Abstract
Meningiomas are the most commonly diagnosed benign intracranial adult tumors. Subsets of meningiomas that present with extensive invasion into surrounding brain areas have high recurrence rates, resulting in difficulties for complete resection, substantially increased mortality of patients, and are therapeutically challenging for neurosurgeons. Exciting new data have provided insights into the understanding of the molecular machinery of invasion. Moreover, clinical trials for several novel approaches have been launched. Here, we will highlight the mechanisms which govern brain invasion and new promising therapeutic approaches for brain-invasive meningiomas, including pharmacological approaches targeting three major aspects of tumor cell invasion: extracellular matrix degradation, cell adhesion, and growth factors, as well as other innovative treatments such as immunotherapy, hormone therapy, Tumor Treating Fields, and biodegradable copolymers (wafers), impregnated chemotherapy. Those ongoing studies can offer more diversified possibilities of potential treatments for brain-invasive meningiomas, and help to increase the survival benefits for patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
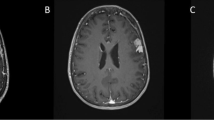
Meningiomas are primary brain tumors that originate from meningothelial (arachnoid) cells [1] and the proportion in all intracranial tumors exceeds 35% [2]. The World Health Organization (WHO) has defined three meningioma subtypes based on the number of mitotic figures and malignant degree: benign, atypical, and anaplastic, and account for 70–80%, 5–20%, and 1–3%, respectively [3]. Benign meningiomas usually do not disrupt the surrounding brain structures. Therefore, complete surgical resection is the preferred treatment for the vast majority of these meningiomas. However, even in benign cases, meningiomas have high recurrence rates, even after curative surgical treatment [4]. It is documented that recurrence rates of benign meningiomas can be up to 20%, even after Simpson I resections, and atypical and anaplastic tumor grades can have recurrence rates of up to 40% and 80%, respectively [5]. The most important factors affecting the recurrence of meningiomas are tumor grade, the extent of surgical resection, and invasion of adjacent brain tissue [6, 7]. Meningiomas with brain invasion generally show obvious edema of brain tissue around the tumor in T2 weighted MRI, and the tumor demonstrates the ability to circumvolute vessels or break through the arachnoid under the microscope during surgery (Fig. 1). Consequences of such a brain-invasion include neurological compromise and a further decrease in the possibility of total surgical resection. The latest classification by WHO of brain tumors proposed brain invasion as an unattached standard for atypia and therefore guide diagnosis and treatment for meningioma [3].
Clinical cases of meningioma exhibiting biological behavior of brain invasion. a Magnetic resonance imaging (MRI) of brain-invasive meningiomas demonstrating vanishment of interface with the brain tissue and marked surrounding edema. b Microscopic images of brain-invasive meningiomas during the surgery. Black arrows highlight the site where the tumors penetrate the arachnoid interface and invade the normal brain tissues. c Pathological images of brain-invasive meningiomas under different magnification
Despite invasion stated explicitly as neoplastic tissue within the adjacent brain, and no separating tissue layers exist in the latest edition [3], there are other evaluating criteria for brain invasion. Brain-invasive meningioma was first defined in the WHO classification in 1993, and its definition was rather vague, partially including [8] or excluding, [9] tumor cells growing along with the Virchow-Robin spaces. Owing to the contributions by Perry et al. [10], a more accurate definition of invasive growth was illustrated as tumor cells invading adjacent brain tissue without separating the connective tissue layer. However, sometimes the surgical specimens used to assess invasion lacked an infiltrative interface with the brain; therefore, Rempel et al. [11] suggested SPARC (secreted protein, acidic and rich in cysteine) as a sign of brain-invasive meningioma. Beyond that, staining for GFAP (glial fibrillary acidic protein), CD44, and EMA (epithelial membrane antigen) were shown to increase the sensitivity of detecting brain invasions [12, 13]. Moreover, some studies further described the pattern of meningioma infiltrative growth: (1) diffuse growth (single cells diffuse into brain tissue) [14, 15]; (2) nests/cluster-like (islands of tumor cells) [14,15,16]; and (3) finger-like/tongue-like tumor expansion into the surrounding brain [13, 14, 16]. Interestingly, infiltration also exhibits gender-specific patterns [14]. Based on previous results, Brokinkel et al. [12] recently recommended a more systematic and accurate detection standard for brain invasion in meningiomas, which contains pre-, intra-, and post-operative methods and should be indicated in communications between neurosurgeons and neuropathologists.
For such invasive meningiomas, gross total resections are not always possible. Meanwhile, adjuvant irradiation strategy in invasive meningiomas has only been investigated in a few studies and has not shown promising prognostic effect yet [12]. In 2014, a study reported by Sun et al. [17] revealed no significant prognostic impacts for radiation therapy in totally resected atypical meningiomas. Consistently, in a larger cohort of 50 patients, whether received radiation therapy or stereotactic radiosurgery for residual meningioma, there is a much higher recurrence risk in the brain-invasive meningioma group [18]. Moreover, atypical meningiomas with spontaneous necrosis appear to be resistant to radiotherapy [18]. Thus, it is essential to review the underlying molecular mechanisms of brain-invasive meningioma tumor cells and describe the current understanding of target treatment options and other promising approaches for brain-invasive meningiomas.
Molecular targets and related agents for brain-invasive meningiomas
After undergoing surgical resection and radiotherapy, patients who relapse with brain-invasive meningiomas have limited therapeutic choices. Next, some critical molecules and related drugs that have potential actions against brain-invasive meningiomas will be reviewed.
Brain invasion of meningioma involving interactions between meningioma cells, normal brain stromal cells, the extracellular matrix (ECM), and basement membranes (BM), which is considered a three-step process, initially degradation of ECM/BM, and tumor cells migration, finally promoting adhesion of meningioma cells to resident cells with the help of growth factors and blood-vessel formation [19, 20], leading to local brain invasion (Fig. 2). Thus, mediators of the invasive behavior of meningioma tumor cells mainly focus on tissue-degrading enzymes, cell adhesion molecules, and various growth factors, which promote tumor proliferation and angiogenesis.
Meningioma brain invasion is considered a three-step process that requires various kinds of proteases to degrade the ECM, adhesion molecules to promote tumor cell migration to resident cells, and different growth factors and neovascularization to feed and support meningioma tumor cells, leading to local brain invasion
Extracellular matrix degradation
Degradation of the ECM/BM is thought to be one of the most important determinants of tumor cell invasion [21]. ECM/BM are rigid structures formed from macromolecules, such as type IV collagen, laminin, entactin, nidogen, fibronectin, and heparin sulfate proteoglycans (HSPG). Proteases, including matrix metalloproteinases (MMPs), serine proteases, cathepsins, and heparanase (HPSE), have the capability of breaking down basal membranes and connective tissue [22,23,24]. Therefore, such enzymes play crucial roles in the process of meningioma invasive growth [25].
MMPs
MMPs are lysosomal endopeptidases involved in ECM degradation [26]. MMPs have been grouped into the following four broad categories based on their substrate specificity: (I) interstitial collagenases (MMP-1, MMP-8, and MMP-13) that degrade fibrillary collagens; (II) type IV collagenases (MMP-2 and MMP-9) that degrade the basement membrane collagens gelatin and elastin; (III) stromelysins (MMP-3, MMP-10, and MMP-11) that degrade proteoglycans, fibronectin, laminin, gelatin, and the globular proteins of type IV collagens; and (IV) membrane-type MMPs (MMP-14, MMP-15, MMP-16, and MMP-17) that contain a unique transmembrane domain in their carboxyl-terminus that localizes these MMPs to the cell surface [27]. There are natural inhibitors of MMPs, inhibitors of matrix metalloproteinases (TIMPs), which form a complex with the active centers of MMPs and control their activities. Specifically, TIMP-1 inhibits MMP-9, whereas TIMP-2 controls MMP-2. Importantly, MMP-2 and MMP-9 are found to be expressed in a broad spectrum of meningiomas and represent valuable prognostic factors in predicting higher risk recurrence in totally resected meningiomas [28, 29]. Recently, Jalali et al. [28] demonstrated that MMP-16 modulates meningioma invasion by positively regulating MMP-2. MMP-3 is positively correlated with meningiomas having an aggressive character [30]. Besides, RNA interference (RNAi)-mediated targeting of MMP-9 significantly regressed cell invasion and orthotopic meningioma formation [31, 32]. Taken together, the results show the potential of MMP inhibitors as a promising therapy for invasive meningioma, and the synthesis of a wide-spectrum inhibitor of MMPs is urgently needed.
The urokinase plasminogen (uPA)-uPA receptor (uPAR) system
The 55-kDa serine protease uPA consists of two disulfide bridges linked to polypeptides. It can be converted from an inactive precursor into an active form by the actions of various proteases, including plasmin, cathepsins B or L [33]. Active uPA interact with receptor uPAR, subsequently requires transmembrane co-receptors for signaling transduction. Integrins family are one of those essential co-receptors [34, 35]. As a result, uPA-uPAR can efficiently convert plasminogen to active plasmin [34]. Plasmin is essential for the degradation of ECM in direct and indirect ways. Additionally, uPA is known to exert additional activities, including the promotion of cellular migration, proliferation, and alteration of cellular adhesive properties [34].
uPA is expressed in many tumor types, including breast, lung, glioblastoma, prostate, and esophageal squamous cell carcinomas [36,37,38,39]. It is documented that synthetic inhibitors target uPA could efficiently inhibit the metastasis of prostate and mammary carcinoma cells [40, 41]. Regulation of uPA is achieved at many levels, including at least two types of fast-acting specific inhibitors, plasminogen activator inhibitor (PAI)-1 and PAI-2. A report by Kandenwein et al. [42] showed that the expression of uPA may be controlled, in part, by promoter methylation and is correlated with pathological meningiomas. Importantly, uPA and uPAR interference by RNAi led to the inhibition of intracranial meningioma formation in a mouse model [43]. Based on these studies, low-molecular-weight inhibitors, which target the uPA-uPAR-PAI-1 system, should be considered as treatment options for invasive meningiomas.
Lysosomal cysteine proteinases
Lysosomal cysteine proteinases are comprised of a large family of papain-like enzymes [44]. Schmitt et al. [45] proposed a proteolytic cascade by cysteine proteases, which starts with the activation of the lysosomal enzymes cathepsin D and cysteine cathepsins B and L, subsequently activating cell membrane-associated pro-urokinase, inducing the extracellular release of plasmin from plasminogen to finally activate various types of MMPs and degrade collagen and other basal lamina proteins. It has been proved that the progression of brain tumors strongly correlated with the high expression of cathepsins B, L, and H [46, 47]. Three types of endogenous inhibitors control their activity: stefins, cystatins, and kininogens [48]. Generally, stefins (stefins A and B) predominantly act intracellularly [47], whereas cystatins (cystatins C) and kininogens act extracellularly [49]. The balance between these inhibitors and cysteine cathepsins seems to be highly relevant and may serve as biomarkers for tumor progression. In brain tumors, the down-regulation of total inhibitory activity of cystatins has been observed and takes responsibility for early meningioma recurrence. Thus, it seems to especially safeguard brain tissues [50]. Consistently, cathepsin B and L are highly expressed in invasive types of benign meningiomas and are considered as diagnostic markers for invasive meningiomas [51]. Collectively, the use of cysteine protease inhibitors may lead to more informed therapeutic strategies in the future.
Heparin sulfate proteoglycans (HSPG) and heparanases (HPSE)
Invasion by meningioma tumor cells is affected by the microenvironment, including components of the ECM/BM, such as HSPG, which is composed of a core protein to which repeating disaccharide units have been added. The specific structures of heparin sulfate chains are achieved through several enzymatic steps that create highly charged, sulfated micro-domains, and are secreted as entities into the ECM or intracellular secretory vesicles [52, 53]. A multitude of growth factors and chemokines bind to HPSG, regulating biological processes, and modulating the adhesion and spread of tumor cells [54]. HPSE is an endo-β-D-glucuronidase that cleaves heparin sulfate chains of HSPG at specific sites, into 5–7 kD sized fragments [55]. Dario Marchetti proposed that HPSE acts as a cellular switch from a non-invasive to invasive phenotype [56]. Indeed, overexpression of HPSE has been found in several human cancers and positively correlates with more extensive tumor invasion and metastasis [57, 58]. Using PG545, a heparanase inhibitor or a glycopolymer of HPSE efficiently reduced tumor cell invasion and metastasis [54, 57]. Another heparanase, M402-necuparanib, is currently under phase II trial investigation in pancreatic cancer [59]. The compound roneparstat has been positively completed in a phase I study with dexamethasone in patients with advanced multiple myeloma [60]. Taken together, HPSE could be a novel therapeutic target for invasive brain tumors. However, the expression of HPSE and its functions on brain invasion in meningiomas still need to be defined, and whether inhibitors of HPSE have any effect on invasive meningiomas is largely unknown.
Cell adhesion molecules
The attachment of cells to their surroundings is important in determining cell shape, proper cell function, and tissue integrity. Cell adhesion is selectively regulated by cell adhesion molecules leading to migration and rearrangement of cell types. According to their migration properties and primary amino acid sequences, four major classes of adhesion molecules have been defined: integrins [61], cadherins [62], immunoglobulin superfamily [63], and selectins [64].
Integrins and their inhibitor, cilengitide
Integrins are cell surface adhesion molecules important for many cellular features, including proliferation and migration. Integrins are composed of different, non-covalently associated α and β chains. These subunits associate to yield a wide variety of heterodimers. Recently, much interest has focused on the role of integrins in carcinomas [65,66,67]. Previous studies have indicated that each histologic meningioma subtype has a specific spectrum of integrin expression, especially αVβ3 and αVβ5 integrins [68], which contribute to invasive growth of meningiomas [69].
Cilengitide is a pentapeptide that targets αVβ3, αVβ5, and αVβ1 integrins by mimicking the Arg-Gly-Asp (RGD)-binding site [70, 71], which has been conducted in phase I, II, and III clinical trials to evaluate therapeutic effects in cancers [72,73,74,75]. Wilisch-Neumann et. al. [76] explored the effects of cilengitide in meningioma cell lines and mouse models and found inhibition of brain invasion in mice after administration of cilengitide.
E-cadherin/β-catenin signaling pathways
Epithelial cadherin (E-cadherin) belongs to the cadherin family, it is a cell-surface glycoprotein that is vital for calcium-dependent cell–cell adhesion and structural rigidity [77, 78]. The extracellular amino-terminus forms a ‘‘zipper-like’’ structure which can act as a tight cell junction. And the intracellular part indirectly associates with cytoskeletal components at cell–cell junctions via catenin [78]. β-catenin, one of the four types of catenins, is a multifunctional protein [77] and forms the E-cadherin/catenin complex [79]. Disruption of this junction will result in diverse disorders, including loose cell-to-cell contacts, and loss of contact inhibition, which are tightly related to tumor invasion [78, 80]. A study conducted by Keiyu et al. revealed that E-cadherin and β-catenin expression closely correlated with grading criteria for meningiomas [81]. Similarly, Ahmed et al. showed that high β-catenin expression was linked to the low incidences of brain invasion and recurrence. Taken together, the E-cadherin/catenin complex could be potential therapeutic targets for meningioma treatment [82].
Selectins
The selectin family represents a group of carbohydrate-binding type I membrane glycoproteins. There are three members, E-, P-, and L-selectin [83]. E-selectin secretion is activated following local stimulation by endothelia of skin and bone marrow and subsequently induced by inflammatory cytokines [84]. P-selectins are stored in alpha granules of the platelet. Through exocytosis, they translocate to the cell surface of activated endothelial cells and platelets [85]. L-selectins are expressed on granulocytes, monocytes, and the majority of lymphocytes and leukocytes [86]. The aforementioned selectins function by interacting with the selectin binding ligand, namely P-selectin glycoprotein ligand-1, which is expressed on the microvilli of activated leukocytes.
Selectins mainly correlate with binding, rotation, and extravasation of activated leukocytes, which commonly take place on the endothelium and also in inflammation reactions [86]. Additionally, some recent studies have implied that selectins could help cancer cells adhere to the endothelium and recruit leukocytes to promote the progression and metastasis of various types of cancer [85, 87]. Consistent with this, a lack of L-selectin was shown to inhibit metastasis [87], and inhibition of P-selectin, which mediates the interaction of thrombocytes and endothelium, significantly reduced metastasis by down-regulating the thrombi assembly [88]. Notably, Atukeren et al. [89] recently evaluated selectins expression in meningiomas and found that all three selectins display higher expression levels in meningiomas compared to control brain tissues, suggesting that selectins are possibly involved in the pathological mechanism of meningioma. Further clinical and experimental studies are needed to demonstrate these current findings.
Growth factors
Growth factors play a seminal role in the brain invasion process, including the promotion of migratory, proliferative, and angiogenesis responses in meningioma cells. Thus, hepatocyte growth factor (HGF), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), transforming growth factor-α (TGF-α), and vascular endothelial growth factor (VEGF), and relevant inhibitors are all reviewed in the next section (Fig. 3).
Selected signaling pathways in meningiomas and molecular targets for drug therapy. ECM the extracellular matrix, HSPG heparin sulfate proteoglycans, HPSE heparanase, MMPs matrix metalloproteinases, TIMP inhibitors of matrix metalloproteinases, uPA/uPAR urokinase plasminogen activator/receptor, PAI plasminogen activator inhibitor, HGF hepatocyte growth factor, PDGF/R Platelet-derived growth factor/receptor, EGF/R epidermal growth factor/receptor, VEGF/R vascular endothelial growth factor/receptor
HGF/c-MET signaling pathways
HGF is a multifunctional protein secreted by mesenchymal cells and has a strong mitogenic effect on hepatocytes. HGF consists of a heavy chain (60 kD) with four domains and a light chain (32 kD). It binds to its tyrosine-kinase receptor (RTK), which is a product of the proto-oncogene c-MET. Mature c-MET is structurally distinct from most other RTKs and exists as a heterodimer containing an extra-cellular α chain and a transmembrane β chain. Once activated by HGF binding, c-MET is auto-phosphorylated and recruits adaptor proteins, activating multiple downstream effector proteins and signaling cascades [90].
Dysregulation of the HGF/c-MET signaling pathway has been known to induce tumor cell proliferation, motility, and invasion in several human cancers, including breast, lung, and hepatocellular carcinomas [91,92,93] and has recently attracted considerable attention. This pathway is widely expressed in human brain tumors, such as gliomas, meningiomas, and schwannomas [94,95,96], and a study has reported that 3 out of 17 c-MET positive meningiomas exhibited brain invasion activity [95]. In the last decades, a big effort has been made to develop related inhibitors and monoclonal antibodies through preclinical, phase I, II, or III clinical trials [90, 97,98,99]. As of yet, there are unfortunately no clinical trials focusing on meningiomas. Given our recent knowledge about HGF/c-MET in cancer cells, future clinical trials focusing on anti-HGF/c-MET agents should take into account whether brain-invasive meningiomas can benefit from these treatments.
PDGF and EGF receptors
PDGF act as a proliferation driver in normal development and multiple cancers [100,101,102]. Increasing evidence of the key role of PDGF in meningioma growth has been reported [103,104,105]. All histological grades of meningiomas express the PDGF ligands AA and BB, Interestingly, only PDGF-β-R receptor was found, which predominantly binds to PDGF-BB. PDGF-BB has been shown to stimulate meningioma growth and activate MAPK and induce c-Fos expression. Conversely, anti-PDGF-BB restrains cell growth [104].
Most meningiomas express both EGF and TGF-α mRNAs [106, 107]. Up-regulated TGF-α activity in meningioma cells and tumor specimens has been proved to correlate with aggressive growth [108]. Meanwhile, over 60% of meningiomas highly expressed EGF receptor (EGFR) [109]. EGF or TGF-α activate their receptors, which promotes the proliferation of meningioma cells in in vitro study [108]. These findings suggest that EGFR activation by autocrine or paracrine mechanisms in human meningiomas may promote tumor growth.
Signal transduction from activated tyrosine kinase receptors, including PDGFR and EGFR, is mediated in part via Ras/Raf/MAPK and PI3K pathways [107], indicating that tyrosine kinase inhibitors may be effective against meningiomas. Imatinib is an oral tyrosine kinase inhibitor, which targets the Bcr-Abl, PDGFR, and c-Kit receptors. Meningiomas often overexpress PDGFR-α and β and are potential targets for imatinib treatment. Moreover, imatinib increases chemo- and radio-sensitivities of different tumor cells in culture, such as glioblastoma cells, soft tissue sarcomas, and leukemic cells [110,111,112], suggesting that imatinib may enhance the activity of other chemotherapeutic agents used to treat brain tumors. Thus, the combination of imatinib and hydroxyurea has been investigated in two phases II trials to test their efficacy in progressive meningiomas [113]. In 2018, data from phase II clinical trials suggest that lapatinib, a dual EGFR/ErbB2 small molecular kinase inhibitor, might have growth-inhibiting effects on meningiomas in NF-2 patients [114]. Although no definite conclusions due to the limited number of patients, well-designed and prospective clinical trials are urgently needed for the therapeutic management of invasive meningiomas. Similarly, recent single-arm phase II studies on the EGFR inhibitors gefitinib (NABTC 00-01) and erlotinib (NABTC 01-03) proved no significant activity against recurrent meningiomas [115].
VEGF/VEGFR and inhibitors
Invasive meningiomas cannot expand beyond the cerebral-pial interface without an adequate blood supply. As meningiomas are rich in blood vessels, their blood supply is predominantly derived from both the external carotid artery and cerebral pial vessels [116]. High expression of VEGF and VEGF receptor-1 was found in meningiomas [117] and was associated with the extent of peritumoral brain edema [118]. A study by Lamszus et al. [119] showed a strong and consistent correlation between VEGF content and meningioma grade, indicating that treating invasive meningiomas may benefit from anti-angiogenic therapy.
Bevacizumab is the anti-VEGF antibody that has been utilized and improved the survival for several malignancies including colorectal, lung, breast, and glioblastomas [120, 121]. To date, bevacizumab has been examined in several retrospective analyses and two phase-II trials involving patients with refractory meningiomas [122]. Generally, these results show promise for patients with recurrent meningioma. Of note, bevacizumab has several important side effects such as high blood pressure, hemorrhage, proteinuria, and colitis. Encouraged by previous results, some researchers have also assessed the efficacy of VEGF inhibitors in patients with relapsed meningiomas. The wide-spectrum tyrosine kinase inhibitor sunitinib, which can target VEGFR, PDGFR, and several other oncogenic pathways, is currently being used in clinical practice for several cancers. This agent was studied in a phase II trial of 36 patients with grade II and III meningiomas who had multiple recurrences and were heavily pretreated [123]. The calculated PFS-6 was 42%. However, toxicity was a significant factor, as four patients developed intratumoral hemorrhages, one of which was fatal. Another two patients developed thrombotic microangiopathy. Further exploration of the role of VEGF/VEGFR inhibitors in invasive meningiomas seems warranted. Hopefully, larger prospective studies of bevacizumab and other VEGF/VEGFR inhibitors will be feasible for this indication in the near future. These agents have the potential to join the list of therapeutic options for treating brain-invasive meningiomas.
Other promising treatment for brain-invasive meningiomas
Immunotherapy for brain-invasive meningiomas
The complex association between the immunology and malignant tumorigenesis has shown huge potential on various types of cancer management such as metastatic melanoma, which have been promisingly identified as sensitive and tolerated to inhibitors of immune checkpoint pathways cytotoxic T lymphocyte antigen 4 (CTLA-4), and programmed death 1 (PD-1)/PD-ligand 1 (PD-L1). This immunotherapeutic approach has been approved by the U.S. Food and Drug Administration [124]. While it currently remains largely unknown about the immune microenvironment in meningioma, immunotherapy possesses perspectival application for managing meningiomas.
A recent report by Du et al. demonstrated the significant reduction of infiltrating T lymphocytes in anaplastic meningiomas, such as CD4 + and CD8 + T cells with PD-1 expressed, which are considered inclined to invade peripheral brain tissue [125]. Additionally, they detected PD-L1 expression in meningioma at either protein or gene levels, and the expression was higher in anaplastic cases [125]. Their study however failed to detect a significant link between PD-L1 levels and survival outcomes due to their cohort was mostly composed of low-grade meningiomas which barely exhibited brain invasion. The prognostic significance of PD-L1 in meningiomas has been investigated to identify the correlation of PD-L1 expression and the infiltrating immune cell population and suggested that PD-L1 possibly plays an important biologic role in the aggressive phenotype of higher-grade meningiomas. Besides, current clinical trials testing anti-PD1 drugs pembrolizumab and nivolumab in recurrent or residual high-grade meningiomas are undergoing [126]. Thus, immunotherapeutic strategies such as checkpoint inhibition, or targeting mesothelin through vaccines/engineered T cells have the potential to be utilized as clinical medication in PD-L1 overexpressing brain-invasive meningiomas (Fig. 4a).
Schematic overview of other promising treatments for brain-invasive meningiomas. a current immunotherapy strategy in meningioma, anti-PD1 drugs pembrolizumab, and nivolumab are undergoing clinical trials in recurrent or residual high-grade meningiomas. b hormone replacement in the treatment of brain-invasive meningiomas. c Tumor treating fields are identified to disrupt the mitotic process in dividing meningioma cells which leads to violent membrane blebbing, subsequently induces immunogenic cell death. d schematic illustration of potential biodegradable copolymers (wafers) impregnated chemotherapy for brain-invasive meningioma
Hormone therapy
According to the epidemiologic evidence, the incidence of meningioma is commonly higher among females, especially when pregnancy and breast cancer occur [127, 128]. So regulation of meningioma growth and development by a sexual hormone is defined. Progesterone and estrogen, which are antagonistic to each other, function in the modulatory mechanism. During hormone replacement therapy (HRT) for some patients in menopause, estrogen-only HRT, but not estrogen + progesterone HRT increased the risk of suffering meningioma according to a large scale clinical study of women [129,130,131]; HRT without supplementing oral contraceptive also play a role in meningioma formation [132].
Progesterone receptor is detected in 58–83% of meningiomas, while estrogen receptor is only reported in 0–8% of this disease [133]. The high expression rate of the progesterone receptor provides a potential therapeutic target for growth inhibition of meningiomas. This concept is supported by a phase II clinical study showing modest clinical regression of meningiomas by blocking progesterone receptors with the anti-progestational agent mifepristone [134]. However, the recent double-blind phase III clinical trial by Ji et al. [135] proved that mifepristone failed to control the unresectable meningioma. The role of the antiandrogenic drug in controlling meningioma is presenting controversial and in need of further evidence to clarify their potential effect [136]. As the antagonist of progesterone, estrogen-like exogenous exposures are associated with a lower risk of meningioma in men [137].
Studies also demonstrate that the status of the GH/IGF-1 axis is significantly associated with the progression rate of meningiomas. Blockade of the GH receptor on the Growth of the tumor cells can be inhibited in vitro by blocking the GH receptor [138]. The antitumor effect of a kind of GH receptor antagonist pegvisomant against intracranial meningiomas has been demonstrated in animal models [139]. But the clinical trial of the GH receptors’ anti-meningioma effect is still lacking. For the correlation between hormone and growth of meningioma, and unsatisfied clinical outcomes of hormone therapy on regressing meningioma, more studies of other hormone receptor inhibitors and the exogenous hormone-like supplement treatment are urgent for providing a novel avenue to curing meningioma (Fig. 4b).
Future perspectives
Brain invasion in meningiomas is correlated with a poor prognosis and an increased risk of recurrence. Once brain invasion has occurred, the therapeutic options for treating meningiomas include surgical resection, radiotherapy, and chemotherapy. The location and biological features of the tumor may limit total resectBesidesition, meningiomas are usually not very sensitive to radiotherapy, and conventional chemotherapy remains controversial. Thus, treating patients with brain-invasive meningiomas, particularly after surgery and radiotherapy, represents an unmet need in neuro-oncology. In this article, we reviewed various aspects of brain invasion in meningiomas, molecular mechanisms of invasion and related targeting agents, and other promising strategies that may use as potential approaches to directly treat brain invasion.
Brain invasion is considered a multiple-step process with several factors involved, such as meningioma tumor cells, microenvironment resident cells, extracellular matrix components, tissue-degrading enzymes, cell adhesion molecules, and various growth factors and receptors. The establishment of meningioma cell lines [140,141,142] and recent animal models [143] will surely provide a good opportunity to explore the molecular mechanisms involved in invasion and design novel therapeutic approaches to prevent repeat surgeries and radiotherapy. Since meningiomas are derived from arachnoidal cells of the leptomeningeal layer, many agents that have failed for other brain tumors due to the low permeability of the blood–brain barrier may be effective for meningiomas. Based on previous results, the number of potential molecular targets, molecular inhibitors, and drug combinations have increased dramatically. However, the selection of the most promising candidates or their combinations for clinical trials is becoming particularly important. It is now established that targeted therapies are active only in the tumor subsets which feature oncogenic activation of targets, as documented by the impressive efficacy of BRAF inhibitors in BRAF-mutant melanomas and anaplastic lymphoma kinase (ALK) inhibitors in ALK-translocated non-small cell lung cancers [144]. Since the molecular pathogenesis of meningiomas and the critical molecular changes driving the brain-invasion of these tumors are still poorly understood, the molecular targeting cores remain to be elucidated. Here, we reviewed the most promising brain invasion targets and related agents (Fig. 3) and described reports predicting or demonstrating the benefits of clinical trials in patients with invasive meningiomas (Tables 1, 2). In the near future, more preclinical studies and clinical trials targeting extracellular matrix degradation enzymes (such as MMPs, uPA-uPAR, lysosomal cysteine, HGF/c-MET), cell adhesion molecules (such as integrins, E-cadherin/β-catenin) and various growth factors (such as EGF/EGFR, TGF-α, PDGF/PDGFR, VEGF/VEGFR) should be conducted to determine the efficiency of related inhibitors for brain-invasive meningiomas. If some of these options show promise, can we further functionally predict whether a meningioma patient will be sensitive to related inhibitors before treatment? For those brain-invasive meningiomas without a predominant genetic marker, a wide-spectrum inhibitor that targets several important signaling pathways may be needed or combined with other approaches including immunotherapy and hormone therapy.
Conclusion
Despite excellent outcomes from surgery and radiotherapy in most meningioma cases, there remains a small subset of patients with brain-invasive meningioma who are refractory to conventional therapies. It is of great importance to identify alternative therapies for these patients. Understanding the crucial mechanisms of brain invasion will promote the development of more effective targeted molecular agents. Moreover, the success of these potential targeted agents and therapeutic options may offer an opportunity to improve the therapeutic strategies for brain-invasive meningiomas.
Abbreviations
- WHO:
-
World Health Organization
- SPARC:
-
Osteonectin or BM-40
- GFAP:
-
Glial fibrillary acidic protein
- EMA:
-
Epithelial membrane antigen
- ECM:
-
Extracellular matrix
- BM:
-
Basement membrane
- HSPG:
-
Heparin sulfate proteoglycans
- MMPs:
-
Matrix metalloproteinases
- HPSE:
-
Heparanase
- TIMPs:
-
Inhibitors of matrix metalloproteinases
- PFS:
-
Progression-free survival
- uPA:
-
Urokinase plasminogen
- uPAR:
-
Urokinase plasminogen receptor
- PAI:
-
Plasminogen activator inhibitor
- HGF:
-
Hepatocyte growth factor
- PDGF:
-
Platelet-derived growth factor
- EGF:
-
Epidermal growth factor
- TGF-α:
-
Transforming growth factor-α
- VEGF:
-
Vascular endothelial growth factor
- MAPK:
-
Mitogen-activated protein kinase
- PI3K:
-
Phosphatidylinositol-3-kinase
- CTLA-4:
-
Cytotoxic T lymphocyte antigen 4
- PD-1/ PD-L1:
-
Programmed death 1/programmed death-ligand 1
- HRT:
-
Hormone replacement therapy
- ALK:
-
Anaplastic lymphoma kinase
References
Preusser M, Brastianos PK, Mawrin C (2018) Advances in meningioma genetics: novel therapeutic opportunities. Nat Rev Neurol 14(2):106–115. https://doi.org/10.1038/nrneurol.2017.168
Harmanci AS, Youngblood MW, Clark VE, Coskun S, Henegariu O, Duran D, Erson-Omay EZ, Kaulen LD, Lee TI, Abraham BJ, Simon M, Krischek B, Timmer M, Goldbrunner R, Omay SB, Baranoski J, Baran B, Carrion-Grant G, Bai H, Mishra-Gorur K, Schramm J, Moliterno J, Vortmeyer AO, Bilguvar K, Yasuno K, Young RA, Gunel M (2017) Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat Commun 8:14433. https://doi.org/10.1038/ncomms14433
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 131(6):803–820. https://doi.org/10.1007/s00401-016-1545-1
Yamasaki F, Yoshioka H, Hama S, Sugiyama K, Arita K, Kurisu K (2000) Recurrence of meningiomas. Cancer 89(5):1102–1110
Saraf S, McCarthy BJ, Villano JL (2011) Update on meningiomas. Oncologist 16(11):1604–1613. https://doi.org/10.1634/theoncologist.2011-0193
Simpson D (1957) The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry 20(1):22–39
Kaley T, Barani I, Chamberlain M, McDermott M, Panageas K, Raizer J, Rogers L, Schiff D, Vogelbaum M, Weber D, Wen P (2014) Historical benchmarks for medical therapy trials in surgery- and radiation-refractory meningioma: a RANO review. Neuro Oncol 16(6):829–840. https://doi.org/10.1093/neuonc/not330
McLean CA, Jolley D, Cukier E, Giles G, Gonzales MF (1993) Atypical and malignant meningiomas: importance of micronecrosis as a prognostic indicator. Histopathology 23(4):349–353
Deen HG Jr, Scheithauer BW, Ebersold MJ (1982) Clinical and pathological study of meningiomas of the first two decades of life. J Neurosurg 56(3):317–322. https://doi.org/10.3171/jns.1982.56.3.0317
Perry A, Stafford SL, Scheithauer BW, Suman VJ, Lohse CM (1997) Meningioma grading: an analysis of histologic parameters. Am J Surg Pathol 21(12):1455–1465
Rempel SA, Ge S, Gutierrez JA (1999) SPARC: a potential diagnostic marker of invasive meningiomas. Clin Cancer Res 5(2):237–241
Brokinkel B, Hess K, Mawrin C (2017) Brain invasion in meningiomas-clinical considerations and impact of neuropathological evaluation: a systematic review. Neuro Oncol 19(10):1298–1307. https://doi.org/10.1093/neuonc/nox071
Zeltner L, Schittenhelm J, Mittelbronn M, Roser F, Tatagiba M, Mawrin C, Kim YJ, Bornemann A (2007) The astrocytic response towards invasive meningiomas. Neuropathol Appl Neurobiol 33(2):163–168. https://doi.org/10.1111/j.1365-2990.2006.00792.x
Spille DC, Hess K, Sauerland C, Sanai N, Stummer W, Paulus W, Brokinkel B (2016) Brain invasion in meningiomas: incidence and correlations with clinical variables and prognosis. World Neurosurg 93:346–354. https://doi.org/10.1016/j.wneu.2016.06.055
Rempel SA, Schwechheimer K, Davis RL, Cavenee WK, Rosenblum ML (1993) Loss of heterozygosity for loci on chromosome 10 is associated with morphologically malignant meningioma progression. Can Res 53(10 Suppl):2386–2392
Mawrin C, Perry A (2010) Pathological classification and molecular genetics of meningiomas. J Neurooncol 99(3):379–391. https://doi.org/10.1007/s11060-010-0342-2
Sun SQ, Kim AH, Cai C, Murphy RK, DeWees T, Sylvester P, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Leonard JR, Evans J, Simpson JR, Robinson CG, Perrin RJ, Huang J, Chicoine MR (2014) Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery 75(4):347–354. https://doi.org/10.1227/NEU.0000000000000461 (discussion 354–345; quiz 355)
Sun SQ, Cai C, Murphy RK, DeWees T, Dacey RG, Grubb RL, Rich KM, Zipfel GJ, Dowling JL, Leuthardt EC, Simpson JR, Robinson CG, Chicoine MR, Perrin RJ, Huang J, Kim AH (2016) Radiation therapy for residual or recurrent atypical meningioma: the effects of modality, timing, and tumor pathology on long-term outcomes. Neurosurgery 79(1):23–32. https://doi.org/10.1227/NEU.0000000000001160
Stetler-Stevenson WG, Liotta LA, Kleiner DE Jr (1993) Extracellular matrix 6: role of matrix metalloproteinases in tumor invasion and metastasis. FASEB Jo 7(15):1434–1441
Arai Y, Kubota T, Nakagawa T, Kabuto M, Sato K, Kobayashi H (1998) Production of urokinase-type plasminogen activator (u-PA) and plasminogen activator inhibitor-1 (PAI-1) in human brain tumours. Acta Neurochir 140(4):377–385 (discussion 385–376)
von Randow AJ, Schindler S, Tews DS (2006) Expression of extracellular matrix-degrading proteins in classic, atypical, and anaplastic meningiomas. Pathol Res Pract 202(5):365–372. https://doi.org/10.1016/j.prp.2006.01.012
Liotta LA, Steeg PS, Stetler-Stevenson WG (1991) Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 64(2):327–336
Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y (1994) Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg 81(1):69–77. https://doi.org/10.3171/jns.1994.81.1.0069
Mignatti P, Robbins E, Rifkin DB (1986) Tumor invasion through the human amniotic membrane: requirement for a proteinase cascade. Cell 47(4):487–498
Nordqvist AC, Smurawa H, Mathiesen T (2001) Expression of matrix metalloproteinases 2 and 9 in meningiomas associated with different degrees of brain invasiveness and edema. J Neurosurg 95(5):839–844. https://doi.org/10.3171/jns.2001.95.5.0839
Chintala SK, Tonn JC, Rao JS (1999) Matrix metalloproteinases and their biological function in human gliomas. Int J Dev Neurosci 17(5–6):495–502
Mignatti P, Rifkin DB (1996) Plasminogen activators and matrix metalloproteinases in angiogenesis. Enzyme Protein 49(1–3):117–137
Jalali S, Singh S, Agnihotri S, Wataya T, Salehi F, Alkins R, Burrell K, Navab R, Croul S, Aldape K, Zadeh G (2015) A role for matrix remodelling proteins in invasive and malignant meningiomas. Neuropathol Appl Neurobiol 41(2):e16-28. https://doi.org/10.1111/nan.12166
Backer-Grondahl T, Moen BH, Arnli MB, Torseth K, Torp SH (2014) Immunohistochemical characterization of brain-invasive meningiomas. Int J Clin Exp Pathol 7(10):7206–7219
Karaarslan N, Gurbuz MS, Caliskan T, Ayan E, Aker FV, Berkman MZ (2016) The effect of matrix metalloproteinase-3 on the prognosis and biological behaviour of meningiomas. Turk Neurosurg 26(5):678–683. https://doi.org/10.5137/1019-5149.JTN.12807-14.1
Tummalapalli P, Gondi CS, Dinh DH, Gujrati M, Rao JS (2007) RNA interference-mediated targeting of urokinase plasminogen activator receptor and matrix metalloproteinase-9 gene expression in the IOMM-lee malignant meningioma cell line inhibits tumor growth, tumor cell invasion and angiogenesis. Int J Oncol 31(1):5–17
Tummalapalli P, Spomar D, Gondi CS, Olivero WC, Gujrati M, Dinh DH, Rao JS (2007) RNAi-mediated abrogation of cathepsin B and MMP-9 gene expression in a malignant meningioma cell line leads to decreased tumor growth, invasion and angiogenesis. Int J Oncol 31(5):1039–1050
Levicar N, Nuttall RK, Lah TT (2003) Proteases in brain tumour progression. Acta Neurochir 145(9):825–838. https://doi.org/10.1007/s00701-003-0097-z
Smith HW, Marshall CJ (2010) Regulation of cell signalling by uPAR. Nat Rev Mol Cell Biol 11(1):23–36. https://doi.org/10.1038/nrm2821
Blasi F, Carmeliet P (2002) uPAR: a versatile signalling orchestrator. Nat Rev Mol Cell Biol 3(12):932–943. https://doi.org/10.1038/nrm977
Pakneshan P, Tetu B, Rabbani SA (2004) Demethylation of urokinase promoter as a prognostic marker in patients with breast carcinoma. Clin Cancer Res 10(9):3035–3041
Zhang B, Zhang Z, Li L, Qin YR, Liu H, Jiang C, Zeng TT, Li MQ, Xie D, Li Y, Guan XY, Zhu YH (2018) TSPAN15 interacts with BTRC to promote oesophageal squamous cell carcinoma metastasis via activating NF-kappaB signaling. Nat Commun 9(1):1423. https://doi.org/10.1038/s41467-018-03716-9
Kwaan HC (1992) The plasminogen-plasmin system in malignancy. Cancer Metastasis Rev 11(3–4):291–311
Muehlenweg B, Sperl S, Magdolen V, Schmitt M, Harbeck N (2001) Interference with the urokinase plasminogen activator system: a promising therapy concept for solid tumours. Expert Opin Biol Ther 1(4):683–691. https://doi.org/10.1517/14712598.1.4.683
Alonso DF, Farias EF, Ladeda V, Davel L, Puricelli L, de Kier B, Joffe E (1996) Effects of synthetic urokinase inhibitors on local invasion and metastasis in a murine mammary tumor model. Breast Cancer Res Treat 40(3):209–223
Evans CP, Elfman F, Parangi S, Conn M, Cunha G, Shuman MA (1997) Inhibition of prostate cancer neovascularization and growth by urokinase-plasminogen activator receptor blockade. Can Res 57(16):3594–3599
Kandenwein JA, Park-Simon TW, Schramm J, Simon M (2011) uPA/PAI-1 expression and uPA promoter methylation in meningiomas. J Neurooncol 103(3):533–539. https://doi.org/10.1007/s11060-010-0411-6
Kondraganti S, Gondi CS, McCutcheon I, Dinh DH, Gujrati M, Rao JS, Olivero WC (2006) RNAi-mediated downregulation of urokinase plasminogen activator and its receptor in human meningioma cells inhibits tumor invasion and growth. Int J Oncol 28(6):1353–1360
Olson OC, Joyce JA (2015) Cysteine cathepsin proteases: regulators of cancer progression and therapeutic response. Nat Rev Cancer 15(12):712–729. https://doi.org/10.1038/nrc4027
Schmitt M, Janicke F, Moniwa N, Chucholowski N, Pache L, Graeff H (1992) Tumor-associated urokinase-type plasminogen activator: biological and clinical significance. Bio Chem Hoppe-Seyler 373(7):611–622
Rao JS (2003) Molecular mechanisms of glioma invasiveness: the role of proteases. Nat Rev Cancer 3(7):489–501. https://doi.org/10.1038/nrc1121
Strojnik T, Kos J, Zidanik B, Golouh R, Lah T (1999) Cathepsin B immunohistochemical staining in tumor and endothelial cells is a new prognostic factor for survival in patients with brain tumors. Clinical Cancer Res 5(3):559–567
Levicar N, Strojnik T, Kos J, Dewey RA, Pilkington GJ, Lah TT (2002) Lysosomal enzymes, cathepsins in brain tumour invasion. J Neurooncol 58(1):21–32
Reese JM, Bruinsma ES, Nelson AW, Chernukhin I, Carroll JS, Li Y, Subramaniam M, Suman VJ, Negron V, Monroe DG, Ingle JN, Goetz MP, Hawse JR (2018) ERbeta-mediated induction of cystatins results in suppression of TGFbeta signaling and inhibition of triple-negative breast cancer metastasis. Proc Natl Acad Sci USA 115(41):E9580–E9589. https://doi.org/10.1073/pnas.1807751115
Sivaparvathi M, McCutcheon I, Sawaya R, Nicolson GL, Rao JS (1996) Expression of cysteine protease inhibitors in human gliomas and meningiomas. Clin Exp Metas 14(4):344–350
Lah TT, Nanni I, Trinkaus M, Metellus P, Dussert C, De Ridder L, Rajcevic U, Blejec A, Martin PM (2010) Toward understanding recurrent meningioma: the potential role of lysosomal cysteine proteases and their inhibitors. J Neurosurg 112(5):940–950. https://doi.org/10.3171/2009.7.JNS081729
Habuchi H, Habuchi O, Kimata K (2004) Sulfation pattern in glycosaminoglycan: does it have a code? Glycoconj J 21(1–2):47–52. https://doi.org/10.1023/B:GLYC.0000043747.87325.5e
Esko JD, Lindahl U (2001) Molecular diversity of heparan sulfate. J Clin Investig 108(2):169–173. https://doi.org/10.1172/JCI13530
Spyrou A, Kundu S, Haseeb L, Yu D, Olofsson T, Dredge K, Hammond E, Barash U, Vlodavsky I, Forsberg-Nilsson K (2017) Inhibition of heparanase in pediatric brain tumor cells attenuates their proliferation, invasive capacity, and in vivo tumor growth. Mol Cancer Ther 16(8):1705–1716. https://doi.org/10.1158/1535-7163.MCT-16-0900
Vlodavsky I, Goldshmidt O (2001) Properties and function of heparanase in cancer metastasis and angiogenesis. Haemostasis 31(Suppl 1):60–63
Marchetti D (2002) Heparanase: a target for therapy of brain invasive tumors? Expert Rev Neurother 2(4):459–463. https://doi.org/10.1586/14737175.2.4.459
Loka RS, Sletten ET, Barash U, Vlodavsky I, Nguyen HM (2018) Specific inhibition of heparanase by glycopolymer with well-defined sulfation pattern prevents breast cancer metastasis in mice. ACS Appl Mater Interfaces. https://doi.org/10.1021/acsami.8b17625
Kundu S, Xiong A, Spyrou A, Wicher G, Marinescu VD, Edqvist PD, Zhang L, Essand M, Dimberg A, Smits A, Ilan N, Vlodavsky I, Li JP, Forsberg-Nilsson K (2016) Heparanase promotes glioma progression and is inversely correlated with patient survival. Mol Cancer Res MCR 14(12):1243–1253. https://doi.org/10.1158/1541-7786.MCR-16-0223
Meirovitz A, Goldberg R, Binder A, Rubinstein AM, Hermano E, Elkin M (2013) Heparanase in inflammation and inflammation-associated cancer. FEBS J 280(10):2307–2319. https://doi.org/10.1111/febs.12184
Galli M, Chatterjee M, Grasso M, Specchia G, Magen H, Einsele H, Celeghini I, Barbieri P, Paoletti D, Pace S, Sanderson RD, Rambaldi A, Nagler A (2018) Phase I study of the heparanase inhibitor roneparstat: an innovative approach for multiple myeloma therapy. Haematologica 103(10):e469–e472. https://doi.org/10.3324/haematol.2017.182865
Horwitz AR (2012) The origins of the molecular era of adhesion research. Nat Rev Mol Cell Biol 13(12):805–811. https://doi.org/10.1038/nrm3473
Hirano S, Takeichi M (2012) Cadherins in brain morphogenesis and wiring. Physiol Rev 92(2):597–634. https://doi.org/10.1152/physrev.00014.2011
Ebnet K (2017) Junctional adhesion molecules (JAMs): cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol Rev 97(4):1529–1554. https://doi.org/10.1152/physrev.00004.2017
Marshall BT, Long M, Piper JW, Yago T, McEver RP, Zhu C (2003) Direct observation of catch bonds involving cell-adhesion molecules. Nature 423(6936):190–193. https://doi.org/10.1038/nature01605
Muthuswamy SK (2006) ErbB2 makes beta 4 integrin an accomplice in tumorigenesis. Cell 126(3):443–445. https://doi.org/10.1016/j.cell.2006.07.020
Pasqualini R, Koivunen E, Ruoslahti E (1997) Alpha v integrins as receptors for tumor targeting by circulating ligands. Nat Biotechnol 15(6):542–546. https://doi.org/10.1038/nbt0697-542
Schmid MC, Khan SQ, Kaneda MM, Pathria P, Shepard R, Louis TL, Anand S, Woo G, Leem C, Faridi MH, Geraghty T, Rajagopalan A, Gupta S, Ahmed M, Vazquez-Padron RI, Cheresh DA, Gupta V, Varner JA (2018) Integrin CD11b activation drives anti-tumor innate immunity. Nat Commun 9(1):5379. https://doi.org/10.1038/s41467-018-07387-4
Bello L, Zhang J, Nikas DC, Strasser JF, Villani RM, Cheresh DA, Carroll RS, Black PM (2000) Alpha(v)beta3 and alpha(v)beta5 integrin expression in meningiomas. Neurosurgery 47(5):1185–1195
Salehi F, Jalali S, Alkins R, Lee JI, Lwu S, Burrell K, Gentili F, Croul S, Zadeh G (2013) Proteins involved in regulating bone invasion in skull base meningiomas. Acta Neurochir 155(3):421–427. https://doi.org/10.1007/s00701-012-1577-9
Haun F, Neumann S, Peintner L, Wieland K, Habicht J, Schwan C, Ostevold K, Koczorowska MM, Biniossek M, Kist M, Busch H, Boerries M, Davis RJ, Maurer U, Schilling O, Aktories K, Borner C (2018) Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nat Commun 9(1):3524. https://doi.org/10.1038/s41467-018-05850-w
Desgrosellier JS, Cheresh DA (2010) Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10(1):9–22. https://doi.org/10.1038/nrc2748
Nabors LB, Mikkelsen T, Rosenfeld SS, Hochberg F, Akella NS, Fisher JD, Cloud GA, Zhang Y, Carson K, Wittemer SM, Colevas AD, Grossman SA (2007) Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol 25(13):1651–1657. https://doi.org/10.1200/JCO.2006.06.6514
Khasraw M, Lee A, McCowatt S, Kerestes Z, Buyse ME, Back M, Kichenadasse G, Ackland S, Wheeler H (2016) Cilengitide with metronomic temozolomide, procarbazine, and standard radiotherapy in patients with glioblastoma and unmethylated MGMT gene promoter in ExCentric, an open-label phase II trial. J Neurooncol 128(1):163–171. https://doi.org/10.1007/s11060-016-2094-0
Nabors LB, Fink KL, Mikkelsen T, Grujicic D, Tarnawski R, Nam DH, Mazurkiewicz M, Salacz M, Ashby L, Zagonel V, Depenni R, Perry JR, Hicking C, Picard M, Hegi ME, Lhermitte B, Reardon DA (2015) Two cilengitide regimens in combination with standard treatment for patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter: results of the open-label, controlled, randomized phase II CORE study. Neuro Oncol 17(5):708–717. https://doi.org/10.1093/neuonc/nou356
Stupp R, Hegi ME, Gorlia T, Erridge SC, Perry J, Hong YK, Aldape KD, Lhermitte B, Pietsch T, Grujicic D, Steinbach JP, Wick W, Tarnawski R, Nam DH, Hau P, Weyerbrock A, Taphoorn MJ, Shen CC, Rao N, Thurzo L, Herrlinger U, Gupta T, Kortmann RD, Adamska K, McBain C, Brandes AA, Tonn JC, Schnell O, Wiegel T, Kim CY, Nabors LB, Reardon DA, van den Bent MJ, Hicking C, Markivskyy A, Picard M, Weller M, European Organisation for R, Treatment of C, Canadian Brain Tumor C, team Cs (2014) Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 15(10):1100–1108. https://doi.org/10.1016/S1470-2045(14)70379-1
Wilisch-Neumann A, Kliese N, Pachow D, Schneider T, Warnke JP, Braunsdorf WE, Bohmer FD, Hass P, Pasemann D, Helbing C, Kirches E, Mawrin C (2013) The integrin inhibitor cilengitide affects meningioma cell motility and invasion. Clin Cancer Res 19(19):5402–5412. https://doi.org/10.1158/1078-0432.CCR-12-0299
Buckley CD, Tan J, Anderson KL, Hanein D, Volkmann N, Weis WI, Nelson WJ, Dunn AR (2014) Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346(6209):1254211. https://doi.org/10.1126/science.1254211
Benham-Pyle BW, Pruitt BL, Nelson WJ (2015) Cell adhesion. Mechanical strain induces E-cadherin-dependent Yap1 and beta-catenin activation to drive cell cycle entry. Science 348(6238):1024–1027. https://doi.org/10.1126/science.aaa4559
Curtis MW, Johnson KR, Wheelock MJ (2008) E-cadherin/catenin complexes are formed cotranslationally in the endoplasmic reticulum/Golgi compartments. Cell Commun Adhes 15(4):365–378. https://doi.org/10.1080/15419060802460748
Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, Sugimura T, Hirohashi S (1994) E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA 91(5):1858–1862
Zhou K, Wang G, Wang Y, Jin H, Yang S, Liu C (2010) The potential involvement of E-cadherin and beta-catenins in meningioma. PLoS ONE 5(6):e11231. https://doi.org/10.1371/journal.pone.0011231
Ahmed RA, Shebl AM, Habashy HO (2017) Expression levels of beta-catenin and galectin-3 in meningioma and their effect on brain invasion and recurrence: a tissue microarray study. Cancer Biol Med 14(3):319–326. https://doi.org/10.20892/j.issn.2095-3941.2017.0024
Kansas GS (1996) Selectins and their ligands: current concepts and controversies. Blood 88(9):3259–3287
Schweitzer KM, Drager AM, van der Valk P, Thijsen SF, Zevenbergen A, Theijsmeijer AP, van der Schoot CE, Langenhuijsen MM (1996) Constitutive expression of E-selectin and vascular cell adhesion molecule-1 on endothelial cells of hematopoietic tissues. Am J Pathol 148(1):165–175
Laubli H, Borsig L (2010) Selectins promote tumor metastasis. Semin Cancer Biol 20(3):169–177. https://doi.org/10.1016/j.semcancer.2010.04.005
Ivetic A, Ridley AJ (2004) The telling tail of L-selectin. Biochem Soc Trans 32(Pt 6):1118–1121. https://doi.org/10.1042/BST0321118
Hollingsworth MA, Swanson BJ (2004) Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 4(1):45–60. https://doi.org/10.1038/nrc1251
Tesfamariam B (2016) Involvement of platelets in tumor cell metastasis. Pharmacol Ther 157:112–119. https://doi.org/10.1016/j.pharmthera.2015.11.005
Atukeren P, Turk O, Yanar K, Kemerdere R, Sayyahmelli S, Eren B, Tanriverdi T (2017) Evaluation of ALCAM, PECAM-1 and selectin levels in intracranial meningiomas. Clin Neurol Neurosurg 160:21–26. https://doi.org/10.1016/j.clineuro.2017.06.002
Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, Wauters E, Walmsley S, Prenen H, Granot Z, Casazza A, Mazzone M (2015) MET is required for the recruitment of anti-tumoural neutrophils. Nature 522(7556):349–353. https://doi.org/10.1038/nature14407
Liu X, Newton RC, Scherle PA (2010) Developing c-MET pathway inhibitors for cancer therapy: progress and challenges. Trends Mol Med 16(1):37–45. https://doi.org/10.1016/j.molmed.2009.11.005
Feng Y, Thiagarajan PS, Ma PC (2012) MET signaling: novel targeted inhibition and its clinical development in lung cancer. J Thorac Oncol 7(2):459–467. https://doi.org/10.1097/JTO.0b013e3182417e44
Ma WW, Adjei AA (2009) Novel agents on the horizon for cancer therapy. CA Cancer J Clin 59(2):111–137. https://doi.org/10.3322/caac.20003
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487(7408):500–504. https://doi.org/10.1038/nature11183
Yun S, Koh JM, Lee KS, Seo AN, Nam KH, Choe G (2015) Expression of c-MET in invasive meningioma. J Pathol Transl Med 49(1):44–51. https://doi.org/10.4132/jptm.2014.10.13
Zhao Y, Liu P, Zhang N, Chen J, Landegger LD, Wu L, Zhao F, Zhao Y, Zhang Y, Zhang J, Fujita T, Stemmer-Rachamimov A, Ferraro GB, Liu H, Muzikansky A, Plotkin SR, Stankovic KM, Jain RK, Xu L (2018) Targeting the cMET pathway augments radiation response without adverse effect on hearing in NF2 Schwannoma models. Proc Natl Acad Sci USA 115(9):E2077–E2084. https://doi.org/10.1073/pnas.1719966115
Parikh RA, Wang P, Beumer JH, Chu E, Appleman LJ (2014) The potential roles of hepatocyte growth factor (HGF)-MET pathway inhibitors in cancer treatment. Onco Targets Ther 7:969–983. https://doi.org/10.2147/OTT.S40241
Peters S, Adjei AA (2012) MET: a promising anticancer therapeutic target. Nat Rev Clin Oncol 9(6):314–326. https://doi.org/10.1038/nrclinonc.2012.71
Finisguerra V, Prenen H, Mazzone M (2016) Preclinical and clinical evaluation of MET functions in cancer cells and in the tumor stroma. Oncogene 35(42):5457–5467. https://doi.org/10.1038/onc.2016.36
Barrow AD, Edeling MA, Trifonov V, Luo J, Goyal P, Bohl B, Bando JK, Kim AH, Walker J, Andahazy M, Bugatti M, Melocchi L, Vermi W, Fremont DH, Cox S, Cella M, Schmedt C, Colonna M (2018) Natural killer cells control tumor growth by sensing a growth factor. Cell 172(3):534-548 e519. https://doi.org/10.1016/j.cell.2017.11.037
Weissmueller S, Manchado E, Saborowski M, Morris JPT, Wagenblast E, Davis CA, Moon SH, Pfister NT, Tschaharganeh DF, Kitzing T, Aust D, Markert EK, Wu J, Grimmond SM, Pilarsky C, Prives C, Biankin AV, Lowe SW (2014) Mutant p53 drives pancreatic cancer metastasis through cell-autonomous PDGF receptor beta signaling. Cell 157(2):382–394. https://doi.org/10.1016/j.cell.2014.01.066
Leder K, Pitter K, LaPlant Q, Hambardzumyan D, Ross BD, Chan TA, Holland EC, Michor F (2014) Mathematical modeling of PDGF-driven glioblastoma reveals optimized radiation dosing schedules. Cell 156(3):603–616. https://doi.org/10.1016/j.cell.2013.12.029
Gupta S, Bi WL, Dunn IF (2018) Medical management of meningioma in the era of precision medicine. Neurosurg Focus 44(4):E3. https://doi.org/10.3171/2018.1.FOCUS17754
Tuchen M, Wilisch-Neumann A, Daniel EA, Baldauf L, Pachow D, Scholz J, Angenstein F, Stork O, Kirches E, Mawrin C (2017) Receptor tyrosine kinase inhibition by regorafenib/sorafenib inhibits growth and invasion of meningioma cells. Eur J Cancer 73:9–21. https://doi.org/10.1016/j.ejca.2016.12.004
Johnson MD, Reeder JE, O’Connell M (2015) MKP-3 regulates PDGF-BB effects and MAPK activation in meningioma cells. J Clin Neurosci 22(4):752–757. https://doi.org/10.1016/j.jocn.2014.10.030
Alexiou GA, Markoula S, Gogou P, Kyritsis AP (2011) Genetic and molecular alterations in meningiomas. Clin Neurol Neurosurg 113(4):261–267. https://doi.org/10.1016/j.clineuro.2010.12.007
Carroll RS, Black PM, Zhang J, Kirsch M, Percec I, Lau N, Guha A (1997) Expression and activation of epidermal growth factor receptors in meningiomas. J Neurosurg 87(2):315–323. https://doi.org/10.3171/jns.1997.87.2.0315
Johnson M, Toms S (2005) Mitogenic signal transduction pathways in meningiomas: novel targets for meningioma chemotherapy? J Neuropathol Exp Neurol 64(12):1029–1036
Andersson U, Guo D, Malmer B, Bergenheim AT, Brannstrom T, Hedman H, Henriksson R (2004) Epidermal growth factor receptor family (EGFR, ErbB2-4) in gliomas and meningiomas. Acta Neuropathol 108(2):135–142. https://doi.org/10.1007/s00401-004-0875-6
Katayama R, Huelsmeyer MK, Marr AK, Kurzman ID, Thamm DH, Vail DM (2004) Imatinib mesylate inhibits platelet-derived growth factor activity and increases chemosensitivity in feline vaccine-associated sarcoma. Cancer Chemother Pharmacol 54(1):25–33. https://doi.org/10.1007/s00280-004-0780-7
Aloyz R, Grzywacz K, Xu ZY, Loignon M, Alaoui-Jamali MA, Panasci L (2004) Imatinib sensitizes CLL lymphocytes to chlorambucil. Leukemia 18(3):409–414. https://doi.org/10.1038/sj.leu.2403247
O’Reilly T, Wartmann M, Maira SM, Hattenberger M, Vaxelaire J, Muller M, Ferretti S, Buchdunger E, Altmann KH, McSheehy PMJ (2005) Patupilone (epothilone B, EPO906) and imatinib (STI571, Glivec) in combination display enhanced antitumour activity in vivo against experimental rat C6 glioma. Cancer Chemother Pharmacol 55(4):307–317. https://doi.org/10.1007/s00280-004-0913-z
Mazza E, Brandes A, Zanon S, Eoli M, Lombardi G, Faedi M, Franceschi E, Reni M (2016) Hydroxyurea with or without imatinib in the treatment of recurrent or progressive meningiomas: a randomized phase II trial by Gruppo Italiano Cooperativo di Neuro-Oncologia (GICNO). Cancer Chemother Pharmacol 77(1):115–120. https://doi.org/10.1007/s00280-015-2927-0
Osorio DS, Hu J, Mitchell C, Allen JC, Stanek J, Hagiwara M, Karajannis MA (2018) Effect of lapatinib on meningioma growth in adults with neurofibromatosis type 2. J Neurooncol 139(3):749–755. https://doi.org/10.1007/s11060-018-2922-5
Norden AD, Raizer JJ, Abrey LE, Lamborn KR, Lassman AB, Chang SM, Yung WK, Gilbert MR, Fine HA, Mehta M, Deangelis LM, Cloughesy TF, Robins HI, Aldape K, Dancey J, Prados MD, Lieberman F, Wen PY (2010) Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J Neurooncol 96(2):211–217. https://doi.org/10.1007/s11060-009-9948-7
Lamszus K (2004) Meningioma pathology, genetics, and biology. J Neuropathol Exp Neurol 63(4):275–286
Baumgarten P, Brokinkel B, Zinke J, Zachskorn C, Ebel H, Albert FK, Stummer W, Plate KH, Harter PN, Hasselblatt M, Mittelbronn M (2013) Expression of vascular endothelial growth factor (VEGF) and its receptors VEGFR1 and VEGFR2 in primary and recurrent WHO grade III meningiomas. Histol Histopathol 28(9):1157–1166. https://doi.org/10.14670/HH-28.1157
Otsuka S, Tamiya T, Ono Y, Michiue H, Kurozumi K, Daido S, Kambara H, Date I, Ohmoto T (2004) The relationship between peritumoral brain edema and the expression of vascular endothelial growth factor and its receptors in intracranial meningiomas. J Neurooncol 70(3):349–357
Lamszus K, Lengler U, Schmidt NO, Stavrou D, Ergun S, Westphal M (2000) Vascular endothelial growth factor, hepatocyte growth factor/scatter factor, basic fibroblast growth factor, and placenta growth factor in human meningiomas and their relation to angiogenesis and malignancy. Neurosurgery 46(4):938–947 (discussion 947–938)
Jain RK, Duda DG, Clark JW, Loeffler JS (2006) Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol 3(1):24–40. https://doi.org/10.1038/ncponc0403
Gilbert MR, Sulman EP, Mehta MP (2014) Bevacizumab for newly diagnosed glioblastoma. N Engl J Med 370(21):2048–2049. https://doi.org/10.1056/NEJMc1403303
Dasanu CA, Alvarez-Argote J, Limonadi FM, Codreanu I (2018) Bevacizumab in refractory higher-grade and atypical meningioma: the current state of affairs. Expert Opin Biol Ther. https://doi.org/10.1080/14712598.2019.1559292
Kaley TJ, Wen P, Schiff D, Ligon K, Haidar S, Karimi S, Lassman AB, Nolan CP, DeAngelis LM, Gavrilovic I, Norden A, Drappatz J, Lee EQ, Purow B, Plotkin SR, Batchelor T, Abrey LE, Omuro A (2015) Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro Oncol 17(1):116–121. https://doi.org/10.1093/neuonc/nou148
Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbe C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363(8):711–723. https://doi.org/10.1056/NEJMoa1003466
Han SJ, Reis G, Kohanbash G, Shrivastav S, Magill ST, Molinaro AM, McDermott MW, Theodosopoulos PV, Aghi MK, Berger MS, Butowski NA, Barani I, Phillips JJ, Perry A, Okada H (2016) Expression and prognostic impact of immune modulatory molecule PD-L1 in meningioma. J Neurooncol 130(3):543–552. https://doi.org/10.1007/s11060-016-2256-0
Apra C, Peyre M, Kalamarides M (2018) Current treatment options for meningioma. Expert Rev Neurother 18(3):241–249. https://doi.org/10.1080/14737175.2018.1429920
Wahab M, Al-Azzawi F (2003) Meningioma and hormonal influences. Climacteric 6(4):285–292
Blitshteyn S, Crook JE, Jaeckle KA (2008) Is there an association between meningioma and hormone replacement therapy? J Clin Oncol 26(2):279–282. https://doi.org/10.1200/JCO.2007.14.2133
O’Shea T, Crowley RK, Farrell M, MacNally S, Govender P, Feeney J, Gibney J, Sherlock M (2016) Growth of a progesterone receptor-positive meningioma in a female patient with congenital adrenal hyperplasia. Endocrinol Diabetes Metab Case Rep. https://doi.org/10.1530/EDM-16-0054
Benson VS, Kirichek O, Beral V, Green J (2015) Menopausal hormone therapy and central nervous system tumor risk: large UK prospective study and meta-analysis. Int J Cancer 136(10):2369–2377. https://doi.org/10.1002/ijc.29274
Shu X, Jiang Y, Wen T, Lu S, Yao L, Meng F (2019) Association of hormone replacement therapy with increased risk of meningioma in women: a hospital-based multicenter study with propensity score matching. Asia Pac J Clin Oncol. https://doi.org/10.1111/ajco.13138
Qi ZY, Shao C, Huang YL, Hui GZ, Zhou YX, Wang Z (2013) Reproductive and exogenous hormone factors in relation to risk of meningioma in women: a meta-analysis. PLoS ONE 8(12):e83261. https://doi.org/10.1371/journal.pone.0083261
Deli T, Orosz M, Jakab A (2019) Hormone replacement therapy in cancer survivors—review of the literature. Pathol Oncol Res. https://doi.org/10.1007/s12253-018-00569-x
Grunberg SM, Weiss MH, Russell CA, Spitz IM, Ahmadi J, Sadun A, Sitruk-Ware R (2006) Long-term administration of mifepristone (RU486): clinical tolerance during extended treatment of meningioma. Cancer Invest 24(8):727–733. https://doi.org/10.1080/07357900601062339
Ji Y, Rankin C, Grunberg S, Sherrod AE, Ahmadi J, Townsend JJ, Feun LG, Fredericks RK, Russell CA, Kabbinavar FF, Stelzer KJ, Schott A, Verschraegen C (2015) Double-blind phase III randomized trial of the antiprogestin agent mifepristone in the treatment of unresectable meningioma: SWOG S9005. J Clin Oncol 33(34):4093–4098. https://doi.org/10.1200/JCO.2015.61.6490
Champagne PO, Passeri T, Froelich S (2019) Combined hormonal influence of cyproterone acetate and nomegestrol acetate on meningioma: a case report. Acta Neurochir 161(3):589–592. https://doi.org/10.1007/s00701-018-03782-4
Schildkraut JM, Calvocoressi L, Wang F, Wrensch M, Bondy ML, Wiemels JL, Claus EB (2014) Endogenous and exogenous hormone exposure and the risk of meningioma in men. J Neurosurg 120(4):820–826. https://doi.org/10.3171/2013.12.JNS131170
Friend KE, Radinsky R, McCutcheon IE (1999) Growth hormone receptor expression and function in meningiomas: effect of a specific receptor antagonist. J Neurosurg 91(1):93–99. https://doi.org/10.3171/jns.1999.91.1.0093
McCutcheon IE, Flyvbjerg A, Hill H, Li J, Bennett WF, Scarlett JA, Friend KE (2001) Antitumor activity of the growth hormone receptor antagonist pegvisomant against human meningiomas in nude mice. J Neurosurg 94(3):487–492. https://doi.org/10.3171/jns.2001.94.3.0487
Puttmann S, Senner V, Braune S, Hillmann B, Exeler R, Rickert CH, Paulus W (2005) Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest 85(9):1163–1171. https://doi.org/10.1038/labinvest.3700307
Baia GS, Slocum AL, Hyer JD, Misra A, Sehati N, VandenBerg SR, Feuerstein BG, Deen DF, McDermott MW, Lal A (2006) A genetic strategy to overcome the senescence of primary meningioma cell cultures. J Neurooncol 78(2):113–121. https://doi.org/10.1007/s11060-005-9076-y
Ragel BT, Couldwell WT, Gillespie DL, Wendland MM, Whang K, Jensen RL (2008) A comparison of the cell lines used in meningioma research. Surg Neurol 70(3):295–307. https://doi.org/10.1016/j.surneu.2007.06.031
Ragel BT, Elam IL, Gillespie DL, Flynn JR, Kelly DA, Mabey D, Feng H, Couldwell WT, Jensen RL (2008) A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg 108(2):304–310. https://doi.org/10.3171/JNS/2008/108/2/0304
Haber DA, Gray NS, Baselga J (2011) The evolving war on cancer. Cell 145(1):19–24. https://doi.org/10.1016/j.cell.2011.03.026
Shih KC, Chowdhary S, Rosenblatt P, Weir AB 3rd, Shepard GC, Williams JT, Shastry M, Burris HA 3rd, Hainsworth JD (2016) A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J Neurooncol 129(2):281–288. https://doi.org/10.1007/s11060-016-2172-3
Karsy M, Hoang N, Barth T, Burt L, Dunson W, Gillespie DL, Jensen RL (2016) Combined hydroxyurea and verapamil in the clinical treatment of refractory meningioma: human and orthotopic xenograft studies. World Neurosurg 86:210–219. https://doi.org/10.1016/j.wneu.2015.09.060
Marincek N, Radojewski P, Dumont RA, Brunner P, Muller-Brand J, Maecke HR, Briel M, Walter MA (2015) Somatostatin receptor-targeted radiopeptide therapy with 90Y-DOTATOC and 177Lu-DOTATOC in progressive meningioma: long-term results of a phase II clinical trial. J Nucl Med 56(2):171–176. https://doi.org/10.2967/jnumed.114.147256
Furtner J, Schopf V, Seystahl K, Le Rhun E, Ruda R, Roelcke U, Koeppen S, Berghoff AS, Marosi C, Clement P, Faedi M, Watts C, Wick W, Soffietti R, Weller M, Preusser M (2016) Kinetics of tumor size and peritumoral brain edema before, during, and after systemic therapy in recurrent WHO grade II or III meningioma. Neuro Oncol 18(3):401–407. https://doi.org/10.1093/neuonc/nov183
Norden AD, Ligon KL, Hammond SN, Muzikansky A, Reardon DA, Kaley TJ, Batchelor TT, Plotkin SR, Raizer JJ, Wong ET, Drappatz J, Lesser GJ, Haidar S, Beroukhim R, Lee EQ, Doherty L, Lafrankie D, Gaffey SC, Gerard M, Smith KH, McCluskey C, Phuphanich S, Wen PY (2015) Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 84(3):280–286. https://doi.org/10.1212/WNL.0000000000001153
Gerster-Gillieron K, Forrer F, Maecke H, Mueller-Brand J, Merlo A, Cordier D (2015) 90Y-DOTATOC as a therapeutic option for complex recurrent or progressive meningiomas. J Nucl Med 56(11):1748–1751. https://doi.org/10.2967/jnumed.115.155853
Simo M, Argyriou AA, Macia M, Plans G, Majos C, Vidal N, Gil M, Bruna J (2014) Recurrent high-grade meningioma: a phase II trial with somatostatin analogue therapy. Cancer Chemother Pharmacol 73(5):919–923. https://doi.org/10.1007/s00280-014-2422-z
Raizer JJ, Grimm SA, Rademaker A, Chandler JP, Muro K, Helenowski I, Rice L, McCarthy K, Johnston SK, Mrugala MM, Chamberlain M (2014) A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J Neurooncol 117(1):93–101. https://doi.org/10.1007/s11060-014-1358-9
Reardon DA, Norden AD, Desjardins A, Vredenburgh JJ, Herndon JE 2nd, Coan A, Sampson JH, Gururangan S, Peters KB, McLendon RE, Norfleet JA, Lipp ES, Drappatz J, Wen PY, Friedman HS (2012) Phase II study of Gleevec(R) plus hydroxyurea (HU) in adults with progressive or recurrent meningioma. J Neurooncol 106(2):409–415. https://doi.org/10.1007/s11060-011-0687-1
Nayak L, Iwamoto FM, Rudnick JD, Norden AD, Lee EQ, Drappatz J, Omuro A, Kaley TJ (2012) Atypical and anaplastic meningiomas treated with bevacizumab. J Neurooncol 109(1):187–193. https://doi.org/10.1007/s11060-012-0886-4
Lou E, Sumrall AL, Turner S, Peters KB, Desjardins A, Vredenburgh JJ, McLendon RE, Herndon JE 2nd, McSherry F, Norfleet J, Friedman HS, Reardon DA (2012) Bevacizumab therapy for adults with recurrent/progressive meningioma: a retrospective series. J Neurooncol 109(1):63–70. https://doi.org/10.1007/s11060-012-0861-0
Johnson DR, Kimmel DW, Burch PA, Cascino TL, Giannini C, Wu W, Buckner JC (2011) Phase II study of subcutaneous octreotide in adults with recurrent or progressive meningioma and meningeal hemangiopericytoma. Neuro Oncol 13(5):530–535. https://doi.org/10.1093/neuonc/nor044
Wen PY, Yung WK, Lamborn KR, Norden AD, Cloughesy TF, Abrey LE, Fine HA, Chang SM, Robins HI, Fink K, Deangelis LM, Mehta M, Di Tomaso E, Drappatz J, Kesari S, Ligon KL, Aldape K, Jain RK, Stiles CD, Egorin MJ, Prados MD (2009) Phase II study of imatinib mesylate for recurrent meningiomas (North American Brain Tumor Consortium study 01–08). Neuro Oncol 11(6):853–860. https://doi.org/10.1215/15228517-2009-010
Bartolomei M, Bodei L, De Cicco C, Grana CM, Cremonesi M, Botteri E, Baio SM, Arico D, Sansovini M, Paganelli G (2009) Peptide receptor radionuclide therapy with (90)Y-DOTATOC in recurrent meningioma. Eur J Nucl Med Mol Imaging 36(9):1407–1416. https://doi.org/10.1007/s00259-009-1115-z
Chamberlain MC, Glantz MJ (2008) Interferon-alpha for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer 113(8):2146–2151. https://doi.org/10.1002/cncr.23803
Chamberlain MC, Glantz MJ, Fadul CE (2007) Recurrent meningioma: salvage therapy with long-acting somatostatin analogue. Neurology 69(10):969–973. https://doi.org/10.1212/01.wnl.0000271382.62776.b7
Fuentes S, Chinot O, Dufour H, Paz-Paredes A, Metellus P, Barrie-Attarian M, Grisoli F (2004) Hydroxyurea treatment for unresectable meningioma. Neurochirurgie 50(4):461–467
Loven D, Hardoff R, Sever ZB, Steinmetz AP, Gornish M, Rappaport ZH, Fenig E, Ram Z, Sulkes A (2004) Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol 67(1–2):221–226
Chamberlain MC, Tsao-Wei DD, Groshen S (2004) Temozolomide for treatment-resistant recurrent meningioma. Neurology 62(7):1210–1212
Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE (2002) Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg 97(2):341–346. https://doi.org/10.3171/jns.2002.97.2.0341
Muhr C, Gudjonsson O, Lilja A, Hartman M, Zhang ZJ, Langstrom B (2001) Meningioma treated with interferon-alpha, evaluated with [(11)C]-L-methionine positron emission tomography. Clin Cancer Res 7(8):2269–2276
Schrell UM, Rittig MG, Anders M, Koch UH, Marschalek R, Kiesewetter F, Fahlbusch R (1997) Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg 86(5):840–844. https://doi.org/10.3171/jns.1997.86.5.0840
Kaba SE, DeMonte F, Bruner JM, Kyritsis AP, Jaeckle KA, Levin V, Yung WK (1997) The treatment of recurrent unresectable and malignant meningiomas with interferon alpha-2B. Neurosurgery 40(2):271–275
Goodwin JW, Crowley J, Eyre HJ, Stafford B, Jaeckle KA, Townsend JJ (1993) A phase II evaluation of tamoxifen in unresectable or refractory meningiomas: a Southwest Oncology Group study. J Neurooncol 15(1):75–77
Acknowledgements
All contributors to this study are included in the list of authors.
Funding
This work was supported by the National Natural Science Foundation of China (Grant number 81802974) and grants from the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (Grant number 2014BAI04B01).
Author information
Authors and Affiliations
Contributions
Generating the idea and writing the manuscript: CQ and MH. Drawing the figures: CQ and MH. Revising the manuscript and modifying the figures: YP. Writing the manuscript and supervising the entire work: WL and QL. Providing final approval for the version to be published: QL, WL, CQ, MH, YP, and YL.
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Qin, C., Huang, M., Pan, Y. et al. Brain-invasive meningiomas: molecular mechanisms and potential therapeutic options. Brain Tumor Pathol 38, 156–172 (2021). https://doi.org/10.1007/s10014-021-00399-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10014-021-00399-x