Abstract
A new voltammetric sensor based on electropolymerization of glycine at glassy carbon electrode (GCE) was developed and applied to determine of pyrazinamide (PZA) by square-wave voltammetry (SWV). The initial cyclic voltammetric studies showed an electrocatalytic activity of poly(Gly)/GCE on redox system of pyrazinamide in 0.1 mol L−1 phosphate buffer solution pH 7.5, with E Pc and E Pa in −0.85 and −0.8 V (versus E Ag/AgCl), respectively. Studies at different scan rates suggest that the redox system of pyrazinamide at poly(Gly)/GCE is a process controlled by diffusion in the interval from 10 to 100 mV s−1. Square-wave voltammetry-optimized conditions showed a linear response of PZA concentrations in the range from 0.47 to 6.15 μmol L−1 (R = 0.998) with a limit of detection (LOD) of 0.035 μmol L−1 and a limit of quantification (LOQ) of 0.12 μmol L−1. The developed SWV-poly(Gly)/GCE method provided a good intra-day (RSD = 3.75 %) and inter-day repeatability (RSD = 4.96 %) at 4.06 μmol L−1 PZA (n = 10). No interference of matrix of real samples was observed in the voltammetric response of PZA, and the method was considered to be highly selective for the compound. In the accuracy test, the recovery was found in the range of 98.2 and 104.0 % for human urine samples and pharmaceutical formulation (tablets). The PZA quantification results in pharmaceutical tablets obtained by the proposed SWV-poly(Gly)/GCE method were comparable to those found by official analytical protocols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
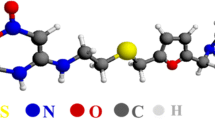
Pyrazinamide (pyrazine-2-carboxamide) (PZA; Fig. 1) is a first-line anti-tuberculosis (TB) drug administrated in the first 2 months of standard TB treatment. Pyrazinamide is also important in the treatment of multidrug-resistant (MDR) TB (MDR-TB) (resistant to at least isoniazid and rifampin). The drug has a unique sterilizing effect by killing the semi-dormant Mycobacterium tuberculosis population [1, 2]. After oral administration, pyrazinamide is almost completely systematically absorbed and eventually enters the pulmonary cavities containing M. tuberculosis [3]. The mechanism of the action of PZA is poorly understood: pyrazinoic acid (POA), the active moiety of PZA, has been shown to inhibit various functions at acid pH in M. tuberculosis [4]. PZA has a plasma half-life of 10 h and is metabolized to pyrazinoic acid and other metabolites by the liver. Renal clearance is low (<2 mL/min), and urinary elimination accounts for less than 4 % of the ingested dose [5]. Hepatotoxic effects attributable to anti-TB therapy are unique among drug-related liver problems, because almost all first-line anti-TB medications have these adverse effects. Adverse effects, such as rhabdomyolysis, exanthemas, and kidney failure, are an important clinical consideration for patients undergoing treatment with first-line anti-TB medications, and it requires discontinuation of medication [6, 7].
In this sense, the development of a reproducible, sensitive, and reliable analytical method to determine pyrazinamide in biological fluids and pharmaceutical formulations has been crucial to monitor the efficacy of the MDR-TB treatment and also to ascertain whether a drug has the proper content of active substance. To date, some analytical methodologies such as spectrophotometry [8–11], capillary electrophoresis [12], chromatographic methods [13–20], and electrochemical methods [21–25] have been applied to quantify PZA in pharmaceutical formulations and body fluids. The electrochemical methods display good advantages for drug detection, for example, high sensitivity, accuracy, precision, simplicity, low cost, and elimination of laborious sample preparation steps. Cheemalapati, Devadas, and Chen [21] developed an electrochemically active film which contains poly-l-methionine (PMET) and electrochemically reduced graphene oxide (ERGO) on glassy carbon electrode (GCE) for PZA detection in urine, plasma, and pharmaceuticals by differential pulse voltammetry (DPV). The linear range was obtained from 0.4 to 1129 μmol L−1 and the limit of detection was found of 0.16 μmol L−1. This method showed acceptable reproducibility and good repeatability with appreciable selectivity.
Devadas et al. [22] developed an electrochemical method for simultaneous determination of isoniazid, PZA, and buspirone hydrochloride at graphene oxide (GO)/poly-l-arginine (PAG)-modified GCE by DPV. The linear range for PZA was from 25 to 1600 μmol L−1, and the limit of detection was found of 3.28 μmol L−1. The method was applied successfully in blood serum and pharmaceuticals. Cheemalapati et al. [23] proposed an electrochemical method for determination of hydralazine hydrochloride, isoniazid, ethambutol hydrochloride, and PZA in pharmaceuticals, human urine, and blood serum using a tyrosine (Tyr)-modified glassy carbon electrode by DPV. The proposed modified electrode showed strong electrocatalytic activity in the analysis of these drugs. The results of PZA analysis indicated a linear range from 10 to 900 μmol L−1, and the limit of detection and limit of quantification were found of 5.13 and 15.55 μmol L−1, respectively.
Bergamini, Santos, and Zanoni [24] studied the electrochemical behavior of pyrazinamide using a poly-histidine modified screen-printed electrode. The modified electrode showed excellent electrocatalytic activity, thus promoting a decrease in PZA reduction potential and improving the voltammetric response. The linear range was obtained from 0.9 to 100 μmol L−1 by DPV. The limit of detection estimated was of 0.57 μmol L−1. The method was applied successfully in human urine samples. Mani et al. [25] determined PZA in pharmaceutical tablets and human blood serum by DPV at multiwalled carbon nanotubes/graphene oxide hybrid composite fabricated electrode. The response of PZA was linear over the concentration range from 37.5 to 1800 μmol L−1, with a limit detection of 5.54 μM. The proposed electrochemical sensor showed good results toward the commercial pharmaceutical formulated PZA samples. Lately, the use of poly amino acid has attracted attention in electroanalysis. The electropolymerization of amino acid-modified electrodes, especially poly(glycine), have been used for determination of pharmaceuticals [26–30]. Recently, the use of electropolymers as a strategy for analytical purposes has been reported in the literature [31–34]. However, there are no reports in the literature yet about the use of poly(glycine)-modified glassy carbon electrode as a electrochemical sensor. Thus, the objective of the present work was to investigate the electrochemical behavior of PZA on a poly(glycine)-modified glassy carbon electrode (poly(Gly)/GCE) and to develop an electroanalytical methodology for quantifying this antibiotic. So far, to the best of our knowledge, an electrochemical determination of PZA on this perspective electrode has not been previously described in the literature. The practical applicability of the method was verified by analysis of pharmaceutical tablets and human urine samples.
Experimental
Reagents and solutions
Pyrazinamide was purchased from Sigma-Aldrich (St Louis, MO, USA). The stock solution of PZA (1000 μmol L−1) was prepared in aqueous solution, and it was stored in a refrigerator at 4–6 °C. The 0.1 mol L−1 phosphate buffer solution (PBS) was used as a supporting electrolyte and were prepared by mixing the salts disodium phosphate and sodium hydrogen phosphate, and then, the pH was adjusted with 1.0 mol L−1 sodium hydroxide (99.0 % Proquimios, Rio de Janeiro, Brazil) solution. The glycine was purchased from Sigma-Aldrich (St Louis, MO, USA). Lauryl sulfate sodium, talc, and povidone were purchased from Isofar (Duque de Caxias, Brazil). Starch, magnesium stearate, and microcrystalline cellulose were acquired from Valdequímica (São Paulo, Brazil). Ascorbic acid and uric acid were purchased from Isofar (Duque de Caxias, Brazil). All the chemicals used were of analytical grade, and all the solutions were prepared with deionized water.
Apparatus
The voltammetric measurements were carried out on an Autolab® PGSTAT 128N potentiostat/galvanostat (Utrecht, Netherlands) operating with the software Autolab Nova version 1.10 for data collection and analysis. The three-electrode electrochemical cell was set with poly(Gly)/GCE as a working electrode, an Ag/AgCl/ 3 mol L−1 KCl electrode as a reference electrode, and a 1.0-cm2 platinum foil as a counter electrode.
All measurements were carried out at room temperature. The pH measurements were done with a Metrohm 827 pH lab pH meter (Metrohm) calibrated with standard buffers at room temperature. The methodology used for comparison with the electrochemical method proposed in this study was performed according to the US Pharmacopeia [35]. Thus, spectrophotometric analysis was carried out on a UV-VIS 800 XI spectrophotometer (FEMTO). UV detection was performed at a wavelength of 268 nm.
Preparation of poly(gly)/GCE
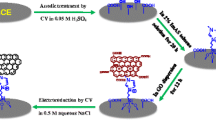
The electropolymerization of glycine in GCE surface was carried out by cyclic voltammetry. In 1.0 mmol L−1 concentration of glycine prepared in pH 7.0 PBS, the GCE surface was completely electropolymerized throughout of 20 cycles in the potential range of −0.5 to +1.8 V at 100 mV s−1 [29]. As shown in Fig. 2, in the initial scan, an anodic peak was observed at +1.6 V; however, the currents decreased with increasing numbers of cycles. This phenomenon indicates that the species obtained after the first electron transfer underwent a chemical reaction of N-terminal of amino group with carbon of GCE [26]. The –NH2 and –COOH groups of amino acids are playing a key role in the electropolymerization process on the electrode surface [36].
Determination of voltammetric parameters
After electropolymerization procedure, the poly(Gly)/GCE was rinsed in deionized water. Then, a volume of 10.0 mL of 0.1 mol L−1 PBS (pH 7.5) containing 0.1 mmol L−1 PZA was placed in the glass electrochemical cell, and the electrochemical behavior of PZA at poly(Gly)/GCE was investigated by cyclic voltammetry (CV) at a scan rate of 50 mV s−1 over the potential range from −0.6 to −1.1 V. Also, the scan rate was varied from 10 to 100 mV s−1. In order to study the effect of pH on the electrochemical behavior of PZA at poly(Gly)/GCE, cyclic voltammograms were also recorded for 0.1 mmol L−1 PZA in 0.1 mol L−1 PBS with pH varying from 6.5 to 9.0 at a scan rate of 50 mV s−1.
The analytical method was developed by square-wave voltammetry (SWV). The potential pulse amplitude (a), potential step increment (ΔE s), and frequency (f) were considered as parameters to assess the optimum experimental performance for quantification of PZA using the poly(Gly)/GCE. A volume of 10.0 mL of 0.1 mol L−1 PBS (pH 7.5) containing 0.1 mmol L−1 PZA was placed in the glass electrochemical cell, and the potential pulse amplitude was varied from 10 to 150 mV (with a frequency and potential step increment fixed at 50 s−1 and 1 mV, respectively), the potential step increment (ΔE s) was varied from 1 to 5 mV (with potential pulse amplitude and frequency fixed at 90 mV of 30 s−1, respectively), and the frequency was varied from 10 to 100 s−1 (with potential pulse amplitude and the potential step increment fixed at 90 and 1 mV, respectively). The optimized square-wave voltammetric parameters were as follows: potential pulse amplitude of 90 mV, potential step increment of 1 mV, and frequency of 60 s−1.
The best experimental condition for PZA analysis using the poly(Gly)/GCE was obtained in 0.1 mol L−1 PBS with pH 7.5 at potential pulse amplitude of 90 mV, potential step increment of 1 mV, and frequency of 60 s−1. The linearity of the method was evaluated by preparing 13 PZA solutions with concentrations varying from 0.47 to 6.15 μmol L−1 at three different days. The results were plotted as a calibration curve, and the linear correlation coefficient was determined by linear fitting.
The limits of detection (LOD) and limits of quantification (LOQ) were determined using the ratio of 3σ/b and 10σ/b, respectively, where b is the slope of the calibration curve and σ is the standard deviation value from ten voltammograms of the blank determined according to the IUPAC recommendations [37]. Before each measurement, the oxygen was removed by bubbling nitrogen through the solutions for 4 min. All the electrochemical experiments were carried out at room temperature. These experimental conditions and the optimized square-wave voltammetric parameters were applied to quantify PZA in pharmaceutical formulation and human urine.
Voltammetric analysis of PZA in pharmaceutical formulation and human urine
The developed voltammetric method was tested for determination of PZA in pharmaceutical formulations (tablets) and human urine. PZA tablets were purchased from a local drugstore. According to the manufacturer’s information, each tablet contained 500 mg of PZA. Twenty tablets were powdered, and then, an amount of the powder equivalent to about 100 mg of PZA was accurately weighed, transferred to a 500-mL volumetric flask, and then added 300 mL of water. The mixture was sonicated for 10 min, and after, it was diluted with water to the final volume of the volumetric flask. This solution was filtrated and an aliquot of 10 mL was transferred to a 100-mL volumetric flask and diluted with water (this solution was named “test solution”). In the analysis, 0.1 mL of the test solution was diluted in 9.9 mL of 0.1 mol L−1 PBS (pH 7.5) in the electrochemical cell and the PZA concentration was determined by the standard addition method. The results obtained were compared with an official US Pharmacopeia protocol [35].
The suitability of the developed voltammetric method for analysis of PZA in biological fluids was also investigated by direct analysis of PZA in human urine. For this purpose, a sample of human urine (25 mL) was collected from a volunteer and stored at temperature of approximately 4 °C. The urine sample was fortified by adding an aliquot of 300 μL of 1.0 mmol L−1 PZA standard stock solution, and a final concentration of 12.0 μmol L−1 PZA was obtained. An aliquot of 1000 μL of the 12.0 μmol L−1 PZA solution was added to a 10-mL volumetric flask, and the final volume was completed with 0.1 mol L−1 supporting electrolyte (0.1 mol L−1 PBS, pH 7.5). This solution was added to the electrochemical cell, and the PZA concentration was determined by the standard addition method.
Results and discussion
Electrochemical behavior of PZA on poly(Gly)/GCE
The electrochemical behavior of 0.1 mmol L−1 PZA in 0.1 mol L−1 PBS (pH 7.5) at poly(Gly)/GCE was investigated by CV at 50 mV s−1 over the potential range from −0.6 to −1.1 V. Figure 3 shows the cyclic voltammograms obtained with bare GCE in absence (curve a) and presence (curve c) of 0.1 mmol L−1 PZA and also shows the cyclic voltammograms of the poly(Gly)/GCE in the absence (Fig. 3, curve b) and presence (Fig. 3, curve d) of 0.1 mmol L−1 PZA in 0.1 mol L−1 PBS (pH 7.5). At bare GCE, the reduction peak occurs at −0.86 V, and no evidence of an oxidation peak in reverse scan was observed. Under identical conditions, the poly(Gly)/GCE produce a significant increase in the reduction peak current of the PZA. In addition, the use of the poly(Gly)/GCE showed a reversible electron transfer process of PZA, with the reduction and oxidation peaks obtained at potential values of −0.85 and −0.80 V, respectively. The evident enhancement of the peak current provided by the poly(Gly)/GCE shows a remarkable catalytic effect of this modified electrode in the electroactivity of the PZA.
Studies of PZA redox system on poly(Gly)/GCE
The influence of the solution pH on the PZA electrochemical redox system was investigated by CV in 0.1 mol L−1 PBS with pH varying from 6.5 to 9.0 at 50 mV s−1. It was observed that an increase of the pH significantly increased the cathodic and anodic current response from 6.5 to 7.5 pH values, and the anodic and cathodic peak potentials of PZA were shifted to a more negative potential with the increase of pH values. For pH values higher than 7.5, however, the cathodic and anodic peak current decreased. Thus, the pH of 7.5 was chosen for the further analysis of PZA. It was also observed that the cathodic peak potential (E Pc) exhibited a dependence on the solution pH (Fig. 4, insert).
The transference of protons in aqueous medium from or toward organic molecules is usually considered to be a fast process, meaning that protons are in equilibrium in solution near the electrode [38], thereby justifying this investigation. A linear correlation was obtained from pH 6.5 to 9.5 in the E Pc versus pH curve with a slope of 0.091 V/pH at 25 °C according to the following equation: E Pc (V) = −(0.213 ± 0.044) − (0.092 ± 0.005) pH (R 2 = 0.993).
The number of electrons in the PZA can be determined applying the following equation: E P − E P/2 = 59 mV/n [39, 40]. Using the voltammogram “d” obtained in Fig. 3, the values of E Pc and E Pc/2 for PZA were −860 and −826 mV, respectively, and the number of electrons transferred (n) in the reduction of PZA was estimated to be equal to 1.74. Furthermore, according to the equation ΔE/ΔpH = (59.1 mV/n) × N H+ (n = 2) and using the slope obtained, we can suggest that the number of protons (N H+) transferred may be three. These results agree with the electrochemical behavior of pyrazine in the mercury electrode [41, 42]. These authors related that the stability of the product formed is dependent on the characteristics of the substituent. For pyrazine compounds containing electron-withdrawing ligands, a reversible behavior was observed, involving two electrons and three protons. In this sense, Fig. 5 shows proposed electrochemical reduction of PZA at poly(Gly)/GCE. The elucidation of reduction mechanism of this analyte requires studies that are more detailed.
Proposed electrochemical reduction of PZA at poly(Gly)/GCE as suggested by Bergamini et al. [24]
The effect of the potential scan rate on the poly(Gly)/GCE electrochemical response was also investigated (Fig. 6), and a plot of the cathodic peak current (I Pc) versus square root of the potential scan rate (ν 1/2) for 0.1 mmol L−1 PZA solution in 0.1 mol L−1 PBS (pH 7.5) resulted in a straight line for both I Pc and I Pa (Fig. 6, insert) being expressed by the following equations: I Pc (μA) = −(10.16 ± 0.41) ν 1/2 / (V s−1)1/2 − (0.500 ± 0.097) (μA) (R 2 = 0.994) and I Pa (μA) = (11.35 ± 0.40) ν 1/2 / (V s−1)1/2 − (0.446 ± 0.094) (μA) (R 2 = 0.997), where suggesting that the electrochemical process is controlled by diffusion [39, 43].
Influence of the potential pulse amplitude, potential step increment, and frequency of square-wave voltammetry
The square-wave voltammetry was selected as a sensitive pulse voltammetric technique for better applicability in the analysis of redox system. The optimization of operating parameters was carried out in order to obtain current response for electrochemical determination of PZA with the highest magnitude and best peak shape. The potential pulse amplitude, potential step increment, and frequency were considered as parameters to assess the optimum experimental performance for quantification of 0.1 mmol L−1 PZA in 0.1 mol L−1 PBS at pH 7.5 using the poly(Gly)/GCE. The variation of potential pulse amplitude in the range from 10 to 150 mV (with a frequency and potential step increment fixed at 50 s−1 and 1 mV, respectively) indicated that the current response of redox system PZA increased and slight shift to the less negative potential values.
Above potential pulse amplitude value of 90 mV, the resultant current of PZA started to shift significantly with observation of substantial broadening of the peak. Therefore, the best voltammetric sensitivity was obtained with potential pulse amplitude 90 mV, which was selected for further investigations.
The values of peak current were also found to vary with respect to the potential step increments (1–5 mV) applied on SWV (potential pulse amplitude and frequency fixed at 90 mV of 30 s−1, respectively). The potential step increment of 1 mV was selected as it sets the best voltammetric profile owing higher sensitivity and selectivity for PZA determination. The effect of the frequency from 10 to 100 s−1 on the SWV response of the poly(Gly)/GCE for 0.1 mmol L−1 PZA was also evaluated (potential pulse amplitude and the potential step increment fixed at 90 and 1 mV, respectively). The values of peak current showed an increase when the frequency was increased from 10 to 60 s−1; above the frequency value of 60 s−1, the resultant peak current decreased. Thus, the frequency of 60 s−1 was chosen for further studies. The optimized parameters of the SWV technique were as follows: pulse amplitude of 90 mV, potential step increment of 1 mV, and frequency of 60 s−1. These optimized parameters were used for calibration curve construction.
Analytical determination of PZA
The applicability of the proposed SWV-poly(Gly)/GCE method for quantification of PZA was examined by calibration curve by plotting resultant peak current against concentration of PZA at optimized experimental conditions. Figure 7 shows the square-wave voltammograms registered after the addition of aliquots of standard solutions (triplicate) containing increasing concentrations of PZA (from 0.47 to 6.15 μmol L−1). The calibration curve with the linear relationship between resultant peak current and the concentration of PZA (C PZA) is showed in the inset of Fig. 7 and is expressed by the following equation: −I P (μA) = (0.561 ± 0.013) C PZA / μmol L−1 − (0.246 ± 0.011) (μA).
Square-wave voltammograms of PZA reduction obtained using the poly(Gly)/GCE under optimized conditions. PZA concentrations: 0.00 μmol L−1 (a), 0.47 μmol L−1 (b), 0.90 μmol L−1 (c), 1.66 μmol L−1 (d), 2.30 μmol L−1 (e), 2.85 μmol L−1 (f), 3.75 μmol L−1 (g), 4.11 μmol L−1 (h), 4.56 μmol L−1 (i), 4.94 μmol L−1 (j), 5.32 μmol L−1 (k), 5.65 μmol L−1 (l), 5.96 μmol L−1 (m), and 6.15 μmol L−1 (n). Optimized parameters: a = 90 mV, f = 60 s−1, and ΔE S = 1.0 mV. Insert: calibration plot
The correlation coefficient was 0.998 (for n = 13). The LOD and LOQ values determined with basis on the ratio of 3σ/b and 10σ/b, respectively, (where b is the slope of the calibration curve and σ is the standard deviation value from ten voltammograms of the blank) were 0.035 and 0.12 μmol L−1, respectively.
The linear range and limits of detection of the proposed electroanalytical method were similar or better than those reported in earlier reports on electroanalytical techniques (Table 1). In addition, the reaction occurring at the poly(Gly)/GCE reached a dynamic equilibrium very rapidly upon addition of PZA, leading to a response time to reach 100 % of signal shorter than 1 s. The advantages exhibited by poly(Gly)/GCE for PZA reduction, principally the high sensitivity, low detection limit, fast response time, and simplicity, demonstrate that this electrode could be potentially used as a electrochemical detector for determination of PZA in HPLC and capillary electrophoresis systems, for example.
Intra-day and inter-day repeatability
The intra-day repeatability of the peak current magnitude was determined by successive measurements (n = 10) on the 4.06 μmol L−1 PZA solution in PBS (pH 7.5, 0.1 mol L−1). When these repeated peak current values were compared with the initial peak current value, a relative standard deviation (RSD) value of 3.75 % was obtained for PZA, indicating good intra-day repeatability of the proposed method.
The inter-day repeatability for the peak current magnitude of 4.06 μmol L−1 PZA solution in PBS (pH 7.5, 0.1 mol L−1) was evaluated over a period of 10 days. A good RSD value of 4.96 % was also obtained. Hence, it is possible to conclude that the SWV-poly(Gly)/GCE approach for PZA determination provides results with adequate precision.
Interference study
The selectivity of the proposed method for PZA determination was tested by the assessment of the effect of possible interferents commonly occurring in pharmaceutical formulations (such as stearate magnesium, starch, microcrystalline cellulose, talc, sodium lauryl sulfate, and povidone) and in human urine (such as uric acid and ascorbic acid). Solutions of these compounds were freshly prepared at a PZA solution/interferent compound concentration ratio of 1:100 under the same conditions used for 4.94 μM PZA in 0.1 mol L−1 PBS at pH 7.5. The analytical response was monitored and compared with the signal obtained for the pure PZA solution (Table 2). The results revealed that the proposed method is highly selective for PZA, once the interferents did not affect the cathodic current of the antibiotic under the concentration tested.
Application of the method in determination of PZA in pharmaceutical formulations, human urine, and recovery tests
The poly(Gly)/GCE was applied to determine PZA in pharmaceutical tablets and human urine; each experiment was conducted in triplicate and using the standard addition method. For comparison purposes, the concentration of PZA in the tablets was also determined by the official spectrophotometric protocol [35]. The data were statistically compared through the paired t test and F test [44], and the results are summarized in Table 3.
It was possible to observe that there was no statistical difference between these two methods at a confidence level of 95 %, indicating that the poly(Gly)/GCE can be successfully used for voltammetric determinations of PZA in pharmaceutical formulations.
The accuracy of the SWV-poly(Gly)/GCE method and the possibility of matrix interferences were further checked by performing analytical recovery experiments. Precise amounts of PZA were added to pharmaceutical tablet sample, and the recovery percentage values were calculated from the actual and added PZA concentrations (Table 4). It can be clearly observed that the matrix of the samples had no influence on the response obtained by the proposed SWV-poly(Gly)/GCE method.
The recovery of the spiked sample ranged between 98.2 and 104.0 % for human urine and pharmaceutical samples (Table 4). This indicates that the poly(Gly)/GCE is effective and can be used to quantify PZA without interference of the urine matrix. It is noticeably demonstrated that the proposed SWV-poly(Gly)/GCE method is a feasible, sensitive, and good alternative for the determination of PZA both in pharmaceutical formulations and human urine.
Conclusions
This work described the development and the application of a novel electroanalytical approach for the direct determination of PZA using a poly(Gly)/GCE as a perspective electrochemical sensor. Cyclic voltammetry and square-wave voltammetry were applied to the study of electrochemical behavior and quantification of PZA. The developed SWV-poly(Gly)/GCE method provided a wide linear concentration range from 0.47 to 6.15 μmol L−1, a low limit of detection (0.035 μmol L−1), which was similar to or better than many reported analytical methods for PZA determination, and a good intra-day and inter-day repeatability (RSD = 3.75 and 4.96 %, respectively, at 4.06 μmol L−1 PZA, for n = 10). The developed SWV-poly(Gly)/GCE method was applied to the determination of PZA in commercial pharmaceutical tablets and human urine samples. Additionally, the concentrations of PZA found in pharmaceutical tablets by the developed SWV-poly(Gly)/GCE method were equivalent to those obtained by UV-VIS spectrophotometry at a confidence level of 95 %. Satisfactory recovery results were obtained in the determination of PZA in human urine, indicating that the SWV-poly(Gly)/GCE method was also successfully applied in this kind of sample. The SWV involving poly(Gly)/GCE is a simple, environmentally friendly, sensitive, rapid, accurate, and precise approach that does not need sophisticated instruments or any separation step, allowing the analysis of PZA without laborious and time-consuming procedures.
References
Syre H, Øvreas K, Grewa HMS (2010) J Antimicrob Chemother 65:704–712
Zhang Y, Mitchison D (2003) Int J Tuberc Lung Dis 7:6–21
Gumbo T, Dona CSWS, Meek C, Leff R (2009) Antimicrob Agents Chemother 53:3197–3204
Sriram D, Yogeeswari P, Reddy SP (2006) Bioorg Med Chem Lett 16:2113–2116
Conte JE Jr, Lin E, Zurlinden E (2000) J Chromatogr Sci 38:33–37
Arbex MA, Varella MDCL, Siqueira HRD, de Mello FAF (2010) J Bras Pneumol 36:626–640
Shih T, Pai C, Yang P, Chang W, Wang N, Hu OY (2013) Antimicrob Agents Chemother 57:1685–1690
Espinosa-Mansilla A, Valenzuela MIA, de la Peña AM, Salinas F, Cañada FC (2001) Anal Chim Acta 427:129–136
Madan J, Dwivedi AK, Singh S (2005) Anal Chim Acta 538:345–353
Goicoechea HC, Olivieri AC (1999) J Pharm Biomed Anal 20:681–686
Marcellos LF, Faria AF, de Souza MVN, Almeida MR, Sabin GP, Poppi RJ, de Oliveira MAL (2012) Cent Eur J Chem 10:1808–1816
Faria AF, Souza MVN, Bruns RE, Oliveira MAL (2010) Talanta 82:333–339
Wu J, Shih H, Wang S, Tsai T (2004) Anal Chim Acta 522:231–239
Husain S, Sarma PN, Sajjad SM, Narsimha R, Subrahmanyam M (1990) J Chromatogr 513:83–91
Mehmedagica A, Vérité P, Ménager S, Tharasse C, Chabenat C, André D, Lafontb O (1997) J Chromatogr B 695:365–372
Zhou Z, Wu X, Wei Q, Liu Y, Liu P, Ma A, Zou F (2013) Anal Bioanal Chem 405:6323–6335
Conte JE, Lin E, Zurlinden E (2000) J Chromatogr Sci 38:33–37
Bhanushali CJ, Zidan AS, Rahman Z, Habib MJ (2013) AAPS PharmSciTech 14:1313–1320
Khuhawar MY, Rind FMA (2002) J Chromatogr B 766:357–363
Shewiyo DH, Kaale E, Risha PG, Dejaegher B, Smeyers-Verbeke J, Vander Heyden Y (2012) J Chromatogr A 1260:232–238
Cheemalapati S, Devadas B, Chen S (2014) J Colloid Interface Sci 418:132–139
Devadas B, Cheemalapati S, Chen S, Ajmal Ali M, Al-Hemaid FMA (2015) Ionics 21:547–555
Cheemalapati S, Devadas B, Chen S, Ajmal Alib M, Al-Hemaid FMA (2014) Anal Methods 6:6774–6782
Bergamini MF, Santos DP, Zanoni MVB (2013) J Electroanal Chem 690:47–52
Mani S, Cheemalapati S, Chen S, Devadas B (2015) Int J Electrochem Sci 10:7049–7062
Li Y, Liu X, Wei W (2011) Electroanalysis 23:2832–2838
Gilbert O, Kumara Swamy BE, Chandra U, Sherigara BS (2009) J Electroanal Chem 636:80–85
Thomas T, Mascarenhas RJ, Swamy BK, Martis P, Mekhalif Z, Sherigara BS (2013) Colloids Surf B 110:458–465
Narayana PV, Reddy TM, Gopal P, Naidu GR (2014) Anal Methods 6:9459–9468
Ma X, Wang Z, Wang X, Xu L (2013) J Solid State Electrochem 17:661–665
Jin G, Chen L, Hang G, Yang S, Wu X (2010) J Solid State Electrochem 14:1163–1169
Wen Y, Duan X, Xu J, Yue R, Li D, Liu M, Lu L, He H (2012) J Solid State Electrochem 16:3725–3738
Liu H, Chen Y, Liu Y, Yang Z (2013) J Solid State Electrochem 17:2623–2631
Taei M, Jamshidi M (2014) J Solid State Electrochem 18:673–683
The United States Pharmacopeia (2015) The national formulary, USP 38-NF 33. United States Pharmacopeia Convention, Rockville
Zhang F, Gu S, Ding Y, Zhou L, Zhang Z, Li L (2013) J Electroanal Chem 698:25–30
Analytical Methods Committee (1987) Analyst 112:199–204
Laviron E (1981) J Electroanal Chem Interfacial Electrochem 124:1–7
Bard AJ, Faulkner LR (2001) Electrochemical methods: fundamentals and applications. Wiley, New York
Brett CMA, Brett AMO (1993) Electrochemistry: principles, methods, and applications. Oxford university press, Oxford
Ni Y, Kokot S, Selby M, Hodgkinson M (1995) Anal Chim Acta 316:233–238
Swartz J, Anson FC (1980) J Electroanal Chem 114:117–128
Gosser DK (1994) Cyclic voltammetry: simulation and analysis of reaction mechanisms. VCH Publishers, New York
Triola MF (2013) Introdução à Estatística, eleventh ed. LTC, Rio de Janeiro
Acknowledgments
The authors thank the National Counsel of Technological and Scientific Development (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), and Financiadora de Estudos e Projetos (FINEP) for the financial support and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG) for the scholarship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferraz, B.R.L., Leite, F.R.F. & Malagutti, A.R. Highly sensitive electrocatalytic determination of pyrazinamide using a modified poly(glycine) glassy carbon electrode by square-wave voltammetry. J Solid State Electrochem 20, 2509–2516 (2016). https://doi.org/10.1007/s10008-015-3072-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-015-3072-8