Abstract
Based on the modulated electronic properties of Fe3O4-graphene (Fe3O4/GN composite) as well as the outstanding complexation between Pb2+ and natural substances garlic extract (GE), a novel electrochemical sensor for the determination of Pb2+ in wastewater was prepared by immobilization of Fe3O4/GN composite integrated with GE onto the surface of glassy carbon electrode (GCE). Fe3O4/GN composite was employed as an electrochemical active probe for enhancing electrical response by facilitating charge transfer while GE was used to improve the selectivity and sensitivity of the proposed sensor to Pb2+ assay. The electrochemical sensing performance toward Pb2+ was appraised by cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and square wave voltammetry (SWV). Under the optimized condition, the sensor exhibited two dynamic linear ranges (LDR) including 0.001 to 0.5 nM and 0.5 to 1000 nM with excellent low detection limit (LOD) of 0.0123 pM (S/N = 3) and quantification limit (LOQ) of 0.41 pM (S/N = 10). Meanwhile, it displayed remarkable stability, reproducibility (RSD of 3.61%, n = 3), and selectivity toward the assay for the 100-fold higher concentration of other heavy metal ions. Furthermore, the novel sensor has been successfully employed to detect Pb2+ from real water samples with satisfactory results.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Garlic (Allium sativum L.) is a worldwide cultivated Alliaceae species and of economic benefits in food and medicine. In the previous research, garlic is effective in treating lead intoxication for workers who are exposed to environmental lead. In general, garlic and its derivatives have been gradually recommended as a promising agent for lead treatment because of the negative effect of the chelating agents that can deplete the body of essential metals resulting in secondary injury for health [1]. The positive effects of garlic on human health are ascribed to the presence of bioactive substances such as organosulfur compounds [2,3,4,5], flavonoids, and vitamins [6]. The organosulfur compounds mainly include diallyl trisulfide, diallyl tetrasulfide, s-allylcysteine, vinyldithiines, allylpropyl disulfide, ajonens ((E) - and (Z)-4, 5, 9–trithiadodeca-1, 6, 11–triene-9- oxides), and allicin [5, 7,8,9]. The components are likely to work synergistically to provide the largest range of garlic’s health benefits. To the best of our knowledge, Fatima and Ahmad [10] have utilized the antioxidant enzymes of garlic as biomarkers of heavy metals for detecting the heavy metals in wastewater. Cheng et al. [11] and Zhou et al. [12] have fabricated electrochemical sensors for determining Pb2+ based on L-cysteine. However, garlic has not been explored in the field of electrochemical sensor to detect the presence of Pb2+ in wastewater, which is expected to attract particular attention for the detection of Pb2+ by virtue of their simple operation modes, rapid response, high sensitivity, and selectivity [13]. Hence, in this study, garlic extract (GE), as a natural product, may provide a sensitive electrochemical interface in the field of electrochemical sensor and pre-concentrate Pb2+ to produce stable complexes for the recognition of Pb2+ in wastewater.
Nonetheless, the preponderances of GE heavily rely on the supporting materials which are in possession of high specific surface area, superior electronic conductivity, and easy functionalization. Based on these criterions, graphene is an ideal supporting material [14]. Graphene (GN) has a unique nanostructure and interesting properties (such as large surface, excellent conductive property, prominent thermal stability) [15], which results in widespread potential applications, like supercapacitors [16] or serving as absorbent of heavy metal ions [17]. However, it has been widely reported that the graphene-metal oxide nanomaterials have better functionalization and performance in their applications than graphene or metal oxide nanomaterials solely [14, 18], resulting from inheriting the advantages of two component materials [19,20,21]. Therefore, it is an interesting and appealing method that Fe3O4 nanoparticles are grown and anchored on GN sheets as supporting material for the electrode of electrochemical sensor, combining with the fascinating merits of Fe3O4 NPs and GN and exhibiting a better electrochemical performance compared with the native phase [21,22,23,24,25,26,27]. Suryawanshi et al. [28] have successfully prepared a Fe3O4 hierarchically perforated graphene nanosheets composite with enhanced electronic conductivity. Zhou et al. [29] have synthesized a well-organized flexible interleaved GNS/Fe3O4 composite through in situ reduction of iron hydroxide between graphene nanosheets (GNS), behaving superior electrochemical performance. Furthermore, the incorporation of Fe3O4 NPs onto graphene sheets tackled the aggregation of Fe3O4 nanoparticles and preserved the electrochemically active surface of Fe3O4 NPs [30]. Thus, the Fe3O4/GN composite is a remarkable electrode material for electrochemical sensor. Based on this background, in this study, Fe3O4/GN composite as an electrochemical active probe could enhance the electrical response by facilitating charge transfer.
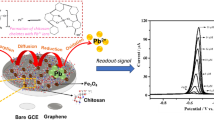
The main purpose of this investigation was to develop a simple, sensitive, selective, and inexpensive electrochemical method for the determination of Pb2+ in wastewater. In the current work, a simple and novel electrochemical sensor with high sensitivity and selectivity has been on the spotlight for Pb2+ determination in wastewater by using Fe3O4/GN/GE-modified glassy carbon electrode (GCE). The electrode preparation is described in Scheme 1. The GE and Fe3O4/GN composite are chosen as the modifier for GE is an economical nature product and Fe3O4/GN composite is environmentally friendly, respectively. To the best of our knowledge, this is the first work describing and integrating the unique features of both Fe3O4/GN composite and GE through fabricating a sensor to exploit their synergy for the electrochemical detection of Pb2+. It was found that the proposed sensor is allowed to select optimal conditions for the detection of Pb2+, showing satisfactory linearity, detection limit, sensitivity, selectivity, and high reproducibility and stability. Relative to the reported methods [31,32,33,34], the established method has superior electrochemical properties. Furthermore, the experiment results of determination Pb2+ in real samples by the developed sensor are also acceptable. Thus, not only does Fe3O4/GN/GE sensor represent a new electrochemical platform for designing environment-friendly sensors but also could meet the needs of practical analysis.
Experiment
Chemicals and apparatus
Aged garlic was purchased from a local market (Chongqing, China). Graphene oxide (GO) was provided by Sigma-Aldrich. All other chemicals, such as FeSO4·7H2O, NaOH, and N2H4·H2O, were of analytical grade purity and purchased from Tianjin Chemical Technology Co., Ltd. (China) without further purification. Unless otherwise stated, the supporting electrolyte is a 0.1 mol/L acetate buffer solution (ABS, pH 5.50). The double-distilled water was used for all solutions.
The morphologies of the as-prepared samples were acquired on a JSM-7500F (Hitachi Co., Ltd., Japan) scanning electron microscope and Tecnai G20 transmission electron microscope (FEI Company, USA). The crystallographic information was recorded by a XRD-6000 classical powder diffractometer with Cu kα radiation (λ = 0.154 nm). The function groups of the samples were gathered by a Nicolet 550 FTIR Spectrometer (Shimadzu Scientific Instruments) from 400 to 4000 cm−1 at room temperature.
GC–MS analysis of the garlic extract (GE) was performed with a 6890N gas chromatograph (Agilent, American) system equipped with a fused silica capillary Agilent Technology HP-5 ms (5% phenyl methyl siloxane) column (30 m × 0.25 mm i.d., film thickness 0.1 μm) and a 5975C plus mass spectrometer (Agilent, American). For GC–MS detection, 1 μL sample was injected for analysis. In the GC system, injector and detection temperature were set at 280 and 290 °C, respectively. The initial temperature was kept at 40 °C for 2 min and ramped to 130 °C with a rate of 8 °C/min, then increased to 290 °C (10 min) at a rate of 10 °C/min. Carrier gas was helium with flow rate of 1 mL/min. A 70-eV EI mode with an ionic source temperature of 230 °C was used in the MS system. The standard mass spectra of organosulfur compounds were provided by literature data and the software of the GC-MS system (National Institute of Standards and Technology (NIST 05. LIB) libraries date).
A CHI660E electrochemical workstation (ChenHua Instrument Co., Shanghai, China) was used to perform the electrochemical experiments, including cyclic voltammetry (CV) and square wave voltammetry (SWV). Different modified glassy carbon electrodes (Fe3O4/GCE, Fe3O4/GN/GCE, Fe3O4/GN/GE/GCE) or a bare glassy carbon electrode (GCE, 3 mm diameter) were used as the working electrode, with an Ag/AgCl/KCl (3 mol/L KCl saturated with AgCl) and platinum wire serving as the reference and counter electrodes, respectively.
Synthesis of Fe3O4 and Fe3O4/GN
The Fe3O4/GN nanocomposites were synthesized by a solvethermal process with Fe3O4 nanoparticles attached to reduced graphene oxide (GN) sheets, which combine the controllable growth of Fe3O4 NPs and the reduction of GO in one single step. It was slightly modified from the method as reported by Wang et al. [35]. For preparing Fe3O4/GN, 20 mL of FeSO4·7H2O solution (a concentration of ~ 69.5 mg/mL) and 5 mL of NaOH solution (a concentration of ~ 80 mg/mL) were slowly added into the prepared 30-mL GO water suspension (a concentration of ~ 2.67 mg/mL) in order with vigorous stirring. The mixture was stirred at room temperature for 1 h. Then, 20 mL N2H4·H2O was injected into the solution apace and then the mixture was loaded into a 100-mL Teflon lined stainless steel autoclave for hydrothermal reaction at 180 °C for 8 h. The Fe3O4/GN separated by magnetic force from solution was washed with deionized water several times and then dried at 60 °C for 8 h.
For comparison, the same synthetic procedures were carried out in the preparation of Fe3O4 NPs but without adding GO, as the reference.
Extraction of GE
The garlic was peeled, cleaned, and dried at room temperature, followed by chopping. A 10-g chopped edible portion was kept at 50 °C for 2 h, then mixed for 1 h after the addition of 70 mL ethanol solution (anhydrous ethanol/deionized water = 7/1, V/V) at 30 °C for leaching repeatedly. After that, the mixture was filtrated and centrifugated to remove the insoluble substance. Next, the ethanol solution was evaporated from the supernate to obtain the garlic extract (GE). The GE stored at 4 °C until subsequent experiments.
Fabrication of the electrochemical sensor
The GCE (Φ = 3 mm) was polished by 1.0, 0.3, and 0.05 μm alumina slurry, along with successive washing with anhydrous ethanol and deionized water, then dried through nitrogen blowing. One milligram of the synthesized Fe3O4/GN was dispersed in 1 mL deionized water with ultra-sonication for 2 h to acquire a homogenous suspension. Then, another 1 h of ultra-sonication was kept after 50 μL of GE mixing until a homogenously dispersed solution (Fe3O4/GN/GE). Afterwards, 5 μL of the resulting homogenous suspension was drop-casted onto the surface of a polished GCE and dried at room temperature. A Fe3O4-modified GCE sensor (Fe3O4/GCE) and Fe3O4/GN-modified GCE sensor (Fe3O4/GN/GCE) were also fabricated using similar procedures for comparison studies.
Electrochemical measurements
For CV experiments, 5.0 mmol/L K3[Fe(CN)6] with 0.1 mol/L KCl standard solutions were used for evaluating electrochemical sensor performance with a scan rate of 50 mV/s from − 0.2 to + 0.7 V. Electrochemical impedance spectroscopy (EIS) measurements were performed in 5.0 mmol/L K3[Fe(CN)6]/K4[Fe(CN)6] solution to determinate the charge transfer resistance of the modified electrodes over a frequency range of 0.1 Hz~100 kHz with amplitude of 10 mV. SWV measurements were taken in 0.1 mol/L ABS containing different concentrations of Pb2+ solutions (pH 5.50) in the absence of dissolved oxygen.
Results and discussion
Physicochemical characterization
GC–MS analysis of GE
The components of the garlic extract (GE) were analyzed by GC–MS. Figure 1 is a representative total ion current chromatogram of GE and Table 1 summarizes the identified compounds. Table 1 also provides the identified structure of chemical compounds and GC retention time. The garlic extract included mainly ethyl acetate, 3-methyl-butanal, 1,1-diethoxy-ethan homopolymer, 1,3-dithiane, allyl methyl disulphide, diallyl disulphide, 3-vinyl-1,2-dithiacyclohex-4-ene, 3-vinyl-1,2-dithiacyclohex-5-ene, and diallyl trisulfide, which consisted with the previous researches [4, 5].
SEM and TEM
The morphologies of as-prepared Fe3O4 NPs, Fe3O4/GN composite, and Fe3O4/GN/GE composite determined by SEM and TEM were shown in Fig. 2. Figure 2a, b is the SEM and TEM images of the Fe3O4 NPs, respectively. It can be found in the images that the Fe3O4 NPs consisted of cube-shaped structures of NPs with the edge length of 50–150 nm and spherical structures of NPs with the diameter of ~ 50 nm. Figure 2c, d provides the micrographs of Fe3O4/GN composite. It is clearly observed from SEM image that Fe3O4 NPs with hybrid structure were densely and uniformly anchored over the crumpled surface of GN. Meanwhile, the presence of GN did not alter the mixture cubic and spherical morphology of Fe3O4 NPs, but effectively decreased their particles after the composite formation. The TEM image in Fig. 2d further confirmed the present clearer structural information of the Fe3O4/GN sample. Valuably, the synergistic effect between the Fe3O4 and GN in the formation retains the unique structure and prevents the agglomeration of Fe3O4 NPs. In addition, the interaction between Fe3O4 NPs and GN makes possible Fe3O4 NPs strongly anchored on the surface of GN even after a long-time ultra-sonication and does not need additional molecular linker to bridge the Fe3O4 NPs and GN. It is noted that the GN provides large areas. Thereby, we can believe that the Fe3O4/GN composite can facilitate the electron transfer and remain the active surface areas to further boost the electrochemical performance of Fe3O4/GN electrodes benefiting from the unique structure [36]. The SEM image of Fe3O4/GN/GE composite shown in Fig. 2e presents an obvious change in comparison to Fe3O4/GN composite, which has been decorated with a GE layer on the surface of Fe3O4/GN composite. All of these results jointly attest the successful preparation of Fe3O4/GN/GE composite, which could be utilized as a remarkable biosensing platform for Pb2+ determination.
XRD and FTIR
Figure 3a has studied the crystal phase and structure information of the prepared Fe3O4 NPs, Fe3O4/GN, and Fe3O4/GN/GE nanocomposite by XRD measurement. The diffraction peaks at 2θ ≈ 30.19°, 35.62°, 43.39°, 57.01°, and 62.79° can be indexed to (2 2 0), (3 1 1), (4 0 0), (5 1 1), and (4 4 0) reflection planes of Fe3O4, which guaranteed the cubic structure of as-synthesized Fe3O4 nanostructure (JCPDS No. 01-082-1533) [15, 37]. The structure of Fe3O4 in Fe3O4/GN and Fe3O4/GN/GE are consistent with the cubic structure of free Fe3O4 nanostructures, implying that there is no structural change even after the composite preparation. In addition, there is a new diffraction peak at 2θ ≈ 25.77° in Fe3O4/GN and Fe3O4/GN/GE composite, which could be credited to the GN, ensuring the composite fabrication of Fe3O4 with GN sheets. Moreover, there is no change in the crystalline morphology of Fe3O4/GN and Fe3O4/GN/GE composite, enunciating that the GE layer does not change the crystalline morphology of Fe3O4/GN.
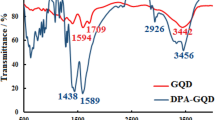
The components responsible for the capping on the prepared Fe3O4/GN/GE nanocomposite are analyzed by FTIR and the FTIR spectrums of Fe3O4 NPs, Fe3O4/GN, and Fe3O4/GN/GE nanocomposite and GE are shown in Fig. 3b. There is an intense peak at ~ 583 cm−1 in the spectrum of Fe3O4 NPs, Fe3O4/GN, and Fe3O4/GN/GE nanocomposite, as shown in Fig. 3b, corresponding to the Fe–O stretching vibration [38]. From GE, the characteristic adsorption peaks located at 524, 1023, 1121, 1270, 1632, 2937, and 3416 cm−1 identify the S–S, C–S, C=S, S–CH2–, –NH, –CH2–, and –CO–NH vibration of GE, respectively, which manifested that the components of GE include organic sulfide, sulfoether compounds, and acid amides. Furthermore, these entire characteristic peaks exit in the FTIR spectrum of Fe3O4/GN/GE, declaring the valid incorporation of GE in the Fe3O4/GN nanocomposite.
Electrochemical characterizations
The cyclic voltammetries (CVs) of different modified electrodes in 5.0 mmol/L K3[Fe(CN)6] containing 0.1 mol/L KCl are shown in Fig. 4a, which have studied the change of electrode behavior of each modified electrodes. All of the electrodes exhibited two well-defined redox peaks of [Fe(CN)6]3−/4-. However, the redox peak currents of each electrode were different. The redox peak current of Fe3O4/GN/GCE was higher than the redox peak current of GCE, while the redox peak current of Fe3O4/GN/GE/GCE was lower than the redox peak current of Fe3O4/GN/GCE. This phenomenon can be ascribed to the Fe3O4/GN enabled fast electron transport through the underlying GN layer to Fe3O4 nanoparticles [35, 39] and the GE layer obstructed the electron transport, respectively (EIS see Supplementary information).
We have studied the electrochemical features and the function for Pb2+ analysis of the Fe3O4/GN/GE by comparing the electrochemical SWV responses on several modified electrodes in the presence of 1 μM target Pb2+ in pH 5.50 ABS, as seen in Fig. 5. After electrochemical accumulation at −1.0 V for 180 s, a well-defined stripping peak appears at the potential values between −0.55 and − 0.60 V with different stripping peak current on all electrodes. There is an inconspicuous stripping peak at −0.57 V on bare GCE, and its stripping peak current for Pb2+ is far less than that of other electrodes. The Fe3O4 and Fe3O4/GN have a larger surface area between the electrode and electrolyte solution. In contrast, the peak current sharply increased upon addition of GE on the Fe3O4/GN, which can form strong complex with Pb2+ and provide more active sites for Pb2+ accumulation. All of those results could certify that the interaction between target Pb2+ and Fe3O4/GN/GE has changed the electronic signal, thus providing a possibility for the recognition of Pb2+.
Optimization of assay conditions
Various experimental parameters were optimized for exploiting the maximum efficacy of Fe3O4/GN/GE/GCE sensor with care, loading of Fe3O4/GN/GE, analyte accumulation time, and pH of buffer solution included. As portrayed in Fig. 6a, the effect of the coated Fe3O4/GN/GE composite on electrode was shown. The peak current increased with the volume of the coated Fe3O4/GN/GE composite on electrode, and then reached maximum when the volume of Fe3O4/GN/GE composite on electrode is 5 μL. Therefore, in the subsequent measurements, 5 μL Fe3O4/GN/GE composite are coated on electrode. As seen from Fig. 6b, the electrochemical signal of the developed biosensor enhanced rapidly as the analyte accumulation time increased and achieved a plateau at 180 s, which may be owing to the increase of composites film and decrease the electron transfer rate of metal stripping. So, an analyte accumulation time of 180 s was employed as the optimum analyte accumulation time. The influence of the acidity of the electrolyte on the determination of Pb2+ in the range of 3.00~6.50 was optimized. As shown in Fig. 6c, it can be seen that the current value increased tardily with the pH of electrolyte in the range from 3.00 to 4.50, while the peak current enhanced rapidly with the pH of electrolyte in the range from 4.50 to 5.50 and obtained the maximum at 5.50. Thus, a pH of 5.50 was chosen as the optimized pH of electrolyte.
Analytical performance
Figure 7 depicts the SWV responses of the Fe3O4/GN/GE biosensor at different concentrations of Pb2+ under the optimal conditions. As portrayed in Fig. 7a, the SWV peaks increased with the increasing target Pb2+ concentrations and reached to plateau at concentration of 1000 nM Pb2+. Furthermore, it is linear with the concentrations of Pb2+ from 0.001 to 0.5 nM and 0.5 to 1000 nM with correlation coefficients of 0.9999 and 0.9975, respectively (Fig. 7b). The linear regression equations were y(μA) = 2.7262 + 23.0585x(nM) and y(μA) = 14.2501 + 0.0102x(nM). The detection limit (LOD) and quantification limit (LOQ) of the biosensor were determined to be 0.0123 and 0.041 pM based on three times the standard deviation of the blank sample/slope and ten times the standard deviation of the blank sample/slope, respectively. The as-prepared biosensor based on novel Fe3O4/GN/GE showed favorable Pb2+ detection performances, compared with that of other Pb2+ biosensors in Table 2, highlighting its sensitivity and acceptability further. Our biosensor provides an attractive detection limit and a broader working range. Besides, the developed biosensor has advantages in fabrication with a facile hydrothermal technique without any surfactants or templates, economy without the use of DNAzyme and with the utilization of the natural substance GE for Pb2+ ion-specific, and environmentally friendly without any toxicant and with the employ of the environmental benignity Fe3O4/GN/GE composite. All of these comparison manifest that the sensing performance of the designed method is superior to the mentioned biosensor for Pb2+ determination.
a SWV curves of Fe3O4/GN/GE/GCE electrodes in 0.1 mol/L ABS (pH = 5.50) containing different concentrations of Pb2+ solution: 0, 0.001, 0.005, 0.01, 0.05, 0.1, 0.5, 1, 5, 10, 50, 100, 500, and 1000 nM. b Plot of the peak current against the concentration of Pb2+. Error bars are the standard deviation for three consecutive measurements
Reproducibility and stability of the proposed sensor
The reproducibility and stability are the extremely important features of the biosensor in its practical applications and development. In our work, the reproducibility of the as-proposed Pb2+ biosensor was evaluated by estimating the variation of the SWV response to 1000 nM Pb2+ for eight different Fe3O4/GN/GE electrodes. According to the experimental results, as shown in Fig. 8a, the SWV response variation of the assays with the same-batch biosensors is 3.61%, revealing the good reproducibility of this method. Additionally, the stability of the as-prepared biosensor was also explored on a 10-week period. The biosensor was stored in the refrigerator (4 °C) when not in use and measured intermittently (per 7 days). The SWV response remained stable (RSD 6.28%) and preserved 86.14% of the initial response after a storage period of 10 weeks for the recognition of 1000 nM Pb2+, as portrayed in Fig. 8b. All of these results indicated that the reproducibility and stability of the developed biosensor were desirable.
a SWV curves of eight different Fe3O4/GN/GE/GCE in 0.1 mol/L ABS (pH = 5.50) containing 1000 nM Pb2+. Inset (a) is the reproducibility of the eight different modified electrodes by SWV. b SWV curves of the different preserved time Fe3O4/GN/GE/GCE in 0.1 mol/L ABS (pH = 5.50) containing 1000 nM Pb2+. Inset (b) is the stability of the proposed sensor. Error bars are the standard deviation for three consecutive measurements
Specificity of the proposed sensor
The specificity of the designed strategy is supposed to be validated to ensure that the biosensor was acceptable before it was applied to the real sample analysis. We challenged the system against other ions including Co2+, Fe3+, Cu2+, Mn2+, Zn2+, Ni2+, Ag+, Hg2+, and Cd2+ to investigate the specificity of our designed strategy. Figure 9 depicts the histograms of peak current changes for the biosensor after reaction with other interfering metal ions. Obviously, the current response was much higher with the target Pb2+ of 0.5 nM than those of other interfering metal ions (10 nM). The results clearly demonstrated that the sensing strategy could monitor Pb2+ in the presence of other metal ions selectively.
Application for the analysis of samples
We have determined Pb2+ in real samples (tap water, rain water, and river water from Jialing River located in Chongqing, China) by measuring the recovery of Pb2+ to evaluate the utility of the fabricated biosensor. To get rid of insoluble substance, all samples were filtered by a filter membrane before detected. We have summarized the recovery values of the real samples, as shown in Table 3. All recovery values range from 91.7 to 99.8% and RSD were 2.33~5.7%, which verify that the developed sensing strategy was potentially applicable for practical detection in real samples.
Conclusions
In this study, we have fabricated a novel cheap and sensitive electrochemical sensor using Fe3O4/GN composite and GE as electrode modification materials for Pb2+ determination. It is first reported that natural substance garlic extract was utilized as Pb2+ specific-receptor and modified with Fe3O4-graphene composite for establishing advanced sensing devices. The novel sensor not only showed high sensitivity and selectivity for Pb2+ but also exhibited broad linearity, low detection limit, and satisfactory reproducibility and stability. Additionally, the practical application in detecting real water samples shows a satisfactory result. In general, this novel sensor possesses superior electrochemical detection performance and less cost compared with other published electrochemical sensor.
References
Aposhian HV, Maiorino RM, Ramirez DG, Charles MZ, Xu Z, Hurlbut KM, Munoz PJ, Dart RC, aposhian MM (1995) Mobilization of heavy metals by newer, therapeutically useful chelating agents. Toxicology 97(1-3):23–38
Kim JM, Ghang N, Kim WK, Chun HS (2006) Dietary S-allyl-L-cysteine reduces mortality with decreased incidence of stroke and behavioral changes in stroke-prone spontaneously hypertensive rats. Biosci Biotechnol Biochem 70(8):1969–1971
Lee YJ, Lee D, Shin SM, Lee JS, Chun HS, Quan FS, Shin JH, Lee GJ (2017) Potential protective effects of fermented garlic extract on myocardial ischemia-reperfusion injury utilizing in vitro and ex vivo models. J Funct Foods 33:278–285
Calle MM, Capote FP, Luque de Castro MD (2017) Headspace−GC–MS volatile profile of black garlic vs fresh garlic: evolution along fermentation and behavior under heating. LWT-Food Sci Technol 80:98–105
Tocmo R, Wu Y, Liang D, Fogliano V, Huang D (2017) Boiling enriches the linear polysulfides and the hydrogen sulfide-releasing activity of garlic. Food Chem 221:1867–1873
Piątkowska E, Kopeć A, Leszczyńska T (2015) Basic chemical composition, content of micro- and macroelements and antio xidant activity of different varieties of garlic’s leaves Polish origin. ŻYWNOŚĆ. Nauka. Technologia. Jakość 1:181–192
Szychowski KA, Binduga UE, Rybczyńska-Tkaczyk K, Leja ML, Gmiński J (2016) Cytotoxic effects of two extracts from garlic (Allium sativum L.) cultivars on the human squamous carcinoma cell line SCC-15. Saudi J Biol Sci. doi: https://doi.org/10.1016/j.sjbs.2016.10.005
Lanzotti V (2006) The analysis of onion and garlic. J Chromatogr A 1112(1-2):3–22
Bhandari PR (2012) Garlic (Allium sativum L.): a review of potential therapeutic application. International Journal of Green Pharmacy (Medknow Publications 6: 118
Fatima RA, Ahmad M (2006) Certain antioxidant enzymes of Allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci Total Environ 346:256–273
Cheng Y, Fa H, Yin W, Hou C, Huo D, Liu F (2015) A sensitive electrochemical sensor for lead based on gold nanoparticles/nitrogen-doped graphene composites functionalized with l-cysteine-modified electrode. J Solid State Electr 20:327–335
Zhou W, Li C, Sun C, Yang Y (2016) Simultaneously determination of trace Cd2+ and Pb2+ based on L-cysteine/graphene modified glassy carbon electrode. Food Chem 192:351–357
Abnous K, Danesh NM, Alibolandi M, Ramezani M, Sarreshtehdar EA, Zolfaghari R, Seyed MT (2017) A new amplified π-shape electrochemical aptasensor for ultrasensitive detection of aflatoxin B1. Biosens Bioelectron 94:374–379
Su J, Cao M, Ren L, Hu C (2011) Fe3O4–graphene nanocomposites with improved lithium storage and magnetism properties. J Phys Chem C 115(30):14469–14477
Boruah PK, Sharma B, Hussain N, Das MR (2017) Magnetically recoverable Fe3O4/graphene nanocomposite towards efficient removal of triazine pesticides from aqueous solution: investigation of the adsorption phenomenon and specific ion effect. Chemosphere 168:1058–1067
Wang B, Park J, Wang C, Ahn H, Wang G (2010) Mn3O4 nanoparticles embedded into graphene nanosheets: preparation, characterization, and electrochemical properties for supercapacitors. Electrochim Acta 55:6812–6817
Chandra V, Park J, Chun Y, Lee JW, Hwang I, Kim KS (2010) Water-dispersible magnetite-reduced graphene oxide composites for arsenic removal. ACS Nano 4(7):3979–3986
Pumera M (2010) Graphene-based nanomaterials and their electrochemistry. Chem Soc Rev 39(11):4146–4157
He Y, Sheng Q, Zheng J, Wang M, Liu B (2011) Magnetite–graphene for the direct electrochemistry of hemoglobin and its biosensing application. Electrochim Acta 56(5):2471–2476
Gao Y, Ma D, Hu G, Zhai P, Bao X, Zhu B, Zhang B, Su DS (2011) Layered-carbon-stabilized iron oxide nanostructures as oxidation catalysts. Angew Chem 50(43):10236–10240
Hsieh CT, Lin JY, Mo CY (2011) Improved storage capacity and rate capability of Fe3O4–graphene anodes for lithium-ion batteries. Electrochim Acta 58:119–124
Zhuo L, Wu Y, Wang L, Ming J, Yu Y, Zhang X, Zhao F (2013) CO2–expanded ethanol chemical synthesis of a Fe3O4@graphene composite and its good electrochemical properties as anode material for Li-ion batteries. J Mater Chem A 1(12):3954–3960
He J, Zhao S, Lian Y, Zhou YM, Wang L, Ding B, Cui S (2017) Graphene-doped carbon/Fe3O4 porous nanofibers with hierarchical band construction as high-performance anodes for lithium-ion batteries. Electrochim Acta 229:306–315
Wang D, Li Y, Wang Q, Wang T (2012) Nanostructured Fe2O3–graphene composite as a novel electrode material for supercapacitors. J Solid State Electr 16(6):2095–2102
Wu B, Ren Y, Mu D, Liu X, Zhao J, Wu F (2013) Enhanced electrochemical performance of LiFePO cathode with the addition of fluoroethylene carbonate in electrolyte. J Solid State Electr 17(3):811–816
Zhang W, Wang L, Zheng X (2014) Indicator-free electrochemical genosensing originated from the self-signal of poly-xanthurenic acid enhanced by Fe3O4/reduced graphene oxide. J Solid State Electr 18(9):2367–2373
Liu HD, Zhang JL, Xu DD, Huang LH, Tan SZ, Mai WJ (2015) Easy one-step hydrothermal synthesis of nitrogen-doped reduced graphene oxide/iron oxide hybrid as efficient supercapacitor material. J Solid State Electr 19(1):135–144
Suryawanshi A, Aravindan V, Mhamane D, Yadav P, Patil S, Madhavi S, Ogale S (2015) Excellent performance of Fe3O4-perforated graphene composite as promising anode in practical Li-ion configuration with LiMn2O4. Energy Storage Materials 9:152–157
Zhou G, Wang D, Li F, Zhang L, Li N, Wu Z, Wen L, Lu GQ (Max), Cheng H (2010) Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem Mater 22: 5306–5313, 18
Liang CL, Liu Y, Bao RY, Luo Y, Yang W, Xie BH, Yang MB (2016) Effects of Fe3O4 loading on the cycling performance of Fe3O4/rGO composite anode material for lithium ion batteries. J Alloy Compd 678:80–86
Buica GO, Lazar IG, Saint AE, Tecuceanu V, Dumitriu C, Anton IA, Stoian AB, Ungureanu EM (2017) Ultrasensitive modified electrode based on poly(1H -pyrrole-1-hexanoic acid) for Pb(II) detection. Sens Actuat B: Chem 246:434–443
Qin D, Gao S, Wang L, Shen H, Yalikun N, Sukhrobov P, Wagberg T, Zhao Y, Mamat X, Hu G (2017) Three-dimensional carbon nanofiber derived from bacterial cellulose for use in a Nafion matrix on a glassy carbon electrode for simultaneous voltammetric determination of trace levels of Cd(II) and Pb(II). Microchim Acta 184(8):2759–2766
Pérez-Ràfols C, Bastos-Arrieta J, Serrano N, Díaz-Cruz JM, Ariño C, Pablo JD, Esteban M (2017) Ag nanoparticles drop-casting modification of screen-printed electrodes for the simultaneous voltammetric determination of Cu(II) and Pb(II). Sensors-Basel 17(6):1458–1469
Dönmez KB, Çetinkaya E, Deveci S, Karadağ S, Şahin Y, Doğu M (2017) Preparation of electrochemically treated nanoporous pencil-graphite electrodes for the simultaneous determination of Pb and Cd in water samples. Ana bioanal Chem 409(20):4827–4837
Wang Q, Jiao L, Du H, Wang Y, Yuan H (2014) Fe3O4 nanoparticles grown on graphene as advanced electrode materials for supercapacitors. J Power Sources 245:101–106
Li L, Gao P, Gai S, He F, Chen Y, Zhang M, Yang P (2016) Ultra small and highly dispersed Fe3O4 nanoparticles anchored on reduced graphene for supercapacitor application. Electrochim Acta 190:566–573
Atarod M, Nasrollahzadeh M, Sajadi SM (2015) Green synthesis of a Cu/reduced graphene oxide/Fe3O4 nanocomposite using Euphorbia wallichii leaf extract and its application as a recyclable and heterogeneous catalyst for the reduction of 4-nitrophenol and rhodamine B. RSC Adv 5(111):91532–91543
Rani GJ, Babu KJ, kumar GG, Rajan MAJ (2016) Watsonia meriana flower like Fe3O4/reduced graphene oxide nanocomposite for the highly sensitive and selective electrochemical sensing of dopamine. J Alloy Compd 688:500–512
Shi W, Zhu J, Sim DH, Tay YY, Lu Z, Sharma Y, Srinivasan M, Zhang H, Hng Huey H, Yan Q (2011) Achieving high specific charge capacitances in Fe3O4/reduced graphene oxide nanocomposites. J Mater Chem 21(10):3422–3427
Zhou Y, Zhang J, Tang L, Peng B, Zeng G, Luo L, Gao J, Pang Y, Deng Y, Zhang F (2017) A label-free GR-5DNAzyme sensor for lead ions detection based on nanoporous gold and anionic intercalator. Talanta 165:274–281
Zhang Y, Xiao S, Li H, Liu H, Pang P, Wang H, Wu Z, Yang W (2016) A Pb2+-ion electrochemical biosensor based on single-stranded DNAzyme catalytic beacon. Sens Actuat B: Chem 222:1083–1089
Zhou Q, Lin Y, Lin Y, Wei Q, Chen G, Tang D (2016) Highly sensitive electrochemical sensing platform for lead ion based on synergetic catalysis of DNAzyme and Au-Pd porous bimetallic nanostructures. Biosens Bioelectron 78:236–243
Zhou Y, Tang L, Zeng G, Zhang C, Xie X, Liu Y, Wang J, Tang J, Zhang Y, Deng Y (2016) Label free detection of lead using impedimetric sensor based on ordered mesoporous carbon-gold nanoparticles and DNAzyme catalytic beacons. Talanta 146:641–647
Ge S, Wu K, Zhang Y, Yan M, Yu J (2016) Paper-based biosensor relying on flower-like reduced graphene guided enzymatically deposition of polyaniline for Pb2+ detection. Biosens Bioelectron 80:215–221
Taghdisi SM, Danesh NM, Lavaee P, Ramezani M, Abnous K (2016) An electrochemical aptasensor based on gold nanoparticles, thionine and hairpin structure of complementary strand of aptamer for ultrasensitive detection of lead. Sens Actuat B: Chem 234:462–469
Morales GR, Silva TR, Galicia L (2003) Carbon paste electrodes electrochemically modified with cyclodextrins. J Solid State Electr 7(6):355–360
Salmanipour A, Taher MA (2011) An electrochemical sensor for stripping analysis of Pb(II) based on multiwalled carbon nanotube functionalized with 5-Br-PADAP. J Solid State Electr 15(11–12):2695–2702
Raghu GK, Sampath S, Pandurangappa M (2012) Chemically functionalized glassy carbon spheres: a new covalent bulk modified composite electrode for the simultaneous determination of lead and cadmium. J Solid State Electr 16(5):1953–1963
Simionca IM, Arvinte A, Ardeleanu R, Pinteala M (2012) Siloxane-crown ether polyamide based electrode for electrochemical determination of lead(II) in aqueous solution. Electroanal 24(10):1995–2004
Morante-Zarcero S, Pérez-Quintanilla D, Sierra I (2015) A disposable electrochemical sensor based on bifunctional periodic mesoporous organosilica for the determination of lead in drinking waters. J Solid State Electr 19(7):2117–2127
Funding
This work was supported by the National Natural Science Foundation of China (No. 31101284), the Graduate Research and Innovation Foundation of Chongqing, China (No. CYS17017), Chongqing Science and Technology Commission (No. CSTC2015shmszxl20097 and CSTC2017shmsA100010), the Fundamental Research Funds for the Central Universities (No. 2018CDXYHG0028), and the Chongqing University Student Research Training Program (No. CQU-SRTP-2016330 and CQU-SRTP-2016337).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 93 kb)
Rights and permissions
About this article
Cite this article
He, B., Shen, Xf., Nie, J. et al. Electrochemical sensor using graphene/Fe3O4 nanosheets functionalized with garlic extract for the detection of lead ion. J Solid State Electrochem 22, 3515–3525 (2018). https://doi.org/10.1007/s10008-018-4041-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-4041-9