Abstract
A sensitive and selective electroanalytical method for the determination of tadalafil (TAD) using adsorptive stripping square wave voltammetry at multiwalled carbon nanotube paste electrode (MWCNTPE) and modified TiO2-multiwalled carbon nanotube paste electrode (TiO2-MWCNTPE) was presented. The calibration curves were linear in the concentration range of 3.6–8.1 and 12.7–61.1 μM on MWCNTPE, 0.27–15.2 μM on TiO2-MWCNTPE. The recommended method was successfully applied to the determination of the drug in tablets and human serum samples with good recoveries. The selectivity of the proposed method was considered in the presence of Ca2+, K+, Na+, 2-mercapto benzimidazole, thiourea, and dopamine by means of recovery tests. Interfering agents did not show considerable effect on TAD determination. No electroactive interferences from the tablet excipients and endogenous substances from biological material were detected. The possible electrooxidation pathway and the number of transferred electrons were also investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
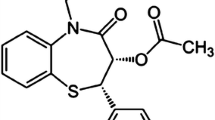
Tadalafil (TAD) (Scheme 1), (6R,12aR)–6-(1,3-benzodioxol-5-yl)-2-methyl-2,3,6,7,12,12a-hexahydropyrazino[1′, 2′:1,6] pyrido[3,4-b] indole-1,4-dione, is an oral drug that is used for treating impotence (the inability to attain or maintain a penile erection) and benign prostatic hyperplasia (BPH). It is in a class of drugs called phosphodiesterase inhibitors that also includes sildenafil and vardenafil. However, its chemical structure differs from that of sildenafil and vardenafil, reflecting differing pharmacological properties.
The mechanism whereby TAD improves the symptoms of BPH is not clear, but phosphodiesterase-5 is present in the muscles of the bladder and the prostate, and it has been suggested that the relaxation of these muscles may make the passage of urine less difficult (www.medicinenet.com/tadalafil/article.htm). Tadalafil is absorbed rapidly at mean C max (0.973 μM for 20 mg) observed at 2 h, thereafter, concentrations declined nearly mono-exponentially with the mean t 1/2 at about 17.5 h. Therefore, development of more effective analytical method is required for the routine analysis of TAD in biological fluids [1].
In the literature, there are several studies on the determination of TAD in pharmaceutical or biological samples. The methods in already published studies are liquid chromatography with UV detection [2–10], fluorescence detection [11], mass detection [12], gas chromatography with mass spectrometry (MS) detection [13], capillary chromatography [14], capillary electrophoresis [15], spectrophotometry [16, 17], and spectrofluorometry [18]. The reported methods have many shortcomings such as potential loss of drugs in the re-extraction procedure and the need of lengthy, tedious, and time-consuming plasma sample preparation and extraction process.
Among several analytical methods for detecting species in a particular solution, electroanalysis is one of the most promising methods for its numerous advantages, such as low cost, sensitivity, reliability, accuracy, consistency, and its simplicity in use [19–21]. Since their discovery, many researchers in academics and industry have developed a widespread interest in using nanomaterial especially carbon nanotubes (CNT) as a biosensor. The reason for widespread utilization of CNTs in electrochemistry is their unique structural, mechanical, and electronic properties, which include the possession of hollow cores for storing guest molecules, high chemical and thermal stability, and high elasticity and conductivity [22]. CNTs show much better transfer characteristics with metal matrix composites. Efficient load transfer between a matrix and CNTs play a key role in the mechanical properties of composites and can lead to development of many super strong nanocomposites [23]. Nano-sized materials support constructively the catalytic sensitivity of CNT due to the combination of their electronic, adsorptive, mechanical, and thermal properties. Similarly, it has been demonstrated that titanium dioxide (TiO2) nanoparticles is one of the most capable materials, which has good potential interest as a sensor electrode in electrochemistry [24, 25]. The combination of CNT and TiO2 can provide significant effect for enhancing catalytic process [26]. With continued developments in the synthesis and production of CNTs, composite materials containing nanotubes are near-term applicable and will see innovations that take advantage of their special properties. In recent years, modified electrodes with nanoparticles have been used as a new type fabrication composite electrode, as they have many particular properties such as high chemical stability, excellent ionic conductivity, and wide electrochemical windows [27–30]. Moreover, high conductors that are multifunctional (electrical and structure) and highly anisotropic insulators and high-strength, porous ceramics are more examples of new materials that can come from nanotubes [31].
Even though a class of drugs called phosphodiesterase inhibitors such as sildenafil [32–34] and vardenafil [35] were studied by voltammetric methods, as yet, there has been no literature report for the electrooxidative behavior and redox properties of TAD, neither bare nor modified electrodes. In this paper, a composite paste electrode that has been made of multiwalled carbon nanotube (MWCNT) and incorporated into TiO2 nanoparticles for determination of TAD is reported for the first time. The aim of this work is to carry out a detailed investigation on the electrochemical behavior and possible oxidation mechanism of TAD with the proposed TiO2-MWCNTPE by using cyclic (CV) and adsorptive stripping square wave (AdSSWV) voltammetric techniques. This work was also aimed to develop a new, fully validated, rapid, and simple voltammetric method for the direct determination of TAD in bulk material, pharmaceutical dosage forms, and serum samples without any time-consuming extraction, evaporation, or separation steps prior to drug analysis.
Experimental
Apparatus
Voltammetric records were obtained by a Bioanalytical Systems-Epsilon potentiostat/galvanostat (BAS, Epilson) analyzer connected with a BAS C-3 solid electrode cell stand. In all voltammetric measurements, BAS MF-2010 model carbon nanotube paste electrode (Ø = 3 mm, A = 0.071 cm2) was used as a working electrode, a platinum wire (BAS MW 1032) as the counter electrode, and an Ag/AgCl (3 mol L−1 KCl) was used as a reference electrode. AdSSW voltammetric conditions were given as follows: pH 3.0 BR buffer solution as supporting electrolyte, ΔE s = 4 mV, f = 200 Hz, ΔE = 40 mV, t acc = 40 s, and E acc = 0 mV, due to the highest sensitivity, and selectivity pH measurements were performed by a Hanna pH meter with combined glass electrode. All experiments were conducted under room conditions.
Reagents
TAD was obtained from Abdi Ibrahim Pharm. Ind. Istanbul, Turkey. The commercial Lifta® tablets containing 20 mg of TAD were acquired from a local pharmacy. The following 0.04 M H3BO3, 0.04 M H3PO4, and 0.04 M CH3COOH were used to prepare Britton–Robinson buffer (BR buffer) solutions extending from pH 2.0 to 10.0. All chemicals were provided from Merck, Darmstadt, Germany. MWCNT powder (mesh size, −270, <53 μm) was obtained from Merck. TiO2 used for modified electrode (1 % Mn doped; nanopowder, <100 nm (BET), ≥97) was provided form Sigma Aldrich. TAD stock solution was prepared daily by dissolving 50 mg TAD in 10.0 mL acetonitrile and kept from the light to avoid possible decomposition. Dilute solutions were prepared freshly by distilled water.
Preparation of the MWCNTPE and TiO2-MWCNTPE
MWCNTPE was prepared by mixing MWCNT powder and mineral oil with 70 % and 30 % mass percentages, respectively. The homogenized paste was inserted in a plastic syringe needle using a 3-mm diameter copper wire (BAS MF 2010). Then, TiO2- multiwalled carbon nanotube paste (MWCNTP) electrodes were prepared with different mass percentages of TiO2 such as 1, 5, 10, and 20 % to find the optimum blend. Modified-MWCNTP electrode prepared as suitable composition by mixing 0.65 g of MWCNT powder with 0.05 g of TiO2 nano-powder and 0.30 g mineral oil. Mineral oil was added as before and mixed again to get a homogenous modified TiO2-MWCNTP electrode (5 % TiO2 by mass).
Tablet assay procedure
Five Lifta® tablets, containing 20 mg TAD, were accurately weighed and crushed to a homogeneous fine powder in a mortar. An accurate weight of this powder equivalent to one tablet content was weighed and transferred into a 100-mL calibrated flask, diluted with pH 3.0 BR buffer solution, and then sonicated for 10 min. The content of TAD was calculated from the corresponding regression equation.
Analysis of human serum samples
Human blood obtained from healthy volunteer was used for the TAD determination. Blood sample was centrifuged at 3,000 rpm at nearly 10 min at room temperature, and proteins in blood were separated from the serum. The obtained serum samples were stored at −4 °C until assay. For the analysis of serum, 3.6 mL of serum samples were spiked with 1.0 mL of TAD stock solution (5 mg mL−1) and completed with 5.4 mL acetonitrile up to 10.0 mL, so that the final concentration of TAD was 500 μg mL−1.
Recovery studies
Since other inactive ingredients of the pharmaceutical dosage forms may interfere with the analysis or affect its accurate quantification, potential effects from matrix components must be investigated. For this purpose, a known amount of the pure TAD was added to the pre-analyzed tablet formulation of TAD. The recovery results were obtained by using the related calibration equation for six repeated measurements.
Results and discussion
No previous electrochemical study about the sensitive, selective, and simple electroanalytical determination of TAD with neither MWCNTPE nor TiO2-MWCNTPE has been performed. To demonstrate the usefulness of a MWCNTP electrode for the determination of TAD which may offer advantages for the use of such electrodes as sensors, voltammetric behaviors of TAD on both MWCNTPE and TiO2-MWCNTPE were investigated in detail by CV and AdSSWV.
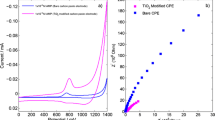
AdSSWV methods have been applied to numerous drug active compounds, by which the low detection and determination limits could be achieved. Effect of “stripping” was proved by obtaining voltammograms using SWV on both electrodes. SW and AdSSW voltammograms of 5 μg mL−1 TAD solutions with MWCNTPE and TiO2-MWCNTPE were shown in Fig. 1a and b, respectively. When used adsorptive stripping voltammetric conditions, about 1.5 times increasing in the current response was obtained with TiO2-MWCNTP electrodes when compared with MWCNTPE. In addition, peak current of TAD at TiO2-MWCNTPE using AdSSWV is 2.6-fold bigger than the peak current of TAD at MWCNTPE using SWV (Fig. 1) (ΔE s = 4 mV, f = 200 Hz, ΔE = 40 mV, t acc = 40 s and E acc = 0 mV pH 3.0 BR buffer solution). The resulting enhancement in kinetics of TAD oxidation, together with the considerable decrease in the background current probably makes TiO2-MWCNTPE more sensitive using either SWV or AdSSWV for the determination of trace amount of TAD. The superiority of AdSSWV, and hence sensitivity, could be attributed to the contribution of adsorption phenomenon of TAD during electro-deposition process.
Effect of potential modulation parameters
Peak currents for 5 μg mL−1 TAD in pH 3.0 BR buffer were obtained at MWCNTP electrode under different accumulation potentials within the ranges of 0–800 mV. As shown Fig. 2a, the peak currents depend on accumulation potentials, and 0 mV was chosen as an optimum potential due to the its maximum sensitivity.
The influence of the accumulation time has been tested between 0 and 120 s in pH 3.0 BR buffer solution. As shown in Fig. 2b, the peak currents did not significantly change at the initial stages of accumulating time but noticeably decreased for further applications after 80 s, which could be attributed to the saturation of the target molecule on the electrode surface. The accumulation time was therefore selected as 40 s for subsequent analysis. The influence of amplitude has been tested in the potential range from 10 to 70 mV. The TAD peak signal reached up to the the maximum current as the amplitude value approached 40 mV and remained nearly constant up to 70 mV (Fig. 2c). The influence of step potential on the TAD peak exhibited that the highest and best-regular response appeared with step potential 4 mV (Fig. 2d). The increments of frequency from 25 to 300 Hz caused both peak increments and potential shifts, respectively. On the other hand, frequency more than 200 Hz caused the peak shape of TAD to be broader and deformed. Thus, frequency was chosen as 200 Hz for subsequent analysis.
In order to compare their performances, the same optimum parameters were used for TiO2-MWCNTP electrode. In summary; accumulation potential, accumulation time, potential step, frequency, and pulse amplitude were E acc = 0 mV, t acc = 40 s, ΔE s = 4, f = 200 Hz and ΔE = 40 mV, respectively.
Influence of composition of TiO2
In order to find the most effective composition of TiO2-MWCNT powders for the sensitive detection of TAD, various modified electrodes with different compositions were tested by SWV in the BR buffer at pH 3.0. The most regular peak responding to TAD had appeared using the electrode prepared with the 5 % (w/w) of TiO2 nano particles. When mass percentage of TiO2 in the modified electrode was increased with respect to MWCNT such as 10 % and 20 %, the peak current of TAD was dramatically decreased. This can be attributed to the strong adsorption behavior of TiO2 nanoparticles. The maximum sensitivity for the oxidation peak was recorded for the composite electrode containing 5 % TiO2 by mass. When the higher amounts of TiO2 were introduced into the carbon nanotubes the electron transferring on the surface of the composite electrode had difficulties due to the higher than 5 % TiO2 by mass.
Influence of scan rate
In order to understand whether the electrode process is diffusion- or adsorption-controlled, scan rate influence on the peak current was investigated within the ranges of 20 to 400 mV s−1 on both electrodes at pH 3.0 BR buffer solutions. Following equations were obtained within the range 20 to 250 mV s−1 for both electrodes.
The logarithm of oxidation peak currents versus scan rates exhibited a linear relationship with a slope of 0.43 and 0.63, for the MWCNTP and TiO2-MWCNTP electrodes, respectively. The 0.43 value is close to the theoretical one of 0.5 for an ideal diffusion-controlled electrode process for MWCNTPE. For TiO2–MWCNTPE, the experimental slope (0.63) than the theoretical one may be attributed to the partial involvement of the diffusive drug molecules in the electrode reaction of the adsorbed ones. The overall electrode process is mixed diffusion-controlled with adsorption of the drug molecules at the electrode surface [36].
The oxidation peak potentials were simultaneously shifted (73 mV for MWCNTPE, 84 mV for TiO2-MWCNTPE) to more positive potentials by the scan rates increments on the both electrodes. The linear relations between the peak potential and the logarithm of scan rate can be expressed as:
In order to determine the kinetic parameters of the electron-transfer process for the TAD oxidation on the MWCNTPE and TiO2-MWCNTPE, cyclic voltammetric experiments were performed at different scan rates. The Laviron’s theory for irreversible processes was then applied to calculate the number of electron transfered and the heterogeneous electron-transfer rate constant (k o).
where E o’ is the formal potential, T is the temperature in degrees Kelvin (298 K), α is the transfer coefficient, k o is the rate constant for the interfacial electron transfer process (per second), n is the number of electrons transferred in the rate determining step, v is the scan rate, F is the Faraday constant (96,480 C mol−1), and R is the universal gas constant (8.314 K−1 mol−1).
Herein, for the MWCNTPE and TiO2-MWCNTPE, slopes and αn values were 0.051, 0.056 and 1.16, 1.06, respectively. Generally, α is assumed to be 0.5 in totally irreversible electrochemical behavior. Therefore, the values of n = 2.32 and 2.12 (~2) were obtained for the oxidation peak at MWCNTPE and TiO2-MWCNTPE, respectively.
The value of E 0 = 0.969 and 0.964 V were obtained from the intercepts of a plot of E p versus v at MWCNTPE and TiO2-MWCNTPE, respectively. From this, k o was calculated to be 96.07 s−1 for MWCNTPE and 178.29 s−1 for TiO2-MWCNTPE. These indicates the efficiency of the MWCNTP and TiO2-MWCNTP electrodes in promoting the electron transfer between TAD and the electrode surfaces.
In this study, the forward peak was shifted at 53 and 54 mV per tenfold change in scan rate for MWCNTPE and TiO2-MWCNTPE, respectively. According to Gosser [37], if both electrode kinetics and chemical kinetics are slow, the cyclic voltammograms are irreversible.
Influence of pH for MWCNTPE and TiO2-MWCNTPE
In most analytical determination, it is well known that the pH of the supporting electrolyte has a major impact on the response of organic and inorganic drug compounds. This parameter was examined to detect involvement of the proton for the oxidation process on MWCNTPE and TiO2-MWCNTPE.
For this aim, the effects of pH on both peak potential and current were studied within pH 2.0 to 10.0 using SWV for both electrodes. The peak potentials were shifted to less positive direction by increasing pH, indicating that the oxidation of TAD was pH-dependent (Fig. 3a and b). The linear relationship between E p and pH for both electrodes exhibited a linear segment in the pH range of 2.0 to 10.0 as follows:
The E p versus pH plot consists of three straight portions with slope values of 29.2 and 36.3 mV, MWCNTP and TiO2-MWCNTP electrodes, respectively. The slope value = (0.059 m/αn), where α is the symmetry transfer coefficient and m and n are the number of protons and electrons involved in the electrode reaction, respectively. The αn values were calculated from Laviron equation. Hence, the m values were calculated as 0.48 and 0.61 (~1) for MWCNTP and TiO2-MWCNTP electrodes, respectively [38, 39].
The influence of pH on the peak currents at both electrodes was also studied in detail, and the experimental results indicated that the proper curves and the maximum peak currents were obtained in pH 3.0 buffer solutions for both electrodes. Accordingly, due to the highest sensitivity and selectivity, the peak current obtained at pH 3.0 BR buffer solution was preferred for analytical purposes.
Electrochemical behavior of TAD
In order to characterize the electrochemical oxidation behavior of TAD, cyclic voltammetry (CV) was carried out in the potential range of 0 to 1.4 V. In this study, electrochemical oxidations behaviors of TAD on the MWCNTPE and the TiO2-MWCNTPE were investigated.
The cyclic voltammetric measurements of 300 μg mL−1 TAD solution for both electrodes indicated irreversible behaviors (Fig. 4). Once the potential scanning was carried out from 0 to 1.4 V in the positive direction, single anodic response was detected on MWCNTPE and TiO2-MWCNTPE at about +1.02 and +1.0 V, respectively. By reversing the potential scan from 1,400 to 0 mV, no reduction peak or wave corresponding to the anodic response of TAD was observed. After the second or further cycles, the TAD oxidation response was decreased and finally remained unchanged. This phenomenon may be due to the adsorption of TAD or its oxidative products at the electrode surface. After all, TAD exhibited an irreversible electrochemical behavior on both electrodes. Anodic peak current of TAD appeared at 1.0 V on TiO2-MWCNTPE which was about 0.02 V less positive (not significant change) than observed at MWCNTPE. These results on TiO2-MWCNTPE demonstrate that a strong enhancement in electron transfer rate of TAD is taking place. TiO2 nanoparticles together with MWCNT provide an excellent electrochemical reactivity.
The SW voltammograms of 5 μg mL−1 TAD was shown in Fig. 1 on MWCNTPE and TiO2-MWCNTPE using both techniques. TAD exhibited an anodic peak which obtained peak current as 2.4 μA at MWCNTPE with an oxidation peak potential at +1.02 V. Using TiO2-MWCNTPE, the peak current at +1.0 V was increased to 3.6 μA, and this indicates the presence of a synergy between TiO2 nanoparticles and MWCNT (Fig. 1).
Oxidation mechanism of TAD
CV techniques are among the most suitable methods for the investigation of redox behavior of drug active compounds that can give insights into its metabolic fate [21, 40–45]. Electrochemical responses from the redox properties of drugs and biomolecules might have profound effects on the understanding of the redox mechanism related to the activity. In order to identify the possible functional group responsible from the electro oxidation of TAD, the CV experimental results and their curves were compared with those of some selected model compounds containing indole and benzodioxol groups.
Although the exact oxidation mechanism was not determined, some conclusions about the potentially electroactive centers under working conditions could be reached. Some model compounds were used for understanding the electrooxidation mechanism of TAD. For this, indole, fluvastatin sodium, which contains indole ring in their structures and etoposide, which contains benzodioxol ring in its structure of TAD, has been studied. Several measurements with different electrochemical techniques were performed using various pH values (pH 3.0 and 7.0 BR-buffer solution) for all these model compounds and TAD in order to obtain information about the oxidation behavior. CV measurements on the anodic direction showed the irreversible nature of the oxidation process with model compounds (Fig. 4). TAD and model compounds (except etoposide which contains benzodioxol ring and methoxy group) exhibit anodic responses nearly at the same potentials. This indicates that the oxidation of TAD is due to the indole moiety. From the CV curves, the voltammetric behavior of indole derivatives, which are structurally related to TAD, may be postulated by the oxidation of this group [21, 40–51]. Our results on model compounds show that the electro active center responsible from the anodic peak was the nitrogen atom on the indole ring. In all studied pH values, indole response was obtained clearly and separately. The anodic oxidative behavior of TAD was also comparable to the indole oxidation of the model compounds as reported in the literature [52]. Fluvastatin sodium is C-3 indole derivatives. Two oxidation peaks were observed like other C-3 indole derivatives [52]. Electrochemical behavior of fluvastatin sodium investigated as details in our previous paper. It gave two oxidation peaks at +0.8 and at +0.9 V in pH 3 phosphate buffer. Electrochemical reaction at boron-doped diamond electrodes involves two electrons and two protons [53]. Chemical structure of TAD is different than fluvastatin sodium molecule. For this reason, its oxidation mechanism is a little bit different. Hence, we may suggest that TAD is irreversibly oxidized with two electrons and one proton at electrodes. Comparative studies on indole, fluvastatin sodium, were realized by CV as a function of pH in order to identify the oxidation process of TAD. Considering the aforementioned comparison for the oxidation process of TAD, and bearing the oxidative process of nitrogen atom on the indole ring in mind, leads finally to hydroxylation of the benzene ring [54] (Scheme 2).
Calibration curve using MWCNTP and TiO2-MWCNTP electrodes
The results of pre-trial showed that it was possible to apply SWSV techniques to the quantitative analysis of 3.6 μM TAD using MWCNTPE and TiO2-MWCNTP electrodes. In the calibration curve, there were two linear sections for MWCNTPE extending from 3.6 to 8.05 μM and 12.7 to 61.1 μM (Table 1). To our advantage, we had focused to the first section because these drugs generally are found at a low concentration in human body. On the other hand, there was a single linear part on TiO2-MWCNTP electrodes with a much lower detection limit compared with the bare electrode (Fig. 5). The linear equations for both electrodes could be displayed as follows:
Effect of some interference substances at TiO2-MWCNTPE
The selectivity of the proposed method was considered in the presence of some cations and bio-molecules by means of recovery tests. The interfering effects were estimated by comparing the peak currents in the presence of the interfering species to that in their absence. The recoveries for a 10.0 μg mL−1 TAD in the presence of 2-mercapto benzimidazole, thiourea, dopamine, uric acid, or Ca2+, K+, and Na+ are presented in Table 2. Dopamine is a member of the catecholamine family that plays active roles in the brain as a neurotransmitter. As shown in Fig. 6, dopamine exhibited an oxidation peak at about 0.76 V in pH 3.0 BR buffer solution and did not show serious interfering effect on the determination of TAD. The influence of some cationic species found commonly in living organisms, such as Ca2+, K+, and Na+ was also studied. They displayed no interfering effects, since they could not be further oxidized near the peak potential of TAD at +1.0 V. The recovery of 10.0 μg mL−1 TAD in the presence of Ca2+, K+, and Na+, and biomolecules with the mass ratios of 1:1, 1:2, 1:5, and 1:10 was extended from 80.3 % to 100.2 %. The interfering species such as urea, uric acid, and their derivatives have also vital importance as physiological compounds for life. These interfering agents did not show considerable effect on the TAD quantization. The degrees of the recoveries in the presence of latter species were consequently between 93.4 % and 99.5 % in their 1:1 mass ratios.
AdSSWV determination of TAD in the presence of dopamine a 10.0 mL pH 3.0 BR buffer solution, b 10.0 μg mL−1 TAD, c 10.0 μg mL−1 TAD + 10.0 μg mL−1 dopamine, d 10.0 μg mL−1 TAD + 20.0 μg mL−1 dopamine, e 10.0 μg mL−1 TAD + 50.0 μg mL−1 dopamine, (ΔE s = 4 mV, f = 200 Hz, ΔE = 40 mV, t acc = 40 s and E acc = 0 mV)
Application of analyte
Determination of TAD from pharmaceutical dosage forms and spiked human serum samples were studied in details using both electrodes. Also, studies on tablets and spiked serum samples were applied to investigate the reliability of the developed voltammetric methods.
Determination of TAD in pharmaceutical dosage forms and recovery studies
In order to investigate the applicability of the proposed methods in pharmaceutical dosage form, a commercial Lifta® tablets were used. To determine whether recipients in the pharmaceutical dosage form interfere with the analysis, the accuracy of the proposed methods were evaluated by recovery tests after addition of known amounts of pure drug to the pre-analyzed formulations of TAD (Table 3). A tablet, “commercial pharmaceutical dosage forms Lifta®”, claims to contain 20 mg TAD was dissolved in 40.0 mL of acetonitrile and was used daily and prepared by dilution of the stock solution to 10 μg mL−1 with supporting electrolyte. The obtained results showed (Table 3) that the validity of the proposed methods was applied successfully to the quantitative for determination of TAD on both electrodes. According to the literature survey, voltammetric determination of TAD has not yet been reported, and therefore, the present method was compared with the other techniques. To evaluate the performance of the present method on the basis of its linearity range, detection limit, and recoveries, it was compared with some other methods reported in the literature for TAD determination, and the results were summarized in Table 4. These results make it evident that the developed method is superior to previously reported ones in terms of dynamic concentration range [3–7, 10, 11, 14–18], detection limit [10, 11, 14, 15], and recoveries [5, 6, 9, 11–14, 18].
Determination of TAD in spiked human serum samples
The optimized AdSSWV method was also successfully applied to determination of TAD spiked to human samples in pH 3.0 BR buffer solution. Only obtained serum results were shown in Table 5. The mean percentage recoveries of TAD based on an average of five replicate measurements was found to be equal to 100.3 and 100.9, MWCNTPE and TiO2-MWCNTPE, respectively (Table 5). The results are obviously accurate and precise.
Conclusions
In the presented work, the electrochemical behavior of TAD is investigated by CV and AdSSWV using MWCNTPE and TiO2-MWCNTPE. The analytical performances of TiO2-MWCNT were better than the bare MWCNTP electrode. The peak current of TAD at TiO2-MWCNTPE using AdSSWV was 2.6-fold higher than that of TAD at MWCNTPE using SWV. TiO2–MWCNT composites with good oxidation catalysis for TAD would promote the ability of the electrode to eliminate the interferences from some cations and bio-molecules. The possible electrooxidation pathway and the number of transferred electrons were investigated as details. From the CV curves, the voltammetric behaviors of indole derivatives, which are structurally related to TAD, were determined, and it was postulated that the anodic peak was due to the oxidation of this group. This method provides a new way to construct a modified electrode for sensitive and selective determination of TAD from tablet dosage forms and human serum samples compared with MWCNTPE. A simple and convenient precipitation procedure makes this proposed method more feasible for the determination of TAD in human serum. The applicability of this method has been demonstrated by successfully analyzing the serum samples of the clinical study. The developed methods provide a selective, fast, experimentally convenient, cost-effective, high-throughput, and simple approach to the determination of TAD in tablet dosage forms and human serum, without the necessity of sample pretreatment or any time-consuming extraction and evaporation steps prior to the analysis. When comparing with the already published methods (Table 4), the proposed method shows the similar sensitivity, determination limit, and linearity ranges. But some of the published methods, especially liquid chromatography (LC)-MS and fluorimetric methods, are more sensitive than our proposed method. However, the proposed electroanalytical assay has many advantages such as simplicity, selectivity, less solvent consumption, and the lack of extraction processes. The proposed assay might be an alternative to the LC techniques in therapeutic drug monitoring, or the experimental data might be used for the development high-performance liquid chromatography-electrochemical detection method. Furthermore, the presented method could possibly be adopted for pharmacokinetic studies, as well as clinical and quality control laboratories.
References
Forgue ST, Patterson BE, Bedding AW, Payne CD, Phillips DL, Wrishko RE, Mitchell MI (2006) Br J Clin Pharmacol 61:280–288
Mehanna MM, Motawaa AM (2012) J AOAC Int 95:1064–1068
Mohammad Y, Kumar BP, Sreenivas R, Gupta R (2010) J Chem Pharm Sci 3:258–261
Barot TG, Patel PK (2010) J AOAC Int 93:516–522
Kannappan N, Yada D, Yada D, Shashikanth MR (2010) Int J Chem Tech Res 2:329–333
Mathpati MM, Sangshetti JN, Rane VP, Patil KR, Shinde DB (2009) Chem Anal 54:679–689
Subba Rao DV, Radhakrishnanand P, Himabindu V (2008) Chromatographia 67:183–188
Rabbaa-Khabbaz L, Daoud RA (2006) J Appl Res 6:170–175
Cheng CL, Chou CH (2005) J Chromatogr B 822:278–284
Aboul-Enein HY, Ali I (2005) Talanta 65:276–280
Farthing CA, Farthing DE, Koka S, Larus T, Fakhry I, Xi L, Kukreja RC, Sica D, Gehr TW (2010) J Chromatogr B 878:2891–2895
Karavadi T, Challa BR (2012) Der Pharm Lettre 4:1401–1413
Nikolaou P, Papoutsis I, Athanaselis S, Alevisopoulos G, Khraiwesh A, Pistos C, Spiliopoulou C (2011) J Pharm Biomed Anal 56:577–581
Flores JR, Nevado JJB, Penalvo GC, Diez NM (2004) J Chromatogr B 811:231–236
Ali I, Aboul-Enein HY (2004) Chromatographia 60:187–191
Yunoos M, Sankar DG, Kumar BP, Hameed S (2010) E-J Chem 7:833–836
Lakshmi VN, Kumar DR, Vardhan SVM, Rambabu C (2009) Orient J Chem 25:791–794
Rajput SJ, Patel SG (2008) Indian Drugs 45:490–492
Welch CM, Compton RG (2006) Anal Bioanal Chem 384:601–619
Dogan-Topal B, Bozal-Palabıyık B, Uslu B, Ozkan SA (2013) Sensors Actuators B Chem 177:841–847
Ozkan SA (2012) Electroanalytical methods in pharmaceutical analysis and their validation. HNB Publishing, New York
Jorio A, Dresselhaus G, Dresselhaus MS (2008) Carbon nanotubes: advanced topics in the synthasis, structure, properties and applications. Springer, Berlin
Alexander M, Pandian K (2013) J Solid State Electrochem 17:1117–1125
Zhang DW, Li XD, Chen S, Tao F, Sun Z, Yin XJ, Huang SM (2010) J Solid State Electrochem 14:1541–1546
Li H, Li M, Guo W, Di H, Fang C, Yang B (2014) J Solid State Electrochem 18:477–485
Perenlei G, Tee TW, Yusof NA, Kheng GJ (2011) Int J Electrochem Sci 6:520–531
Zhang J, Tan X, Zhao D, Tan S, Huang Z, Mi Y, Huang Z (2010) Electrochim Acta 55:2522–2526
Majidi MR, Baj RFB, Naseri A (2013) Cent Eur J Chem 11:1172–1186
Zhua Z, Lib X, Wangb Y, Zenga Y, Sunb W, Huanga X (2010) Anal Chim Acta 670:51–56
Sun W, Jiang Q, Wang Y, Jiao K (2009) Sensors Actuators B 136:419–424
Zhang X, Ju H, Wang J (2008) Electrochemical sensors, biosensors and their biomedical applications, 1st edn. Elsevier, New York
Ozkan SA, Uslu B, Zuman P (2004) Anal Chim Acta 501:227–233
Zuman P (2006) J Solid State Electrochem 10:841–851
Stefan-van Staden R, Van Staden JF, Aboul-Enein HY (2010) J Solid State Electrochem 14:997–1000
Ghoneim MM, Hassanein AM, Salahuddin NA, El-Desoky HS, Elfiky MN (2013) J Solid State Electrochem 17:1891–1902
Laviron E, Roullier L, Degrand CJ (1980) J Electroanal Chem 112:11
Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanisms. Wiley-VCH, USA
Compton RG, Banks CE (2007) Understanding voltammetry. World Scientific, London
Beltagi AM, Abdallah OM, Ghoneim MM (2008) Talanta 74:851–859
Karadas N, Sanli S, Gumustas M, Ozkan SA (2012) J Pharm Biomed Anal 66:116–125
Wang J (1988) Electroanalytical techniques in clinical chemistry and laboratory medicine. VCH Publishers, New York
Kissinger PT, Heineman WR (1996) Laboratory techniques in electroanalytical chemistry, 2nd edn. Marcel Dekker, New York
Hart JP (1990) Electroanalysis of biologically important compounds. Ellis Horwood Ltd, England
Ozkan SA, Uslu B, Aboul-Enein HY (2003) Crit Rev Anal Chem 33:155–181
Grimshaw J (2002) Electrochemical reactions, mechanism in organic chemistry. Elsevier Sci Pub Inc, New York
Lund H, Hammerich O (2001) Organic electrochemistry, 4th edn. Marcel Dekker Inc Pub, New York
Greef R, Peat R, Peter LM, Pletcher D, Robinson J (1990) Instrumental methods in electrochemistry. Ellis Horwood, England
Goyal RN, Kumar N, Singhal NK (1998) Bioelectrochem Bioenerg 45:47–53
Jennings P, Jones AC, Mount AR, Thomson AD (1997) J Chem Soc Faraday Trans 93:3791–3797
Suzen S, Demircigil BT, Buyukbingol E, Ozkan SA (2003) New J Chem 27:1007–1011
Kul D, Gumustas M, Uslu B, Ozkan SA (2010) Talanta 82:286–295
Enache TA, Olivera-Brett AM (2011) Electroanalysis 23:1337–1344
Dogan B, Tuncel S, Uslu B, Ozkan SA (2007) Diam Relat Mater 16:1695–1704
Yola ML, Atar N, Qureshi MS, Üstündag Z, Solak AO (2012) Sensors Actuators B Chem 171:1207–1215
Acknowledgments
We would like to thank Gazi University for supporting to this project (Grant No. BAP-05/2013-1) financially.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Demir, E., Inam, R., Ozkan, S.A. et al. Electrochemical behavior of tadalafil on TiO2 nanoparticles–MWCNT composite paste electrode and its determination in pharmaceutical dosage forms and human serum samples using adsorptive stripping square wave voltammetry. J Solid State Electrochem 18, 2709–2720 (2014). https://doi.org/10.1007/s10008-014-2529-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-014-2529-5