrations were used to determine discharge (Q, L s−1), water velocity (u, m s−1), and dilution due to groundwater input. Discharge was determined by dilution gauging (Gordon and others 1992) using plateau samples. Water velocity (u) was calculated as reach length divided by nominal travel time (that is, time required for conservative tracer to reach ½ plateau concentration, sensu Triska and others 1989).
Nutrient Spiraling and NO3–N uptake
Reach-scale NO3–N uptake was characterized by nutrient cycling metrics. Three related measures of NO3–N uptake were calculated for each solute addition (uptake length, uptake velocity, and areal uptake rate) from the longitudinal decline in non-conservative solute according to Equation (1):
where C x and C o are background and dilution corrected concentrations of NO3–N at distance x downstream and at the most upstream station, respectively. The longitudinal uptake coefficient (k, m−1) represents the fraction of added nutrient load removed per meter of downstream travel (Stream Solute Workshop 1990) derived from the linear regression of ln [NO3–N] and distance downstream. S w is the negative reciprocal of k, with units of length (m). Uptake velocities (v f, m s−1) for NO3–N were calculated by (2)
where z is stream depth (m) and was derived from discharge by (3)
where w = stream width (m). At HWC, direct measurements of depth (n = 100) over the reach were used to derive v f. Areal uptake rate (U, μg NO3–N m−2 h−1) per unit area of stream bottom was calculated as (4)
where [NO3–N] bkg is the mean background NO3–N concentration.
In the case of one nighttime solute addition at Rio Calaveras, concentrations of nitrate did not decrease downstream after accounting for background concentration and dilution. Uptake rate was assumed to be below detection limit for this one injection, and both U and v f were assigned a value of zero.
Whole-stream Metabolism
Whole-stream metabolism was measured using a modified open channel method (Odum 1956; Marzolf and others 1994). Dissolved oxygen (DO) concentrations were measured at an upstream and downstream station (1–5 min intervals, 36–60 h) with YSI 600XLM meters equipped with rapid-pulse oxygen sensors. Barometric pressure was concurrently logged every 5 min using a barometer and datalogger (Campbell CR-10, Logan, UT). Photon flux density (μmol m−2 s−1) of photosynthetically active radiation (PAR) was measured using a LI-COR quantum sensor (5 min average values, LI-COR Inc., Lincoln, NE, USA) positioned immediately next to the stream channel in the middle of the reach. Daylight hours were identified by PAR above 0.3 μmol m−2 s−1 and were used to determine daily total photon flux.
Oxygen reaeration coefficients were determined using the addition of a dissolved volatile tracer (propane) (Marzolf and others 1994; Young and Huryn 1998) during one of the solute additions at each site. Plateau was judged to be reached using the conservative solute tracer, and samples of stream water then were collected in 60 mL plastic syringes at three or more sampling stations. An equal volume of gas (either air or argon) was added to the syringe, and the syringe shaken to promote headspace equilibration. Headspace propane concentrations were determined in the laboratory using a gas chromatograph equipped with a flame ionization detector (Shimadzu GC-9A, Torrance, CA). Reaeration coefficient (kpropane, min−1) was determined following standard procedures described by Marzolf and others (1994), converted to koxygen, and multiplied by the oxygen deficit and reach travel time to calculate oxygen flux due to reaeration.
Effects of groundwater inputs on metabolism measures (McCutchan and others 1998, 2002; Mulholland and others 2001) were quantified using lateral inflow (Qupstream – Qdownstream, sensu Triska and others 1989; Stream Solute Workshop 1990) and subsurface DO concentrations. Groundwater DO was measured in shallow (less than 50 cm depth) near-stream wells. Groundwater DO was determined from a well network in the adjacent catchment of West Fork of Walker Branch at EFWB (P. Mulholland, unpublished data). Accordingly, groundwater DO concentrations were set at 1 ppm for HWC, 2 ppm for GC, 3 ppm for RC, and 7 ppm for EFWB.
Once corrected for groundwater inputs and atmospheric exchange, changes in DO during the night represent ecosystem R, whereas day time changes reflect rates of net ecosystem productivity (Marzolf and others 1994). Measures were scaled to a 24 h period of time by calculating the sum of all the time intervals for R plus an estimate of daytime R for each interval during the day derived from linear regression of pre-dawn and post-dusk R values (Marzolf and others 1994). GPP was determined as the sum of daytime intervals plus calculated daytime R. Relative magnitude of primary production and respiration was assessed by calculating GPP/R ratios (sensu Odum 1956). Net ecosystem production (NEP) was calculated as the difference between GPP and R over a 24 h period of time. For EFWB and HWC, metabolic measures were averaged from two consecutive 24 h periods.
Benthic Chamber Measurements of Metabolism and NO3–N Uptake
Benthic chambers (sensu Bott and others 1978) were used to assess metabolism and NO3–N uptake at ambient NO3–N concentrations. Chamber measurements were conducted at each site during the same week that whole-stream measures were made. Plastic trays (85 cm2, 2 cm depth) filled with benthic sediment were placed in the stream bed at least 3 weeks prior to use. For each stream, four trays were incubated separately in re-circulating metabolic chambers (Truckee River model, Rapid Creek Research, Inc., Boise, ID, USA) submerged in the stream to moderate temperature fluctuations. Dark and light incubations were run consecutively and dark conditions were achieved by covering the chambers with opaque material. Chambers were equipped with DO sensors (Orion Model 840, Thermo Electron, Water Analysis/Orion Products, Beverly, MA, USA) and incubations lasted until detectable changes in DO were observed (ca. 2–4 h under dark or light conditions). Light incubations were conducted between the hours of 10 a.m. and 2 p.m. During light incubations, PAR was recorded every five minutes (LI-COR quantum sensor). DO concentrations and temperature also were measured every five minutes.
Chamber R and NEP were calculated from the slope of the linear regression of DO and time during dark and light incubations, respectively. GPP was calculated as NEP + R. Metabolism rates were converted to g O2 m−2 h−1 using tray surface area and chamber volume (2 L). A sub-sample from each sediment tray was analyzed for organic matter (OM) as ash-free dry mass (AFDM, APHA and others 1992). Sediment chlorophyll a was determined on a different sub-sample using extraction with buffered acetone and a standard spectrophotometric (Hewlett-Packard HP8452A, Hewlett-Packard Corporation, Palo Alto, CA, USA) procedure (Wetzel and Likens 1991).
Daily benthic metabolic rates were generated by scaling chamber R to 24 h and GPP to the total number of daylight hours, while accounting for differences between daily insolation and light levels during chamber incubations. Whole-stream data were used to establish instantaneous GPP versus irradiance curves. Values of maximum photosynthesis and irradiance at the onset of photosaturation were visually estimated from these plots and used to adjust chamber GPP during daylight hours. Daily NEP was calculated by subtracting daily R from daily GPP.
Changes in chamber water NO3–N concentrations during benthic sediment incubations were used to quantify NO3–N uptake. Water samples were taken 15–30 min after initiating recirculation, at the end of the dark incubation, and at the end of the light exposure period. Filtered (Whatman GFF, 0.7 μm pore size) samples were frozen until analyzed using ion-chromatography (Dionex DX-100).
Data Analysis
Nitrate uptake was reported as both v f and U for whole-stream measures and as areal uptake rate from benthic chamber measurements. Uptake velocity is considered appropriate for comparisons across sites, particularly when water depth and velocity vary. However, v f is highly dependent on nutrient concentrations, and U may be a more suitable metric for comparing streams with very different nutrient concentrations. Whole-stream areal uptake rates allowed meaningful comparisons to be made with benthic chamber measurements as well as with rates of metabolism. Both U and v f were expected to increase with increasing metabolism. Biotic demand for N should increase as rates of metabolism increase, which would result in a greater mass of NO3–N being consumed per unit area. If concentrations of NO3–N are relatively constant, then v f should follow the same pattern as U. Uptake velocity would also be predicted to increase with increasing metabolism, independently of its relationship with U. Because v f represents the efficiency of NO3–N uptake relative to its concentration in the water column, it should be greater with higher rates of GPP and R because organisms should have more energy available to allocate to the uptake and reduction of NO3–N.
One-way analyses of variance were used to determine if NO3–N uptake differed among sites. Separate ANOVAs were conducted for each measure of uptake (v f, U, and benthic uptake) with site as the main factor (4 levels) and either multiple solute additions at each site as replicates (n = 4–6/site) or chambers as replicates (n = 3 or 4/site). ANOVAs were also conducted on chamber measures of metabolism, sediment organic matter (AFDM), and chlorophyll standing crop. Significant ANOVA results were followed by Tukey’s multiple comparison test (MCT) to test for differences among sites.
Within each site, one-tailed t-tests were performed for each measure of NO3–N uptake to test the prediction that uptake was significantly greater during the day than during night. Benthic chamber values under light and dark conditions were analyzed using one-tailed paired t-tests (paired by chamber, n = 3 or 4). Two-tailed t-tests were used to determine if there were day–night differences in physical and chemical aspects of the streams. Linear regression analysis was used to determine if the magnitude of the day–night differences in NO3–N uptake (v f, U, and benthic uptake; n = 4) increased with increasing GPP/R (whole-stream or mean benthic chamber values).
Linear regression analysis was also used to assess relationships between NO3–N uptake and metabolism. Measures of NO3–N uptake obtained from a specific solute injection were paired with an associated measure of ecosystem metabolism. Appropriate metabolic measures were obtained by treating each period of solute injection as an experimental unit. Hourly metabolic rates were calculated as the average of all intervals within the designated time. All solute additions (n = 20) were used to examine relationship between NO3–N uptake and R and only daytime additions (n = 10) were used for GPP. For benthic chamber measurements, regression analyses were performed between dark and light NO3–N uptake and R (n = 25) and light NO3–N uptake and GPP (n = 13). For whole-stream and benthic chamber uptake and GPP, regression analyses were repeated using only the two sites with open canopies/high autochthonous contributions to compare these relationships with those including all sites. All statistical analyses were carried out with α = 0.05 using SAS, Version 8 (1999).
RESULTS
Hydrologic and Other Physical Conditions
Three of the sites had very similar values for discharge, each averaging approximately 1 L s−1, whereas HWC had a greater value at 3.4 L s−1 (Table 2). All streams were shallow (Mean depth: 1.8–7 cm) and velocity varied four fold from 0.013 m s−1 for EFWB to 0.053 m s−1 for RC. Wetted channels were generally 1 m wide, except at HWC where the much wider stream channel (greater than 5 m) corresponded to an average depth of less than 2 cm.
Mean temperature and background NO3–N concentration varied across sites, but day–night differences within sites were not significant in all cases (t-test, P > 0.05; Table 2). Mean temperatures were approximately 5–6°C greater in the closed canopy streams (17 and 18°C) compared to the high elevation streams in New Mexico (11.7 and 12.5°C). Background NO3–N concentrations varied an order of magnitude from highest to lowest, with values of 168 μg L−1 at RC to 14 μg L−1 at GC. Background concentrations were intermediate in magnitude at the two other streams (both 28 μg L−1).
Daily PAR values reflected the degree of canopy cover with the highest value of 20.0 mol m−2 d−1 at RC and the lowest value of 0.1 at HWC (Table 3). The number of daylight hours at each site varied from 11.2 at HWC to 14.7 at RC.
Metabolism
Whole-stream and benthic chamber values of GPP and GPP/R were much greater at the two open-canopied sites (RC and GC) than at the two closed-canopied sites (EFWB and HWC)(Tables 3, 4). All four study streams were heterotrophic systems, but the extent to which R exceeded GPP varied. RC and GC had GPP/R ratios that were an order of magnitude greater than EFWB and HWC. The magnitude of the inter-site differences for benthic chamber GPP/R was smaller, but values at RC and GC were significantly greater than those at EFWB and HWC (ANOVA, P = 0.001). Benthic chamber values of GPP/R exceeded 1 at RC and GC and were substantially greater than corresponding whole-stream values for each site except HWC.
Light Versus Dark NO3–N Uptake
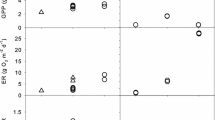
Whole-stream NO3–N uptake was greater during the day than at night at all four sites for v f and U (Figure 1), but none of the differences was statistically significant. The magnitude of the difference between day and night, relative to the mean value for the site, was relatively small for GC and HWC, ranging from 14 to 24% (Table 5). Day–night differences for both metrics were substantially greater at EFWB and RC, with values of over 60% of the mean for EFWB and over 90% for RC.
Comparison of light and dark NO3–N uptake within each study stream. Open symbols represent the mean value obtained from daytime solute additions or benthic chamber incubations under light conditions and solid symbols represent mean values for night time additions or dark benthic chamber incubations (n = 3 for RC and GC; n = 2 for EFWB and HWC for daytime and night time solute injections; n = 4 for RC and EFWB, n = 3 for HWC, and n = 2 for GC for light and dark benthic chamber incubations). Error bars represent the standard error of the mean. Daytime whole-stream NO3–N uptake was not significantly greater than nighttime uptake (v f or U greater; P < 0.05, one-sided t-test), whereas benthic uptake under light conditions was significantly greater than under dark conditions at three sites (indicated with an asterisk; P < 0.05, one-sided t-test).
Within each site, benthic chamber NO3–N uptake rates were significantly greater in light conditions than in dark conditions (Figure 1; one-sided paired t-test, P values RC = 0.050, EFWB = 0.013, HWC = 0.033), with the exception of GC where there was only one chamber from which both light and dark rates had been obtained (chamber NO3–N concentrations dropped below detection limit during some of the incubations). Benthic chamber uptake rates were consistently negative (increase in NO3–N concentration) at HWC and EFWB, but NO3–N production was greater during the night, resulting in higher values of uptake (less negative) during the day.
There were no discernable trends between the magnitude of day–night differences in uptake and the importance of autochthonous production. None of the relationships between day–night differences in uptake (whole-stream v f and U, and benthic uptake) and GPP/R were significant. However, the magnitude of the day–night differences in uptake was generally greater for chamber measurements compared to whole-stream measurements, with the exception of GC (Table 5).
Relationships Between Metabolism and NO3–N Uptake
Nitrate uptake generally increased with increasing metabolism, for both whole-stream and benthic chamber measures, but the relationships between uptake metrics and measures of metabolism varied greatly. Contrary to expectations, R was not a particularly good predictor of NO3–N uptake. Additionally, GPP and uptake appeared to be closely related when all sites were considered, and the relationships were not stronger when the sites with low autochthonous contributions were excluded.
Whole-stream NO3–N uptake as measured by v f generally increased with increasing metabolism, but there were no discernable relationships between U and metabolism (Figure 2). Whole-stream v f increased with increasing GPP, and the strength of the relationship was similar when all sites were considered (P = 0.002, r 2 = 0.72, n = 10) or when only the two sites with substantial autochthonous production were considered (P = 0.021, r 2 = 0.78, n = 6). When a potentially influential point was removed (highest GPP) the relationships were still significant (P = 0.018, r 2 = 0.57, n = 9; P = 0.050, r 2 = 0.77, n = 5). Whole-stream U did not exhibit a significant relationship with GPP for all sites or just RC and GC. Whole-stream v f showed a significant positive relationship with R when all sites were considered (P = 0.006, r 2 = 0.36, n = 20). However, the regression was influenced by a clustering of points with low v f and low R, and at higher values of R, the spread of GC points is suggestive of a negative slope. Similar to the findings for GPP, whole-stream U did not exhibit a significant relationship with R. Within the pair of sites with higher GPP/R values, GC, the site with the higher GPP/R and GPP values of the pair, had higher mean values of v f and U, and the difference was significant for v f (ANOVA, P < 0.0001; Table 3). Within the pair of sites with lower GPP/R values, EFWB, the site with higher rates of GPP and R, had higher mean values of v f and U than HWC, but the differences were not significant.
Relationships between NO3–N uptake and metabolism. Linear regression analysis was used to examine the relationships between NO3–N uptake and corresponding values of GPP and R for both whole-stream and benthic chamber measurements. Values of whole-stream NO3–N uptake (v f, and U) are from individual solute additions conducted during day (open symbols) or night (closed symbols). Whole-stream metabolism measures reflect average DO flux during the period in which solute additions were conducted (generally 1–2 h). For benthic chambers, light (open symbols) and dark (closed symbols) rates of uptake were obtained and paired with rates of metabolism from the same chamber. Values from all sites were analyzed together (solid regression lines), and for relationships with GPP, additional regressions were conducted using only the values from the two sites with substantial autochthonous production (RC and GC; dashed regression lines). Only relationships significant at the P < 0.05 level are shown. Symbols are as follows: circle, East Fork of Walker Branch; triangle, Gallina Creek; square, Hugh White Creek; diamond, Rio Calaveras.
Nitrate uptake in benthic chambers increased with increasing rates of metabolism, and the strongest relationship was between uptake and GPP across all sites (Figure 2; P = 0.0001, r 2 = 0.84, n = 13). When only values from RC and GC chambers were considered, the relationship was weaker but still significant (P = 0.050, r 2 = 0.65, n = 6). The relationship between uptake and R across all sites was significant but relatively weak (P = 0.0008, r 2 = 0.39, n = 25). With the removal of two influential points with the highest R values, the relationship was somewhat steeper (slope of 22.7 compared to 13.0) but still significant (P = 0.004, r 2 = 0.33, n = 23).
DISCUSSION
Importance of Autochthonous Versus Allochthonous Contributions
Different rates of GPP generated a range in the quantity of autochthonous carbon available across the four study streams, and values of GPP/R suggested that the balance between autochthonous and allochthonous carbon sources also varied. To assess the relative importance of autochthonous carbon at the sites, values of GPP/R were compared to the transitional P/R (Rosenfeld and Mackay 1987; Meyer 1989). The transitional P/R has been suggested as a way of characterizing the dependence of the heterotrophic community in a stream on autochthonous versus allochthonous sources of carbon. At GPP/R less than 0.5, R must be supported more by allochthonous than autochthonous sources of carbon, whereas at GPP/R greater than 1, R must be supported more by autochthonous than allochthonous sources. Using these guidelines, the relative importance of autochthonous carbon at the sites matched expectations based on canopy cover/light availability. Both whole-stream and benthic chamber measurements for the closed-canopy sites (HWC and EFWB) indicated that these streams are primarily reliant on allochthonous sources of carbon (GPP/R from 0.03 to 0.17). Whole-stream GPP/R values for the open-canopied sites (RC and GC) were both close to the transitional value of 0.5, indicating an important role for both sources of organic matter. Benthic chamber values of GPP/R for RC and GC were greater than one, and were the only circumstances under which the systems were primarily dependent on autochthonous carbon. Except for HWC, benthic chamber measurements at each site showed greater reliance on autochthonous carbon than whole-stream measurements.
The range in relative importance of autochthonous and allochthonous carbon observed across the sites provided the opportunity to test predictions on how this balance should influence relationships between NO3–N uptake and metabolism, including day–night differences. Based on values of GPP/R, a strong photoautotrophic contribution to uptake would not be expected in EWFB and HWC, but would be likely in RC and GC, especially for the benthic chambers. Large light–dark differences in uptake would be expected for RC and GC, as well as a tighter relationship between uptake and GPP for these two sites compared to all sites. Some, but not all of these predictions were met. Photoautotrophs played a greater role than was expected at the two closed-canopy sites, whereas their influence was not as great as expected at the two open canopy sites.
Contribution of Photoautotrophs to NO3-N Uptake
Photoautotrophs appeared to influence uptake at all four sites, despite low values of GPP/R at two sites. Uptake was greater in daylight than in the dark for both whole-stream and benthic chamber measurements in most cases. Although there were no significant differences between daytime and nighttime whole-stream uptake, daytime means were greater in magnitude at all sites for both uptake metrics. The observed day–night differences in uptake were likely due to photoautotrophic activity and not differences in temperature, NO3–N concentration, or discharge, because day–night differences in these physical/chemical variables were small and not statistically significant. The lack of significant day–night differences, despite the importance of autochthonous production at RC and GC, may be due to photoautotrophic uptake occurring during both day and night. Light enhances uptake of NO3–N by algae that use reductant generated during photosynthesis, but uptake can also occur in the dark when algae use stored carbon compounds (Abrol and others 1983; Huppe and Turpin 1994). A lack of diel variation in NO3–N uptake might also be seen if the magnitude of assimilation by photoautotrophs was small relative to other processes (for example, heterotrophic assimilation), or rates were changing within day and night periods.
Benthic NO3–N uptake rates were significantly greater in incubations conducted under light conditions compared to dark conditions for three of the four sites, reinforcing the trend observed for the whole-stream rates. Photoautotrophic influences should be more dominant in benthic sediment chambers compared to whole stream reaches due to the exclusion of heterotrophs associated with deep sediments (Grimm and Fisher 1984; Naegeli and Uehlinger 1997; Fellows and others 2001). Furthermore, differences in benthic rates may have been easier to detect because replicate chambers were run at the same time of day, in contrast to whole-stream measures that were made at different times of day. Gallina Creek was the only site at which the light–dark difference in rates was not significant, but it is likely that the incubations at this site did not represent an accurate assessment of benthic community uptake. Chamber NO3–N concentrations were already below ambient concentrations by the time the first water samples were collected, and final samples were below detection limit in several cases. As a result, measured rates are probably underestimates, and it is possible that benthic sediments at GC would have shown light-dark differences if uptake rates had been consistently detectable. In NO3–N-amended chamber incubations conducted at this site for a different purpose, light incubations had higher uptake rates than dark incubations (8,250 ± 1,430 μg NO3–N m−2h−1 compared to 5,005 ± 739 μg NO3–N m−2h−1, Fellows 2000). In EFWB and HWC, NO3–N release occurred during light and dark conditions, but rates were lower (that is, uptake less negative) during light conditions. Although light–dark differences in nitrification rates could result in this difference, it is likely that lower rates of NO3–N release under light conditions was due to photoautotrophic uptake, because no day–night differences in stream water NO3–N concentrations were observed.
Benthic chamber light–dark differences in uptake were generally greater than those for whole-stream measures, supporting the prediction that the magnitude of day–night differences would increase with increasing autochthonous contributions. However, across sites, variation in the magnitude of day–night differences was not explained by GPP/R ratios. Light–dark differences in uptake for GC and EFWB did not follow the expected ranking based on increasing autochthonous contributions: greater day–night differences were expected for GC and smaller differences for EFWB. Low stream water NO3–N concentrations at GC precluded detection of light–dark differences in chamber incubations, and also may explain why the relative magnitude of whole-stream day–night differences were not greater. With high rates of GPP and R at GC, metabolic demands are likely to be high relative to NO3–N availability, and may lead to consistently high allocation of resources to N uptake by both autotrophs and heterotrophs throughout the day. The idea that NO3–N uptake is very efficient at this site is reinforced by the significantly higher values of v f at GC compared to the other sites. The pair of closed-canopy streams had the same NO3–N concentrations as each other and very similar GPP/R ratios, but much larger day–night differences in uptake were observed at EFWB compared to HWC. Rates of GPP were greater in EFWB for both whole-stream and benthic chambers, and even though R was also large, photoautotrophic activity appears to have made a large enough contribution to total uptake to cause day–night differences.
In addition to looking for day–night differences in NO3–N uptake, the role of photoautotrophs can also be assessed by the magnitude of their contribution to total NO3–N uptake. Using published stoichiometry values to link metabolism and nutrient assimilation, photoautotrophic demand for N was estimated and compared with measured NO3–N uptake. We assumed a molar C:N content of algal biomass of 12:1 (Grimm 1985; Dodds and Priscu 1990; Webster and others 2003) to calculate a predicted rate of autotrophic N assimilation for each site from values of whole-stream and chamber net primary production (NPP). NPP was assumed to be 50% of GPP (Odum 1957; Webster and Meyer 1997) and the photosynthetic quotient was set to 1 (that is, 1 mol O2 produced:1 mol CO2 fixed). Measured NO3–N uptake rates were scaled to 24 h and then plotted against calculated photoautotrophic demand and compared to a 1:1 line (Figure 3). Values of calculated demand were substantial relative to measured uptake, except for the negative chamber uptake values for EFWB and HWC. Whole-stream uptake was very similar to calculated demand for RC. Measured NO3–N uptake in benthic chambers for GC and RC, as well as whole-stream values for GC, were below the 1:1 line, suggesting that assimilation exceeded measured NO3–N uptake. In contrast, EFWB and HWC whole-stream uptake was greater than estimated autotrophic assimilation. At these two sites, benthic chamber NO3–N release exceeded autotrophic demand.
Comparison of area-specific NO3–N uptake rates to calculated photoautotrophic assimilatory N demand. Rates of net primary production from whole-stream (closed symbols) and benthic chamber (open symbols) measurements were used along with a C:N molar ratio of 12:1 to calculate predicted algal N assimilatory demand (straight line represents the 1:1 line for measured:calculated values; see text for more details). Symbol shapes are as in Figure 2.
The comparison of estimated rates of photoautotrophic assimilation with measured NO3–N uptake rates enable some broad generalizations to be drawn about the relative importance of this process and other processes affecting NO3–N uptake at the sites. Values of NO3–N assimilation by photoautotrophs that equal or exceed 100% of measured values, such as RC and GC whole-stream and chamber rates, suggest that this process may dominate uptake. Failure to detect significant day–night differences at these sites therefore was not likely due to autotrophic uptake being small in magnitude compared to total uptake. When values exceed 100%, another source of N (for example, NH4–N) or production of NO3–N via nitrification may be important to meet assimilatory demand. Estimates of photoautotrophic assimilation that are less than 100% of observed uptake, as was the case for whole-stream uptake at HWC and EFWB, suggest that other processes are involved in NO3–N uptake, such as heterotrophic assimilation and/or denitrification. On the other hand, with values of 50 and 39%, photoautotrophic uptake appears to be a significant process even in these forest streams, at least during summer when the study was conducted. This is consistent with the suggestion by Hall and Tank (2003) that photoautotrophs are likely more important than heterotrophs in NO3–N uptake based on their study of whole-stream NH4–N and NO3–N uptake in 11 Wyoming streams.
Nearly all published examples of short-term solute additions have been conducted during the day (but see Martí and others 1994). Based on the results from this study, measurements of whole-stream NO3–N uptake from daytime additions would slightly overestimate uptake when scaled to 24 h. For the four sites in this study, multiplying the mean daytime value of U by 24 h results in values that exceed those calculated using both day and night time additions by 6% for GC, 10% for HWC, 25% for EFWB, and 37% for RC. One other study has reported on day–night differences in whole-stream nutrient uptake, and found significant differences in day and night uptake rates for NH4–N and phosphate in La Solana, a Mediterranean stream in Spain (Martí and others 1994).
Relationships Between NO3–N Uptake and Metabolism
Nutrient uptake should increase with increasing rates of metabolism due to assimilative demands of in-stream biota, but predictions based on this general hypothesis were not consistently met in this study. We predicted increasing uptake with increasing GPP, especially in systems with substantial autochthonous production, and expected R to be a good predictor of uptake across all systems. No relationships between U and metabolism were observed, and relationships between v f or benthic uptake and R were weak. Relationships between v f or benthic uptake and GPP were stronger than those with R, but were not improved by excluding sites with low autochthonous production.
The two previous studies that have investigated both whole-stream N uptake and metabolism in multiple streams, Hall and Tank (2003) and Webster and others (2003), also found variation in the strength of relationships between uptake and metabolism. In a study of 15N–NH4 uptake in 11 streams across multiple biomes, U ranged aproximately one order of magnitude among streams but did not show a relationship with metabolism (Webster and others 2003). Furthermore, Webster and others (2003) found no significant relationships between any measure of uptake (that is, S w, v f, U) and any metabolic parameter. However, N demand calculated from NPP, microbial production, and measured detrital C:N ratios compared reasonably well to measured assimilative N uptake (NH4–N and NO3–N). They argued that the multiple mechanisms of nitrogen uptake must be taken into account when seeking a relationship between uptake and metabolism. In contrast, Hall and Tank (2003) found that GPP was related to v f for NO3–N, and that both GPP and R were related to v f for NH4–N for 11 Wyoming streams. They suggested that these strong relationships stem from the close geographic proximity of their sites and the higher observed variation in v f compared to Webster and others (2003). The strongest relationship among whole-stream measures for our four streams was between v f and GPP, and the relationship with R was weak but significant. Predictions of total assimilative N uptake should approach measured values as calculations become more detailed, including individual uptake processes, and C:N ratios of organisms. However, many of these values, such as heterotrophic growth efficiency and the ratio between NPP and GPP are taken from the literature as opposed to being site-specific, and the detail of study required at each site to some extent precludes the inclusion of a large number of sites. The relationships between uptake and metabolism may be too complex to allow detection of simple linear relationships in some cases, but it is still worthwhile to consider the circumstances under which relationships might emerge.
The tightest relationships between NO3–N uptake and a single metric of metabolism would be expected at either end of the spectrum of the importance of autochthonous versus allochthonous carbon sources. In systems dominated by autochthonous carbon contributions, total ecosystem uptake of inorganic N will be dominated by autotrophic uptake. A tight relationship between total uptake and GPP is predicted, through autotrophic assimilation, with the slope of the relationship influenced by the C:N of the autotroph. Because GPP and autotrophic R are related, there will also be a tight relationship between uptake and autotrophic R. With heterotrophic R primarily fueled by autochthonous carbon sources, most heterotrophic N demand should be met from N contained in these low C:N substrates. This results in a weak relationship, if any, between heterotrophic N uptake and heterotrophic R. In contrast, in allochthonous-dominated systems, heterotrophs using these high C:N carbon sources will need to take up inorganic N. A tight relationship between heterotrophic uptake and R therefore is expected, with the slope depending on the C:N of the substrate being degraded. Autotrophic activity will be low, and total ecosystem N uptake will not show a relationship with GPP because heterotrophic uptake dominates. The predicted importance of C:N ratios in influencing the relationship between uptake and metabolism for both autotrophs and heterotrophs is consistent with the findings of Dodds and others (2004), who reported that C:N ratio explains a substantial part of the variation in N-specific uptake rates of organisms and detritus across a number of terrestrial and aquatic ecosystems. When both autochthonous and allochthonous sources of carbon are important, relationships between ecosystem uptake and metabolism are likely to be blurred because of the lack of dominance of either autotrophic or heterotrophic uptake, and the influence of having a mix of detrital components with different C:N ratios. To test the relationships suggested by this conceptual model, autochthonous or allochthonous-dominated systems could be targeted based on a priori knowledge of GPP/R ratios. One limitation to testing this model is the uncertainty associated with separating ecosystem R into autotrophic and heterotrophic components, which is currently done by assuming that autotrophic R represents a fixed fraction of GPP.
Simple linear relationships between uptake and metabolism are predicted based on the assumption of constant C:N ratios, but work in lake ecosystems suggests that systematic changes in stoichiometry might be seen with changes in light and nutrient availability (Sterner and others 1997; Sterner and Elser 2002). Sterner and others (1997) found that as the ratio between light and P availability increased, so did the ratio of C:P in biomass. If the C:N ratio of new autotrophic biomass increases with increasing light/nutrient ratios, a flattening of the relationship between uptake and GPP would be seen, with higher values of GPP having lower than expected uptake. If this were the case for GC which had very low NO3–N concentrations, a higher C:N value would reduce the calculated autotrophic demand, effectively moving the points on Figure 3 to the left, closer to the value of measured uptake. It also might explain the trend of decreasing uptake with increasing R for GC and EFWB in Figure 2. Because the concept of the light/nutrient ratio was developed in lakes, using total phosphorus as a metric of nutrient availability, further work in streams is needed, especially to decide what an appropriate measure of nutrient availability would be.
Assimilatory uptake is the focus of this and other studies that have investigated metabolism and N uptake, but dissimilatory processes, such as denitrification and nitrification, may be quantitatively important in some stream ecosystems. Denitrification would show a positive relationship with production of carbon dioxide, but not necessarily any relationship with consumption of oxygen, because it is an anaerobic respiratory process. Denitrification therefore could increase uptake beyond what would be predicted based on rates of metabolism. Most studies assume denitrification is a relatively small component of NO3–N uptake in the oxic environment of streams, especially streams with low NO3–N concentrations (for example, Hall and Tank 2003; Webster and others 2003). A recent study by Mulholland and others (2004) using an addition of 15N–NO3 has confirmed that this is the case for EFWB. On the other hand, the importance of nitrification in streams has been shown to vary greatly, from below detection to close to 100% of measured NH4–N uptake (Bernhardt and others 2002; Webster and others 2003). Nitrification consumes oxygen while producing NO3–N, increasing the apparent rate of R and possibly causing an underestimation of assimilatory uptake. Neither nitrification nor denitrification can be quantified using solute additions of non-labeled NO3–N, in which measured uptake reflects the net demand resulting from the interaction of all organisms present. However, nitrification was observed in this study when benthic sediments were isolated in chambers at the two closed-canopy streams. Recent studies on nitrification in streams have focused on factors that allow nitrifiers to successfully compete with heterotrophs for NH4–N, including availability of labile organic carbon (Strauss and Lamberti 2000, 2002; Bernhardt and others 2002; Bernhardt and Likens 2002) and the concentration of NO3–N (Bernhardt and others 2002). Low-availability of labile organic carbon can allow nitrifiers to out-compete heterotrophs (Strauss and Lamberti 2000, 2002). Because rates of GPP are low at EFWB and HWC, and our measurements in August were approximately 10 months since last autumnal leaf fall, labile organic carbon availability was likely to have been low and may be a factor contributing to the observed benthic nitrification.
Our results add to the growing number of studies confirming the importance of small streams in reducing loads of dissolved inorganic N being transported downstream (Alexander and others 2000; Peterson and others 2001a; Bernhardt and others 2002; Mulholland 2004; Bernhardt and others 2005). All four streams demonstrated uptake of NO3–N over the reaches studied, despite evidence of nitrification in benthic sediments at two of the sites. Day-night differences in NO3–N uptake of stream reaches as well as isolated benthic sediments support the conclusions of Webster and others (2003) and Hall and Tank (2003) that differences in nutrient spiraling are linked to metabolic activity. Differences in the relationship between stream metabolism and nutrient uptake stem in part from the relative availability of autochthonous versus allochthonous carbon sources, which in turn is influenced by interaction of the stream and its catchment. Valett and others (2002) proposed that streams at the two ends of this spectrum, desert and forest streams, exhibit very different nutrient retention over successional time. Nutrient retention in desert streams is dominated by autochthonous processes, and is therefore controlled by the successional stage of in-stream autotrophs (for example, following floods, Grimm 1987). Conversely, the status of the catchment vegetation (seasonally as well as over successional time) controls retention in forest streams, where retention is dominated by processes fuelled by allochthonous carbon. Our findings suggest that with respect to NO3–N uptake, autochthonous processes can play an important role in uptake even in forest streams where they contribute a relatively small portion of the carbon budget.
References
Aber JD, Nadelhoffer KJ, Steudler P, Melillo JM. 1989. Nitrogen saturation in northern forest ecosystems. Bioscience 39:378–86
Abrol YP, Sawhney SK, Naik MS. 1983. Light and dark assimilation of nitrate in plants. Plant Cell Environ 6:595–9
Alexander RB, Smith RA, Schwarz GE. 2000. Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403:758–61
APHA, AWWA, and WEF. 1992. Standard Methods for the Examination of Water and Wastewater, 18th ed. Washington, (DC): American Public Health Association
Baker MA, Valett HM, Dahm CN. 2000. Organic carbon supply and metabolism in a shallow groundwater ecosystem. Ecology 81:3133–48
Beck WS, Liemn KF, Simpson GG. 1991. Life: an introduction to biology. New York: Harper Collins Publishers
Bernhardt ES, Likens GE. 2002. Dissolved organic carbon enrichment alters nitrogen dynamics in a forest stream. Ecology 83:1689–700
Bernhardt ES, Hall RO, Likens GE. 2002. Whole-system estimates of nitrification and nitrate uptake in streams of the Hubbard Brook Experimental Forest. Ecosystems 5:419–30
Bernhardt ES, Likens GE, Hall RO, Buso DC, Fisher SG, Burton TM, Meyer JL, McDowell MH, Mayer MS, Bowden WB, Findlay SEG, Macneale KH, Stelzer RS, Lowe WH. 2005. Can’t see the forest for the stream? – In-stream processing and terrestrial nitrogen exports. Bioscience 55:219–30
Bott TL, Brock JT, Cushing CE, Gregory SV, King D, Petersen RC. 1978. A comparison of methods for measuring primary productivity and community respiration in streams. Hydrobiologia 60:3–12
Davis JC, Minshall GW. 1999. Nitrogen and phosphorus uptake in two Idaho (USA) headwater wilderness streams. Oecologia 119:247–55
Dodds WK, Priscu JC. 1990. A comparison of methods for assessment of nutrient deficiency of phytoplankton in a large oligotrophic lake. Can J Fish Aquat Sci 47:2328–38
Dodds WK, Marti E, Tank JL, Pontius J, Hamilton SK, Grimm NB, Bowden WB, McDowell WH, Peterson BJ, Valett HM, Webster JR, Gregory S. 2004. Carbon and nitrogen stoichiometry and nitrogen cycling rates in streams. Oecologia 140:458–67
Elser JJ, Dobberfuhl DR, MacKay NA, Schampel JH. 1996. Organism size, life history, and N:P stoichiometry: toward a unified view of cellular and ecosystem processes. Bioscience 46:674–84
Elwood JW, Newbold JS, O’Neill RV, Winkle WV. 1983. Resource spiraling: an operational paradigm for analyzing lotic ecosystems. In: Fontaine TD III, Bartell SM, Eds. Dynamics of lotic ecosystems. Ann Arbor (Michigan): Ann Arbor Science
Fellows CS. 2000. Ecosystem metabolism and nitrate retention in headwater streams. PhD thesis, Albuquerque: University of New Mexico
Fellows CS, Valett HM, Dahm CN. 2001. Whole-stream metabolism in two montane streams: Contribution of the hyporheic zone. Limnol Oceanogr 46:523–31
Fisher SG, Likens GE. 1973. Energy flow in Bear Brook, New Hampshire: an integrative approach to stream ecosystem metabolism. Ecol Monogr 43:421–39
Fisher SG, Gray LJ, Grimm NB, Busch DE. 1982. Temporal succession in a desert stream ecosystem following flash flooding. Ecol Monogr 52:93–110
del Giorgio PA, Cole JJ. 1998. Bacterial growth efficiency in natural aquatic systems. Ann Rev Ecol Syst 29:503–41
Gordon ND, McMahon TA, Finlayson BL. 1992. Stream hydrology: an introduction for ecologists. New York: Wiley
Grimm NB. 1985. Roles of primary producers and consumers in nitrogen dynamics of a desert stream ecosystem. PhD Dissertation. Tempe (Arizona): Arizona State University
Grimm NB. 1987. Nitrogen dynamics during succession in a desert stream. Ecology 68:1157–70
Grimm NB, Fisher SG. 1984. Exchange between interstitial and surface-water: implications for stream metabolism and nutrient cycling. Hydrobiologia 111:219–28
Gulis V, Suberkropp K. 2003. Effect of inorganic nutrients on relative contributions of fungi and bacteria to carbon flow from submerged decomposing leaf litter. Microb Ecol 45:11–9
Hall RO Jr, Tank JL. 2003. Ecosystem metabolism controls nitrogen uptake in streams in Grand Teton National Park, Wyoming. Limnol Oceanogr 48:1120–8
Hall RO Jr, Bernhardt ES, Likens GE. 2002. Relating nutrient uptake with transient storage in forested mountain streams. Limnol Oceanogr 47:255–65
Hedin LO, von Fischer JC, Ostrom NE, Kennedy BP, Brown MG, Robertson GP. 1998. Thermodynamic constraints on nitrogen transformations and other biogeochemical processes at soil-stream interfaces. Ecology 79:684–703
Hill WR, Mulholland PJ, Marzolf ER. 2001. Stream ecosystem responses to forest leaf emergence in spring. Ecology 82:2306–19
Huppe HC, Turpin DH. 1994. Integration of carbon and nitrogen metabolism in plant and algal cells. Ann Rev Plant Physiol Plant Mol Biol 45:577–607
Martí E, Armengol J, Sabater S. 1994. Day and night nutrient uptake differences in a calcareous stream. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 25:1756–60
Marzolf ER, Mulholland PJ, Steinman AD. 1994. Improvements to the diurnal upstream-downstream dissolved-oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 51:1591–9
McCutchan JH Jr, Saunders JF III, Lewis WM Jr, Hayden MG. 2002. Effects of groundwater flux on open-channel estimates of stream metabolism. Limnol Oceanogr 47:321–4
McCutchan JH, Lewis WM, Saunders JF. 1998. Uncertainty in the estimation of stream metabolism from open-channel oxygen concentrations. J North Am Benthol Soc 17:155–64
Meyer JL. 1989. Can P/R ratio be used to assess the food base of stream ecosystems? A comment on Rosenfeld and Mackay (1987). Oikos 54:119–21
Meyer JL, Edwards RT. 1990. Ecosystem metabolism and turnover of organic-carbon along a blackwater river continuum. Ecology 71:668–77
Minshall GW. 1978. Autotrophy in stream ecosystems. Bioscience 28:767–70
Minshall GW, Petersen RC, Cummins KW, Bott TL, Sedell JR, Cushing CE, Vannote RL. 1983. Interbiome comparison of stream ecosystem dynamics. Ecol Monogr 53:1–25
Mulholland PJ. 1992. Regulation of nutrient concentrations in a temperate forest stream: roles of upland, riparian, and instream processes. Limnol Oceanogr 37:1512–26
Mulholland PJ. 2004. The importance of in-stream uptake for regulating stream concentrations and outputs of N and P from a forested watershed: evidence from long-term chemistry records for Walker Branch Watershed. Biogeochemistry 70:403–26
Mulholland PJ, Marzolf ER, Webster JR, Hart DR, Hendricks SP. 1997. Evidence that hyporheic zones increase heterotrophic metabolism and phosphorus uptake in forest streams. Limnol Oceanogr 42:443–51
Mulholland PJ, Fellows CS, Tank JL, Grimm NB, Webster JR, Hamilton SK, Marti E, Ashkenas L, Bowden WB, Dodds WK, McDowell WH, Paul MJ, Peterson BJ. 2001. Inter-biome comparison of factors controlling stream metabolism. Freshw Biol 46:1503–17
Mulholland PJ, Valett HM, Webster JR, Thomas SA, Cooper LW, Hamilton SK, Peterson BJ. 2004. Stream denitrification and total nitrate uptake rates measured using a field N-15 tracer addition approach. Limnol Oceanogr 49:809–20
Naegeli MW, Uehlinger U. 1997. Contribution of the hyporheic zone to ecosystem metabolism in a prealpine gravel-bed river. J North Am Benthol Soc 16:794–804
Nealson KH, Stahl DH. 1997. Microorganisms and biogeochemical cycles: What can we learn from layered microbial communities? In: Banfield JF, Nealson KH, Eds. Geomicrobiology: interactions between microbes and minerals. Washington, (DC): Mineralogical Society of America. p 5–34
Newbold JD, Elwood JW, O’Neill RV, Vanwinkle W. 1981. Measuring nutrient spiralling in streams. Can J Fish Aquat Sci 38:860–3
Odum HT. 1956. Primary production in flowing waters. Limnol Oceanogr 1:103–17
Odum HT. 1957. Trophic structure and productivity of Silver Springs, Florida. Ecol Monogr 27:55–112
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Marti E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory S, Morrall DJ. 2001a. Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Peterson CG, Horton MA, Marshall MC, Valett HM, Dahm CN. 2001b. Spatial and temporal variation in the influence of grazing macroinvertebrates on epilithic algae in a montane stream. Archiv für Hydrobiologie 153:29–54
Peterson CG, Valett HM, Dahm CN. 2001c. Shifts in habitat templates for lotic microalgae linked to interannual variation in snowmelt intensity. Limnol Oceanogr 46:858–70
Poff NL, Ward JV. 1989. Implications of streamflow variability and predictability for lotic community structure: a regional-analysis of streamflow patterns. Can J Fish Aquat Sci 46:1805–18
Redfield AC. 1958. The biological control of chemical factors in the environment. Am Sci 46:205–21
Reiners WA. 1986. Complementary models for ecosystems. Am Nat 127:59–73
Romani AM, Butturini A, Sabater F, Sabater S. 1998. Heterotrophic metabolism in a forest stream sediment: surface versus subsurface zones. Aquat Microb Ecol 16:143–51
Rosenfeld JS, Mackay RJ. 1987. Assessing the food base of stream ecosystems: alternatives to the P/R ratio. Oikos 50:141–7
SAS. 1999. The SAS System for Windows, Version 8. Cary (North Carolina): SAS Institute
Schlesinger WH. 1997. Biogeochemistry: an analysis of global change, 2nd ed. San Diego: Academic Press
Smith RA, Alexander RB, Wolman MG. 1987. Water-quality trends in the nations rivers. Science 235:1607–15
Sterner RW, Elser JJ. 2002. Ecological stoichiometry: the biology of elements from molecules to the biosphere. Princeton (New Jersey): Princeton University Press
Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH. 1997. The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am Nat 150:663–84
Strauss EA, Lamberti GA. 2000. Regulation of nitrification in aquatic sediments by organic carbon. Limnol Oceanogr 45:1854–9
Strauss EA, Lamberti GA. 2002. Effect of dissolved organic carbon quality on microbial decomposition and nitrification rates in stream sediments. Freshw Biol 47:65–74
Stream Solute Workshop. 1990. Concepts and methods for assessing solute dynamics in stream ecosystems. J North Am Benthol Soc 9:95–119
Suberkropp K. 1998. Effect of dissolved nutrients on two aquatic hyphomycetes growing on leaf litter. Mycol Res 102:998–1002
Triska FJ, Kennedy VC, Avanzino RJ, Zellweger GW, Bencala KE. 1989. Retention and transport of nutrients in a 3rd-order stream: channel processes. Ecology 70:1877–92
Valett HM, Crenshaw CL, Wagner PF. 2002. Stream nutrient uptake, forest succession, and biogeochemical theory. Ecology 83:2888–901
Valett HM, Morrice JA, Dahm CN, Campana ME. 1996. Parent lithology, surface-groundwater exchange, and nitrate retention in headwater streams. Limnol Oceanogr 41:333–45
Vitousek PM, Howarth RW. 1991. Nutrient limitation on land and in the sea: how can it occur? Biogeochemistry 13:87–115
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG. 1997. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol Appl 7:737–50
Webster JR, Patten BC. 1979. Effects of watershed perturbation on stream potassium and calcium dynamics. Ecol Monogr 49:51–72
Webster JR, Meyer JL. 1997. Stream organic matter budgets. J North Am Benthol Soc 16:3–161
Webster JR, D’Angelo DJ, Peters GT. 1991. Nitrate and phosphate uptake in streams at Coweeta Hydrologic Laboratory. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 24:1681–6
Webster JR, Mulholland PJ, Tank JL, Valett HM, Dodds WK, Peterson BJ, Bowden WB, Dahm CN, Findlay S, Gregory SV, Grimm NB, Hamilton SK, Johnson SL, Marti E, McDowell WH, Meyer JL, Morrall DD, Thomas SA, Wollheim WM. 2003. Factors affecting ammonium uptake in streams – an inter-biome perspective. Freshw Biol 48:1329–52
Wetzel RG, Likens GE. 1991. Limnological analyses, 2nd edn. Berlin Heidelberg New York: Springer
Young RG, Huryn AD. 1998. Comment: improvements to the diurnal upstream-downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquat Sci 55:1784–5
Young RG, Huryn AD. 1999. Effects of land use on stream metabolism and organic matter turnover. Ecol Appl 9:1359–76
Acknowledgements
For unflagging assistance in field and lab work, we thank Jim Thibault, Chelsea Crenshaw, Chris Thomas, Andrea Shriver, Michelle Baker, John Craig, Todd Royer, Laura Bean, Doug Moyer, John Morrice, Miranda Fleig, and Diana Northup. Special thanks to P. Valero and A. Bearce for access to the New Mexico sites. Comments from Nancy Grimm, Wade Hadwen, and two anonymous reviewers improved the manuscript. Funding for this research was provided by NSF DEB grants 9902324 to C.N.D. and C.S.F, 9815868 to H.M.V. and P.J.M., 9816087 to C.N.D and P. Unnikrishna, 9420510 to H.M.V. and M.E. Campana, and a NSF Graduate Fellowship to C.S.F.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fellows, C.S., Valett, H.M., Dahm, C.N. et al. Coupling Nutrient Uptake and Energy Flow in Headwater Streams. Ecosystems 9, 788–804 (2006). https://doi.org/10.1007/s10021-006-0005-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10021-006-0005-5