Abstract
Polymer blend electrolytes comprising of poly(vinyl acetate), poly(vinylidene fluoride-co-hexafluoropropylene), LiClO4, and EC-based plasticizer combinations (EC + PC, EC + GBL, EC + DMP, EC + DBP, EC + DEC) are prepared by solvent casting technique. Ionic conductivities of the electrolytes are determined by ac impedance studies in the temperature range 303–363 K. Among the various combinations of plasticizers, EC + PC added complex exhibits maximum ionic conductivity of the order of 10−4 S cm−1 and the temperature-dependent ionic conductivity plots seem to obey the VTF relation. The structural and complex formations of the prepared samples have been confirmed by X-ray diffraction analysis. DSC technique is used to study the thermal behaviour. The surface images of the sample having maximum ionic conductivity are analyzed with the help of SEM and AFM techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, the requirement of polymer electrolyte increases due to their wide applications in solid state cells, electro chromic devices, super capacitors, gas sensors, etc. The solid polymer electrolytes are mainly used in fabrications of secondary Li-ion battery because of the desirable properties such as high energy density and easy packaging properties [1, 2]. Initially, the polymer electrolytes based on PEO–Li salt complexes were most widely studied by Wright et.al [3]. However, they showed poor ionic conductivity of the order of 10−7 S cm−1 at ambient temperature. Further, efforts have been made to enhance the ionic conductivity of the polymer matrix with the help of various processes such as blending of two polymers [4], addition of plasticizers with the polymer electrolytes to form the plasticized polymer electrolyte [5–8], addition of fillers to make the composite polymer electrolytes [9–12], etc. Among these approaches, plasticized polymer electrolytes have been proved to be promising for the electrolytes of the lithium batteries owing to their high ionic conductivity [13]. Generally, the gel polymer electrolytes are prepared by addition of plasticizers to the polymer host along with the lithium salt in low boiling solvent such as tetrahydrofuran (boiling point 60 °C), and the resultant solution is cast as film after the evaporation of the solvent. However, the plasticized electrolytes also show off drawbacks, such as reactivity of polar solvents with lithium electrode, poor mechanical properties at high degree of plasticization, and solvent volatility [14, 15]. Recently, several works have been made to improve the ionic conductivity of the blend-based polymer electrolytes such as PVC/PEMA [16], PVdF–HFP/PMAML [17], and PVC/PAN [18]. Baskaran et al. [19] reported that PVAc-based polymer electrolytes have conductivity in the range 10−5 S cm−1. The conductivity of PVAc polymer complexes exhibit high values by blending PVAc with other suitable polymers. Ionic conductivity of PVAc with poly(methyl methacrylate) (PMMA) and poly(vinylidene fluoride) (PVdF)-based blend electrolytes were first reported by Baskaran et.al. [20, 21] and they have good compatible nature. Here, we have chosen a co-polymer of vinylidene fluoride and hexafluoropropylene (PVdF:HFP) which contains an amorphous phase capable of trapping large amount of electrolyte and a crystalline phase that acts as a mechanical support for the formation of a free standing film. PVdF-co-HFP exhibits high anodic stability and it shows compatible nature with other polymers [22, 23].The choice of choosing LiClO4 is due to its smaller ionic radius, smaller dissociation energy and the high solubility in most of the organic solvents. The interest on PVAc-based systems increases because PVAc offers good mechanical stability, low glass transition temperature, and easy film formation properties. Poly(vinyl acetate) films also have high tensile strength (29.4 ± 49.0 MPa) [24] and abrasion resistance.
In this study, novel polymer electrolytes based on PVAc/PVdF–HFP/LiClO4/EC + X (where X = PC, GBL, DMP, DBP, and DEC) were prepared for different combinations of plasticizers.
2 Experiment
2.1 Materials
The PVAc and PVdF–HFP with average molecular weights of 14 × 104 and 4 × 105 (Aldrich), respectively, were used as received. LiClO4 anhydride was purchased from Aldrich, USA, and it was dried in vacuum at 100 °C for 24 h before use. The plasticizers and the solvent THF used in this study were purchased from Aldrich, USA and E-Merck, Germany, respectively, and they were used as received. PVAc/PVdF–HFP (6.25:18.75 weight ratio) and LiClO4 (8 wt%) were dissolved in anhydrous tetrahydrofuran separately. These solutions were mixed together and the mixture was continuously stirred for 24 h with the help of a magnetic stirrer and 67 wt% mixture of the plasticizers EC + PC, EC + GBL, EC + DMP, EC + DBP, and EC + DEC were added with the complex system. Then the solvent in the mixture was allowed to evaporate slowly until a homogeneous gel solution was obtained and it was degassed to remove air bubbles. The solution thus obtained was cast on a clean glass plate and PVAc/PVdF–HFP/EC-X-based gel electrolyte films containing LiClO4 was obtained by heating at 60 °C to remove any trace of solvent. Finally, the gel polymer electrolytes of constant ratio of PVAc (6.25):PVdF–HFP (18.75):LiClO4 (8)/EC-X (67 wt%) were prepared with a view to identify the suitable combination of plasticizers for high ionic conductivity and good mechanical stability.
2.2 X-ray diffraction analysis
The structural and complex formation of the electrolytes were confirmed by X-ray diffractometer of model X’pert PRO PANalytical using Cu-Kα radiation as source and operated at 20 kV. The sample was scanned in the 2θ ranging from 10 to 80° for 2 s in the step mode.
2.3 Thermal analysis
The thermal behaviour of the prepared samples was studied using a differential scanning calorimeter (DSC) (Mettler Toledo DSC 822e apparatus). The samples were heated from −50 to 450 °C at a heating rate of 10 °C/min.
2.4 Surface analysis
The electrolyte film exhibiting maximum ionic conductivity was subjected to atomic force microscopy (AFM) and the topography image of the film was depicted using Veeco-diCP-II microscopic model over a scanned area of 2 × 2 μm. The pore size and the rms roughness of the film are measured from the topography image. The surface image of the maximum conductivity film has also been studied using scanning electron microscope (SEM) of model JOEL, JSM-840A.
2.5 Conductivity measurements
The AC conductivity measurements were carried out on PVAc/PVdF–HFP/LiClO4/EC-X systems of about 50–70 μm thickness and 1 cm2 area. Polymer films were sandwiched between two stainless steel (SS) electrodes under an oscillation potential of 10 mV from 1 Hz to 500 kHz using computer controlled micro auto lab III Potentiostat/Galvanostat impedance analyzer in the temperature range of 303–363 K. The conductivity value was determined using the bulk resistance value obtained from the complex impedance plot. The temperature of the cell was controlled using a thermostat and the ionic conductivity was calculated using the relation σ = t/(R b A), where R b is the bulk resistance, t is the thickness of the film, and A is the area of the film.
3 Results and discussion
3.1 Conductivity studies
The typical room temperature complex impedance plot of the sample exhibiting maximum ionic conductivity is shown in Fig. 1. The room temperature conductivity of the prepared samples is found to be ~10−4 S cm−1. It is evident from Table 1 that the conductivity values increase with increasing temperature. The complex impedance plot shows the depressed spike which corresponds to the lower frequency region and the bulk resistance R b value has been obtained from the intercept on the real axis of the impedance plot. It is observed that the semicircular portion (due to bulk electrolyte impedance) corresponding to higher frequency does not appear in Fig. 1. It is quite reasonable since the facile mobility in liquid and gel electrolyte systems, when compared with solid polymer electrolytes, indicates that ions possess dielectric relaxation times and hence the inconsequential capacitive effect of the electrolytes in the spectrum [25].
The conductivity of the electrolyte increases with an increase in temperature. The increase of conductivity with the temperature is interpreted as being due to a hopping mechanism between co-ordinating sites, local structural relaxations, and segmental motions of the polymers [26]. In fact, it is obviously noted from Fig. 2, that the temperature-dependent conductivity plot obeys VTF relation, which describes the movement of ions through the viscous matrix i.e., the ions move through the plasticizers rich-phase which involves the plasticizers, the host polymers, and the salts. Finally, the plasticizers influence the degree of mixing, the polymer plasticizer interaction by providing more charge carriers and more mobile medium for the ions [27], which results in enhancing the conductivity of the plasticized-electrolyte system.
From Table 1, it is concluded that the electrolyte containing EC, PC plasticizers exhibits maximum ionic conductivity of the order of 5.033 × 10−4 S cm−1 which is mainly due to the specific properties of the plasticizers such as higher dielectric constant and lower viscosity. Among the various plasticizers, EC and PC have higher dielectric constants (89 and 64) and their viscosities are 1.90 and 2.53 cp, respectively. In addition, the higher ionic conductivity is mainly attributed to the higher amorphous nature of PVAc and the amorphous HFP phase in P(VdF-co-HFP). This higher amorphicity provides more free volume to the mobile Li+ ions thereby increasing the over all ionic conductivity.
3.2 X-ray diffraction analysis
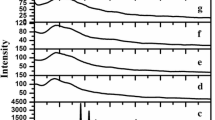
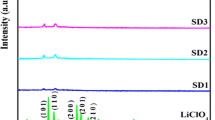
X-ray diffraction pattern of pure PVAc, PVdF–HFP, LiClO4, and PVAc/PVdF–HFP/LiClO4 complexes with plasticizers are shown in Fig. 3a–h. The presence of broad less intense characteristic peak in Fig. 3c reveals the complete amorphous nature of pure PVAc. The characteristic peaks appearing at 2θ = 17, 19, and 38° confirm the semi crystalline nature of P(VdF–HFP) (Fig. 3b). The sharp peaks with high intensity confirm the crystalline nature of LiClO4 salt as shown in Fig. 3a. The diffraction pattern of the plasticized-electrolyte samples are shown in Fig. 3d–h. A higher degree of amorphous phase has been observed in all the complexes; hence, the conductivity is higher which reveals that the ionic conductivity increases only in amorphous phase. It is clearly observed from the characteristic spectrum that the addition of plasticizer and LiClO4 salt decreases the crystallinity of the GPEs. The peaks corresponding to LiClO4 are not observed in the electrolytes indicating that the salt is well complexed in the polymer matrix, and it also confirms that the salt does not contain any separate phase in the electrolytes.
3.3 Thermal analysis
The DSC plots of PVAc/PVdF–HFP/LiClO4/EC-X-based GPE systems are shown in Fig. 4. All the systems exhibited endothermic peaks around 140–150 °C which is attributed to the melting of PVF–HFP [28] and the plots corresponding to the samples D1, D2, D3, D4, and D5 show the transitions at 36, 39, 39, 36, and 34 °C, respectively, which indicate the melting of plasticizer EC (melting point of EC: 38 °C). In the case of EC + DEC-based electrolytes (D5) one broad endothermic peak is found in between 110 and 145 °C which may be due to the overlapping of boiling point of DEC and melting point of PVdF–HFP [29]. The broad exothermic peaks around 266, 288, 260, 276, and 252 °C indicate the complete decomposition of the samples D1, D2, D3, D4, and D5, respectively. It is concluded that the system based on the combination of EC + GBL shows higher thermal stability (288 °C) even though EC + PC exhibits maximum ionic conductivity.
3.4 Scanning electron microscopic analysis
The SEM image of the sample (D1) exhibiting maximum ionic conductivity is shown in Fig. 5. It is observed that the surface of the electrolyte has number of fine pores and these are reasonable for ion hopping. The formation of porous structure is a complex process that depends on the interactions of the solvent and plasticizers with the host polymers such as PVAc and PVdF-co-HFP. It is observed that the numerous pores (dark region) with the size of 1–10 μm are responsible for the high conductivity of the sample. This shows the porous structure of the membrane leads to entrapment of large volume of the liquid in the pores accounting for the increased conductivity [30]. The interconnected micropores in the membrane help in absorbing liquid electrolytes and hence the ionic conductivity of the membrane is enhanced. The presence of pores in the microstructure is mainly due to solvent removal [31, 32].
3.5 Atomic force microscopic analysis
Novel three dimensional topography image of the sample D1 are shown in Fig. 6. From the topography image, the pore size has been estimated of the order of 4 μm and which is in close agreement with the pore size determined from SEM photograph. In addition, the rms roughness of the sample over the scanned area 2 × 2 μm has been obtained and it is in the order of 40 nm [33]. The surface roughness of the sample may play an important role in the conductivity. The micropores and the chain segments of the blended polymers are responsible for the enhancement of ionic conductivity.
4 Conclusions
Polymer electrolytes comprising PVAc/PVdF–HFP/LiClO4 complexes with the different combination of plasticizers are prepared by solvent casting technique. The ionic conductivity of the polymer electrolytes has been obtained in the range 303–363 K. The maximum room temperature conductivity 5.033 × 10−4 S cm−1 has been estimated for EC + PC added complex because of their higher dielectric nature and the temperature-dependent ionic conductivity of electrolytes seems to obey VTF relation. The complex formation of the samples has been confirmed from XRD analysis. It is found that the sample D1 exhibits maximum ionic conductivity and it is thermally stable up to 266 °C.
References
Wang C, Wei Y, Ferment GR, Li W, Li T (1999) Poly(ethylene oxide)–silica hybrid materials for lithium battery application. Mater Lett 39:206–210
Barthel J, Schmid A, Gores HJA (2002) New class of electrochemically and thermally stable lithium salts for lithium battery electrolytes. V. synthesis and properties of lithium bis[2, 3-pyridinediolato(2–)-O, O]borate. J Electrochem Soc 147:21
Fenton DE, Parker JM, Wright PV (1973) Complexes of alkali metal ions with poly ethylene oxide. Polymer 14:589
Inganäs O (1988) Electroactive polymer blends. Br Polym J 20:233–236
Kim D-W, Park J-K, Rhee H-W (1996) Conductivity and thermal studies of solid polymer electrolytes prepared by blending poly (ethyleneoxide) poly (oligo[oxyethylene] oxysebacoyl) and lithium perchlorate. Solid State Ionics 83:49–56
Nagasubramanian G, Attia AI, Halpert G (1994) A polyacrylonitrile-based gelled electrolyte: electrochemical kinetic studies. J Appl Electrochem 24:298–302
Stallworth PE, Greenbaum SG, Croce F, Slane S, Salomon M (1995) Lithium-7 NMR and ionic conductivity studies of gel electrolytes based on poly (methylmethacrylate). Electrochim Acta 40:2137–2141
Abbrent S, Lindgren J, Tegenfeldt J, Wendsjo A (1998) Gel electrolytes prepared from oligo(ethylene glycol)dimethacrylate: glass transition, conductivity and Li+-coordination. Electrochim Acta 43:1185–1191
Croce F, Appetecchi GB, Persi L, Scrosati B (1998) Nanocomposite polymer electrolytes for lithium batteries. Nature 394:456–458
Croce F (1999) Physical and chemical properties of nanocomposite polymer electrolytes. J Phys Chem B 103:10632–10638
Nan C-W (1993) Physics of inhomogeneous inorganic materials. Prog Mater Sci 37:1–116
Fan L, Nan C-W, Dang Z (2002) Effect of modified montmorillonites on the ionic conductivity of (PEO)16LiClO4 electrolytes. Electrochim Acta 47:3541–3544
Ramesh S, Arof AK (2001) Structural, thermal and electrochemical cell characteristics of poly(vinyl chloride)-based polymer electrolytes. J Power Sources 99:41–47
Fan L, Dang Z, Nan C-W, Li M (2002) Thermal, electrical and mechanical properties of plasticized polymer electrolytes based on PEO/P(VDF-HFP) blends. Electrochim Acta 48:205–209
Manuel Stephan A, Gopu Kumar S, Renganathan NG, Anbu Kulandainathan M (2005) Characterization of poly (vinylidene fluoride–hexafluoropropylene) (PVdF–HFP) electrolytes complexed with different lithium salts. Eur Polym J 41:15–21
Han H-S, Kang H-R, Kim S-W, Kim H-T (2002) Phase-separated polymer electrolyte based on poly(vinyl chloride)/poly(ethyl methacrylate) blend. J Power Sources 112:461–468
Wang Z-L, Tang Z-Y (2004) A novel polymer electrolyte based on PMAML/PVDF-HFP blend. Electrochim Acta 49:1063–1068
Rajendran S, Ravi Shanker Babu, Sivakumar P (2007) Effect of salt concentration on poly (vinyl chloride)/poly (acrylonitrile) based hybrid polymer electrolytes. J Power Sources 170:460–464
Selvasekarapandian S, Baskaran R, Kamishima O, Kawamura J, Hattori T (2006) Laser Raman and FTIR studies on Li+ interaction in PVAc–LiClO4 polymer electrolytes. Spectrochim Acta Part A Mol Biomol Spectrosc 65:1234–1240
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ionics 177:2679–2682
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamura J, Hattori T (2006) ac impedance, DSC and FT-IR investigations on (x)PVAc–(1−x)PVdF blends with LiClO4. Mater Chem Phys 98:55–61
Capiglia C, Saito Y, Yamamoto H, Kageyama H, Mustarelli P (2000) Transport properties and microstructure of gel polymer electrolytes. Electrochim Acta 45:1341–1345
Capiglia C, Saito Y, Kataoka H, Kodama T, Quartarone E, Mustarelli P (2000) Structure and transport properties of polymer gel electrolytes based on PVdF-HFP and LiN(C2F5SO2)2. Solid State Ionics 131:291–299
Daniels W (1987) In: Mark HF (ed) Encyclopedia of Polymer Science and Technology, vol 17. Wiley, New York, p 402
Song JY, Wang YY, Wan CC (2000) Conductivity study of porous plasticized polymer electrolytes based on poly(vinylidene fluoride) a comparison with polypropylene separators. J Electrochem Soc 147:3219
Okamoto Y, Yeh TF, Lee HS, Skotheim TA (1993) Design of alkaline metal ion conducting polymer electrolytes. J Polym Sci Part A Polym chem 31:2573–2581
Maccallum JR, Vincent CA (1987) polymer electrolyte review. Elsevier Applied sci, London, p 141
Jiang Y-X, Chen Z-F, Zhuang Q-C, Xu J-M, Dong Q-F, Huang L, Sun SG (2006) A novel composite microporous polymer electrolyte prepared with molecular sieves for Li-ion batteries. J Power Sources 160:1320–1328
Liu Y, Lee JY, Hong L (2002) Synthesis, characterization and electrochemical properties of poly(methyl methacrylate)-grafted-poly(vinylidene fluoride- hexafluoropropylene) gel electrolytes. Solid State Ionics 150:317–326
Hwang YJ, Nahm KS, Prem Kumar T, Manuel Stephan A (2008) Poly(vinylidene fluoride-hexafluoropropylene)-based membranes for lithium batteries. J Mem Sci 310(1-2):349–355
Kim CS, Oh SM (2001) Performance of gel-type polymer electrolytes according to the affinity between polymer matrix and plasticizing solvent molecules. Electrochim Acta 46(9):1323–1331
Manuel Stephan A, Saito Y (2002) Ionic conductivity and diffusion coefficient studies of PVdF–HFP polymer electrolytes prepared using phase inversion technique. Solid State Ionics 148(3–4):475–481
Horcas I, Fernandez R, Gomez-Rodriguez RM, Colchero J, Gomez-Herrero J, Baro AM (2007) WSXM: a software for scanning probe microscopy and a tool for nanotechnology. Rev Sci Instrum 78:013705
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ulaganathan, M., Rajendran, S. Novel Li-ion conduction on poly(vinyl acetate)-based hybrid polymer electrolytes with double plasticizers. J Appl Electrochem 41, 83–88 (2011). https://doi.org/10.1007/s10800-010-0211-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-010-0211-x