Abstract
An attempt has been made to prepare a new blend polymer electrolytes (BPEs) based on PVdF-co-HFP and PVAc doped with Mg (ClO4)2 by using the solvent-casting technique. The physicochemical properties of the as prepared polymer electrolytes were characterized by XRD, FTIR, SEM, TG/DTA, linear sweep voltammetry (LSV), and cyclic voltammetry (CV). The maximum ionic conductivity value 3.85 × 10−5 S cm−1 has been observed for PVdF-co-HFP (69)-PVAc (23)-Mg (ClO4)2 (8 wt%) system at 30 °C using AC impedance spectroscopic technique. The FTIR analysis confirms the complex formation between the polymers and salts. The TG/DTA studies showed the thermal stability of the film. The polymer electrolyte membrane shows a wide electrochemical stability window, and the temperature dependence of ionic conductivity obeys the Arrhenius rule.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, environmental pollution and power crisis have been severely accumulated due to the extreme utilization of fossil fuel resources. To defeat these problems, clear energy resources and related energy conversion and storage materials and devices are highly demanded [1]. Electrical energy storage technologies play an important role in the demand for green and sustainable energy. Rechargeable batteries or secondary batteries, such as Li-ion batteries, Na-ion batteries, and Mg-ion batteries, reversibly convert between electrical and chemical energy via redox reaction, thus storing the energy as chemical potential in their electrodes. [2].
The polymer battery has the advantage of having a higher energy density, solvent-free condition easy process ability, and being leakage proof and light in weight. The interest on solid polymer electrolytes has grown during the last decades together with the search of new and more efficient, high performance devices for energy conversion and storages [3]. Rechargeable lithium metal batteries with high specific capacities are still commercially unavailable. Even though the Li ion battery has the choice in the view of its specific capacity and cycle stability. However, the application of LIBs in electric vehicles (EVs) or renewable energy storage is largely limited due to the finite energy/power density [4]. The lithium ion-conducting gel polymer electrolytes has safety problems, limitations, and relatively expensive, because they are highly reactive in nature and also toxic. Apart from lithium batteries, certain magnesium, zinc, and sodium batteries are reported [5,6,7]. So, to a greater extent, researchers focus on developing solid state Mg2+ ion-conducting electrolytes to achieve recharge ability [8,9,10].
Magnesium possesses a many number of characteristics that make it attractive electrode material for rechargeable batteries, including its low cost, relative abundance (fifth most abundant materials on earth), high safety, ease of handling, and its low toxicity which allows for urban waste disposal [11]. In future, the rechargeable magnesium batteries have a wide scope in the future in the analysis of negligible hazards and enhanced safety [12, 13]. There have been some studies on the solid polymer electrolytes based on magnesium salts [14,15,16,18]. Polymer blending can avoid problems associated with a low ionic conductivity of the system with property that could not be obtained with the use of a single polymer [19] The blending of the polymers is one of the effective methods to improve the mechanical stability and electrical conductivity of the polymeric materials. Many researchers have reported on the blend polymer electrolyte (BPE) system, such as PVdF-HFP/PMMA, PVdF-HFP/ PVAc, PVAc/PMMA, PVdF-HFP/PVAc, PVdF-HFP/PEG, and PVAc/PVdF-HFP [20,20,21,22,23,25] with different lithium salts and ammonium salts, respectively. The review of the literature reveals that attention has been given to the polymer electrolytes based on PVdF-HFP and PVAc BPE for Mg2+ ion battery.
Various methods have been adopted to reduce crystallinity of polymer electrolytes and to increase segmental mobility of the host polymer, such as blending [26, 27], co-polymerization [28,28,29,31], cross linking, and plasticization of polymer matrix [32,32,33,35]. Among the polymer electrolytes, the PVdF-HFP-based gel polymer films have received much attention since they have both crystalline VdF and amorphous HFP units. The amorphous phase is capable of trapping large amount of electrolyte, facilitating ion migration, while the crystalline phase provides a structural integrity to support a free standing film formation. In addition, the PVdF-HFP-based gel polymer electrolyte films are mechanically stiff and strong, thermally, and chemically stable [36,36,37,39]. The PVAc happens to be one of the polymers which possess a large dipole moment and high relaxation time which are due to its side chains connected to ester groups in the side chain [40, 41]. PVAc is a proper contender to achieve high ionic conductivity at ambient temperature, mechanical stability, and comfortable film formation among the various choices. To improve the ionic conductivity, an attempt has been made to identify the effects of Mg (ClO4), on the new PVdF-co-HFP/PVAc-based BPEs.
Materials and instrumentations
Poly(vinylidene fluoride-co-hexafluoropropylene) (PVdF-HFP) with an average molecular weight of (Mw) ~ 400,000 and polyvinyl acetate (PVAc) and magnesium perchlorate Mg(ClO4) salt were obtained from Sigma-Aldrich. All the composite polymer electrolytes were prepared by using a solvent-casting technique. They were dried under vacuum at 80 °C at about 10 h for removing the moisture or impurities. The polymers and the salts were first dissolved in tetrahydrofuran (THF) separately and then mixed together. The complex mixture was stirred continuously about 24 h to ensure the fine and homogeneous mixture. The acquired homogeneous solution was poured into the Petri dish to allow the Petri dish to vacuum oven to evaporate the residual solvent from the complex at 60 °C for 5 h. The obtained composite polymer electrolytes were stored in a vacuum desiccator for further studies. The prepared BPE with flexible and free standing film is shown in Fig. 1.
The structural analysis of the prepared polymer composites was analyzed by XRD using an X’pert PRO PANanlytical X-ray diffractometer. The complex formation between the polymer and salt was confirmed by FTIR using SPECTRA RXI, Perkin Elmer spectrophotometer in the range of 400–4000 cm−1. Thermal stability of the polymer electrolyte was carried out by thermogravimetric/differential thermal analysis (TG/DTA) using SIINT 6300, Japan, with the heating rate of 10 °C min−1 under the nitrogen atmosphere from room temperature to 800 °C. The morphology of the polymer electrolytes was examined by gold sputter-coated scanning electron microscopy (SEM) (FEI Quanta 250 Microscope, Netherlands). Conductivity studies were carried out with the help of stainless steel (SS)-blocking electrodes using a computer-controlled microauto lab type III Potentiostat/Galvanostat in the frequency range of 10 Hz to 100 MHz over the temperature range of 30–90 °C, and the electrochemical stability of the maximum conducting electrolyte was evaluated by means of linear sweep voltammetry (LSV) using SS as the working electrode and reference electrode. The high conducting polymer electrolytes (Q2) from the prepared BPEs has been constructed, and their results are discussed.The composition of the prepared polymer electrolytes is shown in Table 1.
Results and discussion
XRD
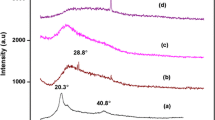
The XRD patterns of the PVdF-HFP, PVAc, Mg(ClO4)2 of the pure polymer, salt, and composite gel polymer electrolytes are shown in Fig. 2. The pure PVdF-HFP shows the semi-crystalline microstructure, i.e., the coexistence of mixed crystalline and amorphous nature of the material with predominant peaks at 2θ = 17°, 20°, and 38° [17]. The PVAc shows the broad hump and which confirm the amorphous nature of the polymer [42]. The diffraction pattern of the (MgClO4) salt showed a high intense peak, which revealed the crystalline nature of the salts and also well matched with the JCPDS (No: 37-0523). The Mg(ClO4)2 crystalline peaks are absent in the polymer composite electrolytes. The diffractogram of a polymer electrolyte shows a broadened peak between 10° and 25°. The PVdF-HFP peak is masked at 20°; the broad peak and the intensity of the peak decreased. These changes reveal that the gel polymer electrolyte is predominantly amorphous and its crystallinity is depressed due to the immobilization of liquid electrolyte in PVdF-HFP. This inciates that the liquid electrolyte solution most likely blends with the PVdF-HFP at the molecular level. This confirms that the interaction takes place between the constituents that are involved in the preparation of the composite electrolytes. The sample Q2 is more amorphous compared to other polymer composites. The amorphous nature which could be the reason for high conductivity of the film Q2.
FTIR analysis
FTIR transmittance spectra of pure and the prepared complexes are presented in Fig. 3. The spectrum of PVdF-HFP, the crystalline phase of VdF unit identified by the vibration bands of 981, 759 and 618 cm−1, is due to a non-polar trans-gauche conformation (i.e., α phase) of the semi-crystalline PVdF-HFP [43,43,45]. The vibration band of PVdF-HFP has been observed at 1400 and 1200 cm−1 for ▬CF and ▬CF2 groups. The peaks at 527 cm−1 can be assigned to the bending vibrations of ▬CF2. The bands at 842 and 879 cm−1, assigned to amorphous phase, appear as symmetrical stretching; vibration and the bands are shifted slightly in the polymer complexes, respectively. These observations indicate the substantial conformational changes in the crystalline texture of the host polymer PVdF-HFP due to the interaction with the PVAc and salt electrolytes. The disappearance of some bands associated with crystalline phase and the appearance of the bands of amorphous phase of the polymer indicate the reduction of crystallinity and dominance of the amorphous phase in the composite gel polymer electrolyte.
The vibration bands at 2928, 1735, 1026, 1369, and 1229 cm−1 are attributed to CH3 asymmetric, C〓O group, C▬O stretching, and ▬CH3 symmetric bending modes of pure PVAc. The peaks at 947, 607, and 794 cm−1 are ascribed to ▬CH bending and CH3(C▬O) group, and the C▬H rocking mode of vibration of PVAc has been confirmed [46, 47]. The stretching vibration mode of ClO4− anion observed in the range of 650–600 cm−1 has been probed to get the information associated with the degree of salt dissociation in the composite gel polymer electrolyte [48, 49]. These peaks are shifted in Q1, Q2, Q3, Q4, and Q5. The changes in the frequency characteristics of the electrolyte membranes are given in Table 2 and confirm the complexation in the measured polymer electrolytes.
TG/DTA analysis
Figure 4a–e shows the TG/DTA analysis of the prepared polymer composite electrolytes. It can be seen that from the TG curves, the initial weight loss occurs from 77 to 83 °C with a weight loss of 5, 3, 8, 4, and 9 wt% for all the samples due to the residual solvent and moisture at the time of loading the sample. It is observed that the second weight loss occurs at 245 to 274 °C for prepared samples, respectively, with the corresponding weight losses which are 17, 16, 19, 18, and 20 wt%. This may be due to the structural decomposition of the polymer blends. The complete decomposition of the sample takes place between 522 and 526 °C with the corresponding weight loss of about 78, 81, 73, 78, and 71 wt%. After nearly 650 °C, no appreciable weight loss is observed.
In DTA curve, the first exothermic peaks occurs in the range of 245 to 274 °C, and the second exothermic peaks occurs in the range of 523 to 528 °C in the prepared polymer electrolytes. This indicates the decomposition of the polymer film is in good agreement with the observed TG results.
SEM
Figure 5a, b shows the SEM image with different magnifications of the maximum conducting BPE (Q2) film. In general, a porous structure is essential for ionic transport from one site to another site. Hence, large porosity and small pore size are fundamental for a good separator, when the surface shows enormous number of pores and hence higher ion migration. The uniformly dispersed pores in the polymer microstructure lead to the retention of liquid electrolyte and formation of their better connectivity through the polymer, giving rise to have possibility of high ionic conductivity. Here, the sample shows the large number of pores which is evident from the picture, and this leads to yield the high conductivity. This is well matched and confirmed from the impedance analysis and also from XRD.
Impedance analysis
Figure 6 shows the Nyquist plot of the prepared polymer composite electrolytes at 30 °C. The ionic conductivity values of the electrolytes are calculated by using Eq. (1)
where l and A are the thickness and the area of the polymer electrolytes film, and Rb is the bulk resistance of the electrolyte film. The bulk resistance can be calculated from the intercept of hig- frequency or low-frequency spike or semi-circle on the Z′ axis or X axis.
The impedance plot shows a high-frequency semi-circle for Q5 sample, and the other sample shows the spike at low-frequency range. The highest ionic conductivity at ambient temperature is found to be 3.85 × 10−5 S/cm for the Q2 sample. The semi-circular portions corresponding to the high-frequency region are absent (inset figure) except Q5. This indicates that the majority of the current carriers in the electrolyte medium are ions, and that the total conductivity is mainly due to the ion conduction [50].
Temperature dependence of ionic conductivity
Figure 7 represents the temperature dependence conductivity for the prepared polymer electrolytes. The conductivity increases with increasing temperature for all the prepared polymer electrolytes, and it follows the Arrhenius type of thermally activated process given by the relation
where σo is the pre-exponential factor, Ea is the activation energy in eV, k is the Boltzmann constant, and T is the absolute temperature. The activation energy Ea is the combination of the energy of defect formation, and the energy of migration is calculated for all the prepared polymer electrolytes by using the Arrhenius plot. The low activation energy for the magnesium ion transport is due to the complete amorphous nature which provides a bigger free volume of the polymer electrolytes. As the amorphous region increases, the polymer chain acquires faster internal modes in which bond rotation produces segmental motion to favor the inter- and intra-chain ion hopping and thus, the conductivity becomes high [51, 52]. The sample Q2 has a higher ionic conductivity (6.27 × 10−4 S/cm at 90 °C) and the lower activation energy (3.37 eV) compared to other compositions.
Dielectric analysis
Figure 8a, b represents the frequency dependence of ɛ′ (ω) and ɛ″ (ω) of the prepared polymer electrolytes at room temperature (30 °C), respectively. From the obtained dielectric constant spectra, the values of the ɛ′ (ω) are high at low frequency and become constant at high frequency. Similarly, the high dielectric loss is obtained at the low frequency, and it decreases when the frequency increases.
At low frequency side, the values of the dielectric constant and also dielectric loss are high; this may due to the free charge carriers have enough time to build up at the interfaces between the electrode and electrolytes, leading to the localization of charge carriers [53]. At low frequency, the high value of dielectric constant is obtained; this is due to the contribution of the electrode polarization effects.
At high frequency, the periodic reversal of the electric field occurs so fast that there is no excess ion diffusion in the direction of the applied field. The fluctuations of the charge carriers in the directions of the field now lag behind, which ensure the low dielectric values for increasing the frequency.
Generally, the spectrum consists of two relaxation process, such as α and β. The α relaxation process is taking place at low frequency. It means it is observably at high temperature, which may be caused by the movements of the main segments of the polymer. Then the β relaxation process takes place at high-frequency region. It means that it is obviously at low temperature, which may be caused due to the side group dipoles and the nearest part of the backbones. The obtained spectrum shows α relaxation process. From the prepared BPEs, the high dielectric constant and dielectric loss are obtained for the sample Q2.
Modulus spectra analysis
Figure 9a, b shows the modulus spectra of the prepared polymer electrolytes. The electric modulus is defined as
where M′ and M″ are the real and imaginary parts of the polymer electrolytes. Both M′ and M″ are observed approximately zero at low frequency side that corresponds to the repression of the electrode polarization effect at the electrode-electrolyte interfaces. A long tail obtained for both the M′ and M″ modulus spectra at low frequency may be convincingly accredited as due to the large capacitance coupled with the electrode, and also due to the occurrence of the negligible electrode polarization contribution, the M′ and M″ decreases towards the low-frequency region.
Further increases of the frequency values of M′ and M″ also increase and reach the maximum value at higher frequency, which implies the distribution of relaxation process over a range of frequency. The M′ and M″ spectrum shows a perfect and distinct dispersion that demonstrate a distinctive increase in high frequency. The observed modulus spectrum for the prepared BPEs at high-frequency region that reveals the electrolytes has a good ionic conductivity. The spectrum at high-frequency region may be due to the bulk effect. The sharp increase in the high-frequency region, which indicates the possibility of the ionic conduction, takes place and contained by these polymer blend electrolytes. The maximum peak shift towards the high frequency for the optimum blend ratio of Q2 sample implies that it has small relaxation time.
Loss tangent spectra
Figure 10 shows the dielectric relaxation parameter of the polymer electrolyte from the variation of tanδ with frequency for the prepared polymer electrolytes at 30 °C. The dielectric loss tangent (tanδ) can be defined by the equation
It has been observed that the tanδ increases with increasing frequency and reaches a maximum. Then, it decreases for further increasing the frequency. For maximum dielectric loss at a particular temperature, the absorption peak is described by the relation ωτ = 1, where τ is the relaxation time and ω is the angular frequency of the applied signal. It is clear that the temperature increase in the charge carriers has been thermally activated and the loss tangent peak shifts towards higher frequency. This indicates that the relaxation time decreases with increases of temperature.
Transference ion number
For energy storage application, the polymer electrolyte should be an ionic conductor. The DC polarization technique is used to evaluate the ion transference number in the polymer electrolytes. The prepared polymer electrolyte was placed between the SS electrode, such as SS/PE/SS cell. In this method, the DC current was measured as a function of time on the application of fixed DC voltage across the cell. Then applying the 1.0 V, the current versus time plot was obtained. The obtained current vs time plot was shown in Fig. 11.
The transference ion number can be calculated from the dc polarization current vs time plot by using the following equation
where tion is the ion transference number, and Ii and If are the initial and final current values of the cells. The ionic transference number for the polymer electrolyte (Q2) is obtained as 0.98, respectively. This suggests that the charge transport in the polymer electrolyte is mainly due to the ions.
Linear sweep voltammetry
The electrochemical stability of BPE is an important parameter for energy storage devices. The electrochemical stability of the maximum conducting BPE (Q2) film which has been measured using LSV and recorded on the SS/BPE/Mg cell was shown in Fig. 12. The voltage is swept from 0 V towards positive values with a scan rate of 5 mV s−1 until a large current is obtained at certain potential, which is due to the electrolyte decomposition at the inert electrode interface. The figure shows that the BPE (Q2) film is stable up to 3.68 V, which is a sufficient range for electrochemical applications especially for Mg batteries, and also compared to Pandey et al. [54], the value is high for blend system.
Cyclic voltammetry
CV tests with Mg/GPE/Mg cells were carried out to check the Mg2+ ion conduction in the GPEs. The potential was swept from − 4 to 4 V at a rate of 5 mV S−1. As can be shown in Fig. 13, the anodic peak appeared at about 2.45 V, implying that the polymer electrolyte has the voltage is more than 2 V. Also, the current peaks are distinctly observed. This reveals that the deposition and resolution of Mg2+ ion occur at the Mg/GPE interface.
From the analysis, the prepared polymer electrolyte has the electrochemical stability for the application of the rechargeable batteries, especially for the Mg ion battery application.
Conclusion
A new Mg+ ion conducting PVdF-HFP-PVAc blend solid polymer electrolyte has been synthesized. The amorphous nature is confirmed by the XRD analysis. FTIR studies reveal the complex formation between the polymer matrix and salt. The AC conductivity vale for all the prepared polymer electrolytes has been evaluated using impedance spectroscopy analysis. The high ionic conductivity has found to be 3.85 × 10−5 S cm−1 at ambient temperature for Q2 sample. The activation energy obtained from conductivity data from Ea decreases with increasing ionic conductivity and vice versa. The electrochemical stability window, estimated from LSV, is found to be ~ 3.68 V, which is sufficient for electrochemical applications. The CV suggests the existence of electrochemical equilibrium between the Mg metal and Mg+ ions in the BPE.
Abbreviations
- PVdF-HFP:
-
poly(vinylidene fluoride-co-hexaflouropropylene)
- PVAc:
-
poly(vinyl acetate)
- XRD:
-
X-ray diffraction,
- FTIR:
-
Fourier-transform infrared spectroscopy
- SEM:
-
scanning electron microscope,
- TG/DTA:
-
Thermogravimetric/differential thermal analysis
- PMMA:
-
poly(methyl methacrylate)
- PEG:
-
polyethylene glycol
References
Mohtadi R, Mizuno F (2014) Magnesium batteries: current state of the art, issues and future perspectives. Beilstein J Nanotechnology 5:1291–1311
Liu C, Zachary GN, Cao G (2016) Mater Today. 19(2):109–123. https://doi.org/10.1016/j.mattod.2015.10.009
Ha CJ, HyukYoon J, IlCho W, HoJang (2004) Electrochemical properties of LiNi0.8Co0.2 − xAlxO2 prepared by a sol–gel method. J Power Sources 136:132–138
Deng H, Nie P, Luo H, Zhang Y, Wang J, Zhang X (2014) Highly enhanced lithium storage capability of LiNi0.5Mn1.5O4 by coating with Li2TiO3 for Li-ion batteries. J Mater Chem A 2(43):18256–18262
Aurbach D, Lu Z, Schechter A, Gofer Y, Gizbar H, Turgeman R, Cohen geman R, Cohen Y, Moshkovich M, Levi E (2000) Prototype systems for rechargeable magnesium batteries. Nature 407(6805):724–727
Pandey GP, Hashmi SA (2009) Experimental investigations of an ionic-liquid-based, magnesium ion conducting, polymer gel electrolyte. J Power Sources 187(2):627–634
Novak P, Imdhof R, Haas O (1999) Magnesium insertion electrodes for rechargeable nonaqueous batteries—a competitive alternative to lithium. Electrochim Acta 45(1-2):351–367
Wu N, Yang ZZ, Yao HR, Yin YX, Gu L, Guo YG (2015) Improving the electrochemical performance of Li4Ti5O12 electrode in a rechargeable Mg battery by lithium magnesium co-intercalation. J Angew Chem Int Ed 54(19):5757–5761
Tao ZL, Xu LN, Gou XL, Chen J, Yuan HI (2004) TiS2 nanotubes as the cathode materials of Mg ion batteries. J Chem Comm 18:2080–2081
Kim J, Lee J, You J, Park MS, Hossain MSA, Yamauchi Y, Kim JH (2016) Conductive polymers for next generation energy storage systems, recent progress and new function. MaterHoriz 3:517–535
Gregory TD, Hoffman RJ, Winterton RC (1990) Nonaqueous electrochemistry of magnesium applications to energy storage. J Electrochem Soc 137(3):775–780
Kumar GG, Munichandriah N (2002) Poly(methylmethacrylate) magnesium triflate gel polymer electrolyte for solid state magnesium battery application. J Electrochim Acta 47(7):1013–1022
Yoshimoto N, Yakushiji S, Ishikawa M, Morita M (2003) Rechargeable magnesium batteries with polymeric gel electrolytes containing magnesium salts. J. Electrochim Acta 48(14-16):2317–2322
Polu AR, Kumar R (2012) Ionic conductivity and discharge characteristic studies of PVA-Mg (CH3COO)2 solid polymer electrolytes. Int J Polymer Mater 62:76–80
Polu AR, Kumar R (2014) Mg2+-ion conducting poly(ethylene glycol)-TiO2composite polymer electrolytes for solid-state batteries. Materials Express 4:79–84
Ulaganathan M, Rajendran S (2010) Preparation and characterization of PVAc/P(VdF-HFP)-based polymer blend electrolytes. Ionics 16:515–521
Pandey GP, Agrawal RC, Hashmi SA (2011) Magnesium ion-conducting gel polymer electrolytes dispersed with fumed silica for rechargeable magnesium battery application. J Solid State Electrochem 15(10):2253–2264
Oh JS, Ko JM, Kim DW (2004) Preparation and characterization of gel polymer electrolytes for solid state magnesium batteries. J Electrochim Acta 50(2-3):903–906
Shakur MF, Ithnin R, Illias HA, Kadir MFZ (2013) Proton conducting polymer electrolyte based on plasticized chotosan-PEO and application in electrochemical devices. Opt Mater 35(10):1834–1841
Kalyanasundaram NT, Subramanian A, Lokesh KS, Muhammed Musthafa OT (2008) Effect of porosity on PVdF-HFP/PMMA based electrolyte. J Materials Chem and Phy 110:11–16
Basri NH, Mohamed NS (2009) Conductivity studies and dielectric behavior of PVdF-HFP/PVC LiClO4 solid polymer electrolyte. Solid State Sci Technol 17:63–72
Rajendran S, Shanthi Bama V, Ramesh Prabhu M (2010) Effect of lithium salt concentration in PVAc/PMMA-based gel polymer electrolytes. Ionics 16(1):27–32
Lee H, Yanilmaz M, Toprakci O, Fu K, Zhang X (2014) PVdF-HFP/PVAc: a review of recent developments in membrane separators for rechargeable lithium-ion batteries. J Energy Environ Sci 7(12):3857–3886
JuHwangSoo Y, KyungJeong KSN, Manuel Stephan A (2007) Electrochemical studies on poly(vinylidene fluoride–hexafluoropropylene) membranes prepared by phase inversion method. Eur Polym J 43:65–71
Ulaganathan M, Rajendran S (2010) Effect of different salts on PVAc/PVdF-co-HFP based polymer blend electrolytes. J Appl Polym Sci 118:646–651
Kirankumar K, Ravi M, Pavani Y, Bhavani S, Sharma AK, VVR NR (2014) Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J Membr Sci 454:200–211
Kim DW, Park JK, Rhee HW (1996) Conducting and thermal studies of solid polymer electrolytes prepared by poly(ethyleneoxide), poly(oligo[oxyethylene]oxysebacoyl) and lithium perchlorate. Solid State Ionics 83(1-2):49–56
Kim CH, Kim HT, Park JK, Moon SI, Yoon MS (1996) Preparation and ionic conductivities of the plasticized polymer electrolytes based on poly(methyl methacrylate-co-alkali metal methacrylate). J polymer Sci: part B:Polymer Phys 34(16):2709–2714
Sung HY, Wang YY, Wan CC (1998) Preparation and characterization of poly(vinyl chloride-co-vinyl acetate)-based gel electrolytes for Li-ion batteries. J Electrochem Soc 145(4):1207–1211
Rhoo HJ, Kim HT, Park JK, Hwang TS (1997) Ionic conduction in plasticized PVCPMMA blend polymer electrolytes. Electrochimca Acta 42(10):1571–1579
Jian-HuaCao, Bao-KuZhu, You-YiXu (2006) Structure and ionic conductivity of porous polymer electrolytes based on PVDF-HFP copolymer membranes. J Membr Sci 281:446–453
Wieczorek W, Florjancy Z, Stevens JR (1995) Proton conducting polymer gels based on a poly acrylamide matrix. Electrochim Acta J 40:2327–2330
Shanmukaraj D, Wang GX, Murugan R, Liu HK (2008) Ionic conductivity and electrochemical stability of PMMA-PEO blend ceramic filler composites. J Phys Chem Solids 69(1):243–248
Prajapathi GK, Roshan R, Gupta PN (2010) Effect of plasticizer on ionic transport and dielectric properties of PVA:H3PO4 proton conducting polymeric electric electrolyte. J Phys and Chem of Solids 71(12):1717–1723
Ulaganathan M, Rajendran S (2011) Novel Li-ion conduction on poly(vinyl acetate)-based hybrid polymer electrolytes with double plasticizers. J Appl Electrochem 41(1):83–88
Gebreyesus MA, Purushotham Y, Sivakumar J (2016) Preparation and characterization of lithium ion conducting polymer electrolytes based on a blend of poly(vinylidene fluoride-co-hexafluoropropylene) and poly(methyl methacrylate). Heliyon 2(7):e00134. https://doi.org/10.1016/j.heliyon.2016.e00134
Abbrent S, Plestil J, Hlavata D, Lindgren J, Tegenfeldt J, Wendsjo A (2001) Crystallinity and morphology of PVdF-HFP-based gel electrolytes. Polymer 42(4):1407–1416
Ataollahi N, Ahmad A, Hamzah H, Rahman MYA, Mohamed NS (2012) Preparation and characterization of PVDF-HFP/MG49 based polymer blend electrolyte. Int J Electrochem Sci 7:6693–6703
Tafur JP, Santos F, Fernández Romero AJ (2015) Influence of the ionic liquid on the gel polymer electrolytes properties. Membranes 5(4):752–771
Baskaran R, Selvasekarapandian S, Hirankumar G, Bhuvaneswari MS (2004) Vibrational ac impedance and dielectric spectroscopic studies of poly(vinylacetate)-N,N-dimethylformamide-LiClO4 polymer gel electrolytes. J Power Sources 134(2):235–240
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2007) Structure, thermal and transport properties of PVAc–LiClO4 solid polymer electrolytes. J Phys and Chem Solids 68(3):407–412
Arunkumar D, Selvasekarapandian S, Baskaran R, Savitha T, Nithya H (2012) Thermal vibrational and AC impedence studies on proton conducting polymer electrolytes based on poly(vinylacetate). J Non crystal solids 358:531–536
Ulaganathan M, Nithya R, Rajendran S, Raghu S (2012) Li-ion conduction on nanofiller incorporated PVdF-co-HFP based composite polymer blend electrolytes for flexible battery applications. Solid State Ionics 218:7–12
Michael MS, Jacob MME, Prabaharan SRS, Radhakrishna S (1997) Enhanced lithium ion transport in PEO-based solid polymer electrolytes employing a novel class of plasticizers. Solid State Ionics 98(3-4):167–174
Ulaganathan M, Chithra MM, Rajendran S (2013) Highly porous lithium ion conducting solvent free poly(vinylidene fluoride-co-hexafluoropropylene)/poly( ethyl methacrylate) based polymer blend electrolytes for Li battery applications. J. Electrochim Acta 93:230–235
Selvasekarapandian S, Baskaran R, Kamishima O, Kawamura J, Hattori T (2006) Laser Raman and FTIR studies on Li+ interaction in PVAc-LiClO4 polymer electrolytes. J Spectrochim Acta part A 65(5):1234–1240
Ulaganathan M, Sundar Pethaiah S, Rajendran S (2011) Li-ion conduction in PVAc based blend electrolytes for lithium battery applications. J Mater Chem Phys 129(1-2):471–476
Tsunemi K, Ohno H, Tsuchida A (1983) A mechanism of ionic conduction of poly(vinylidene fluoride)-lithium perchlorate hybrid films. J. Electrochim Acta 28(6):833–837
Park US, Hong YJ, Oh SM (1996) Fluorescence spectroscopy for local viscosity measurements in polyacrylonitrile (pan)-based polymer gel electrolytes. J Electrochim Acta 41(6):849–855
Missan HPS, Chu PP, Sekhon SS (2006) Ion conduction mechanism in non-aqueous polymer electrolytes based on oxalic acid: effect of plasticizer and polymer. J Power Sources 158(2):1472–1479
Polu AR, Kumar R (2013) Preparation and characterization of PVA based polymer electrolytes for electrochemical cell application. Chinese J Polym Sci 31(4):641–648
Tripathi SK, Jain A, Gupita A, Mishra M (2012) Electrical and electrochemical studies on magnesium ion-based polymer gel electrolytes. J Solid State Electrochem 16(5):1799–1806
Manjuladevi R, Thamilselvan M, Selvasekarapandian S, Mangalam R, Premalatha M, Monisha S (2017) Mg-ion conducting blend polymer electrolyte based on poly(vinyl alcohol)-poly(acrylonitrile) with magnesium perchlorate. Solid State Ionics 308:90–100
Pandey GP, Hashmi SA (2009) Experimental investigations of an ionic-liquid-based magnesium ion conducting polymer gel electrolytes. J Power Sources 187(2):627–634
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOC 38 kb)
Rights and permissions
About this article
Cite this article
Ponmani, S., Kalaiselvimary, J. & Ramesh Prabhu, M. Structural, electrical, and electrochemical properties of poly(vinylidene fluoride-co-hexaflouropropylene)/poly(vinyl acetate)-based polymer blend electrolytes for rechargeable magnesium ion batteries. J Solid State Electrochem 22, 2605–2615 (2018). https://doi.org/10.1007/s10008-018-3971-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-018-3971-6