Abstract
The flake-like Li3V2(PO4)3/C has been successfully synthesized by rheological phase method using polyvinyl alcohol (PVA) as template; the Li3V2(PO4)3/C without PVA assistance has been prepared for comparison. X-ray diffraction analysis shows that the two samples are well crystallized, and no impurity phases are detected. The scanning electron microscopy results reveal that there is a significant difference in morphologies between PVA-assisted sample and sample without PVA; the former shows a flake-like morphology, while the latter presents regular granular shape with some agglomeration. Transmission electron microscopy images reveal that Li3V2(PO4)3 particles are coated with a uniform surface carbon layer. The lattice fringes with a spacing of 0.428 nm can be clearly seen from the high-resolution transmission electron microscopy image. The PVA-assisted sample shows a discharge capacity of 120, 110, and 96 mAh g−1 at 1 C, 20 C, and 50 C, respectively; however, the sample without PVA exhibits a lower discharge capacity. Based on the analysis of electrochemical impedance spectroscopy, the lithium ion diffusion coefficients of Li3V2(PO4)3/C and PVA-assisted Li3V2(PO4)3/C are 4.19 × 10−9 and 4.99 × 10−8 cm2 s−1, respectively. In summary, it is demonstrated that using PVA as a template can obtain flake-like morphology and significantly improve the comprehensive electrochemical performances of Li3V2(PO4)3/C cathode material.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Because the LiCoO2 cathode material has the intrinsic drawbacks of being expensive and noxious, Li transition metal phosphate materials such as LiFePO4 [1, 2], LiMnPO4 [3], LiVPO4F [4], and Li3V2(PO4)3 [5–7] have attracted much attention to acting as alternative cathode materials for lithium ion batteries. Among these materials, monoclinic structure Li3V2(PO4)3 has the advantages of having large theoretical capacity, good cryogenic property, and high operating voltage. However, Li3V2(PO4)3 suffers low electronic conductivity which limits the high-rate performance and hinders its wide application. A variety of efforts has been made to increase the electronic conductivity, such as carbon coating [8–10], metal doping [11], and particle size reduction [12]. It is confirmed that conductive carbon coating is the most efficient way [13]. Carbon coating on the surface of Li3V2(PO4)3 particles can not only enhance the electronic conductivity and suppress the particle agglomeration in the calcination procedure but also prevent the cathode material from directly contacting with the electrolyte solution, thus avoiding vanadium ions dissolving in the electrolyte [14, 15]. As far as we know, the influence of the surface carbon depends greatly on the type of carbon source and the synthesis method. Researchers usually choose the organic or polymer carbon sources for the reason of better dispersal ability, such as citric acid [16], oxalic acid [17], glucose [18], sucrose [19], stearic acid [20], ascorbic acid [21], polyvinyl alcohol [7], sugar [22], and so on. Rheological phase method [23] is an economical and effective soft chemical method that has no long time-consuming and any troublesome processes for preparing compounds or materials from a solid–liquid rheological mixture. Besides, the key of this method is a liquid–solid rheological body including the solid particles and liquid molecules which are uniformly distributed. The surface area of the solid particles can be utilized efficiently due to the close and uniform contact between solid particles and fluid. However, no studies have yet been reported about the synthesis of the Li3V2(PO4)3/C by using stearic acid as carbon source and polyvinyl alcohol (PVA) as template via rheological phase method.
In this work, a novel rheological phase method using PVA as a template has been introduced to synthesize Li3V2(PO4)3/C composites and their electrochemical performances, and especially the high-rate charge–discharge property has been investigated.
Experimental
Preparation of the Li3V2(PO4)3/C composites
Li3V2(PO4)3/C composites were prepared via a novel rheological phase method by using LiH2PO4, with V2O5 as the starting materials. Stearic acid was employed as carbon source, and the residual carbon decomposition from it during calcination process could be the reductant. The polyvinyl alcohol (PVA-1799) acted as a template reagent. The stearic acid and PVA also played an important role in dispersing the raw materials. Stoichiometric amounts of LiH2PO4, V2O5, and 4 wt% PVA were mixed together in proper ethanol by ball milling for 1 h in a high-speed vibrating ball miller (SFM-3, 1,200 rpm, MTI Corporation) in order to get a rheological body. After that, 24.5 wt% stearic acid which was dissolved in ethanol was added to the rheological body, and ball milling was continued for another 1 h to get the pasty precursor. The obtained pasty precursor was dried at 90 °C for 6 h in an air-dry oven to evaporate the ethanol. The dried precursor was reground and finally calcined at 700 °C for 4 h in a tube furnace under Ar atmosphere to obtain the Li3V2(PO4)3/C composites. The Li3V2(PO4)3/C without PVA assistance was prepared by the same process for comparison. The Li3V2(PO4)3/C without PVA assistance was named sample A, and the PVA-assisted Li3V2(PO4)3/C was named sample B.
Material characterizations
Thermogravimetry–derivative thermogravimetry–differential thermal analysis (TG-DTG-DTA) of the precursor was measured by TG-209F1 device at the temperature range from 30 to 1,000 °C at the heating rate of 10 °C min−1 under a N2 flow of 20 ml min−1. The residual carbon content of the material was tested by CS-902 analytical device. The structure and crystalline parameters were analyzed by the X-ray diffraction (XRD, D/max-rB, Rigaku, by using Cu Kα radiation with λ = 0.15046 nm). The morphology and size of Li3V2(PO4)3/C particles were observed by scanning electron microscopy (SEM, SPA400 Seiko Instruments). The microscopic structure of the as-prepared sample was characterized by transmission electron microscope (TEM) and high-resolution transmission electron microscope (HRTEM) (JEM-2100, JEOL, Japan).
The battery preparation and electrochemical measurement
The working cathode is made up of 80 wt% active material (prepared Li3V2(PO4)3/C powder), 13 wt% acetylene black that worked as the conducting agent, and 7 wt% polyvinylidene fluoride which served as the binder, and an appropriate amount of N-methyl-2-pyrrolidine was used as solvent. The obtained slurry was spread uniformly onto a thin and smooth aluminum foil and then dried at 100 °C for 10 h in a vacuum and cut into slices. The lithium metal foil was used as a counter electrode, Celgard 2400 as the separator, and 1 M LiPF6 dissolved in a mixture of ethylene carbonate and dimethyl carbonate of 1:1 in volume as electrolyte. The cells were assembled in a glove box filled with high-purity argon. The calculated total mass loading for the electrodes are about 2.0 mg cm−2. The charge–discharge measurements were recorded in the range of 3.0∼4.5 V under different current densities at room temperature by employing a battery test system (NEWARE, China). Cyclic voltammetry (CV) was performed on a CHI660B electrochemical workstation at a scanning rate of 0.1 mV s−1 under the voltage range of 3.0∼4.5 V. The electrochemical impedance spectroscopy (EIS) was measured by the as-prepared cells and recorded on the same electrochemical workstation over a frequency range from 100 kHz to 10 mHz with an AC amplitude of 5 mV.
Results and discussions
Material characterizations
Figure 1 shows the TG-DTG-DTA curves of sample B precursor from 30 to 1,000 °C. About 33.56 % weight loss of the precursor is observed from 30 to 700 °C. With the temperature increasing, low weight loss appears that it can be entirely neglected. According to the weight loss derivative curve, the weight loss curve can be sketchily divided into three parts. The first part below 160 °C is due to the release and evaporation of the adsorbed water. In previous reports, the pyrolysis of PVA occurred at 160 °C in nitrogen atmosphere and starts to abruptly decompose at 280 °C [24]. The second part between 160 and 400 °C is attributed to the volatilization and further thermal decomposition of stearic acid, which leads to the formation of reductive carbon, as well as the continuous elimination of hydrogen atom and oxygen atom, causing the pyrolysis of partial PVA and forming the conjugated double bond polymer. When the precursor is heated between 400 and 700 °C, a series of complex reactions take place. This period contains the transformation of V5+ to V3+ by the reduction of carbon at the high temperature inside inert atmosphere, along with the release of carbon oxides as well. The well-crystallized Li3V2(PO4)3/C composites are obtained from the reaction of lithium source, vanadium source, phosphate source, and residual carbon. Therefore, based on the results of TG-DTG-DTA, 700 °C is chosen as the calcination temperature.
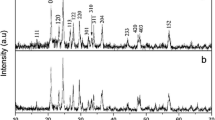
Figure 2 shows the XRD patterns for the as-prepared samples—sample A and sample B. They have the same phase, and no impurity phases are detected. The sharp diffraction peaks indicate that both samples are well crystallized, and they can be indexed well on the basis of monoclinic Li3V2(PO4)3 with a space group of P21/n [PDF no. 47-0107], which are consistent with the previous reports [25, 26]. The refinement structural parameters of sample A and sample B are summarized in Table 1, in which a slight difference exists. Element analysis of the final products reveals that the carbon content of sample A and sample B are 3.05 and 3.18 %, respectively. However, there are no obvious diffraction peaks detected in relation to carbon, which indicates that the residual carbon from stearic acid or PVA is in amorphous state, or the carbon content coating on Li3V2(PO4)3 particles is too small to be detected.
Figure 3 shows the SEM images of sample A and sample B. The morphologies of sample A and sample B have a significant difference. It can be clearly observed that the particles of sample A present regular granular shape with some agglomeration. Moreover, the particle size distribution is narrow which ranges from 200 to 500 nm approximately. Sample B which is assisted with PVA has a flake-like morphology, and the lengths of the flakes are not uniform with the range of 1 to 2 μm; nevertheless, the shorter thicknesses of the flakes are 100 to 300 nm.
Figure 4 shows the TEM, high-resolution TEM (HRTEM), and the selected area electron diffraction (SAED) images of the as-prepared sample B. As shown in Fig. 4a, b, it can be clearly seen that the Li3V2(PO4)3 particles are coated with a uniform surface carbon layer. The surface carbon layer is favorable in enhancing conductivity and forming an efficient barrier to alleviate vanadium dissolution in the electrolyte solution. Figure 4c shows the HRTEM image taken from an individual Li3V2(PO4)3 particle; it can be seen that the lattice fringes appear clearly, indicating that the sample is well crystallized. The observed lattice fringes with a spacing of 0.428 nm corresponding to the (1 2 1) plane clearly confirm the monoclinic Li3V2(PO4)3 structure, which agrees well with the results of XRD. In order to show well the crystallinity of the as-prepared sample, the SAED image is presented in Fig. 4d, which indicates single crystal nature of Li3V2(PO4)3 nanoparticles in the Li3V2(PO4)3/C composites.
From the results of SEM and TEM images, sample A presents a regular granular shape with some agglomeration, while sample B which is assisted with PVA has a flake-like morphology. Many literatures have reported that PVA is a soft template which is widely used in controllable synthesis of nanoparticles [27, 28]; we can make a bold conjecture that PVA acting as a template plays an important role in changing the morphology of the Li3V2(PO4)3/C. PVA has a structure of planar zigzag; what is more, the hydroxyl of PVA can chelate vanadium (V2O5) and form a complex compound. When LiH2PO4, V2O5, and PVA are mixed together in proper ethanol, the starting materials (solid particles, namely LiH2PO4, V2O5, and PVA) can be dispersed efficiently by ethanol (fluid) in the rheological body. The surface area of the starting materials is completely naked to each other, which is a benefit to the reaction. And then, PVA reacts with V2O5 to form a complex compound, and LiH2PO4 attaches to the complex compound and self-assembles. After that, the stearic acid molecules are self-assembled homogeneously on the surface of the PVA template. Finally, the flake-like Li3V2(PO4)3/C sample is obtained by high-temperature calcination via the rheological phase method. PVA plays a role of the template that controls the synthesis of nanoparticles and directs the growth orientation of Li3V2(PO4)3 nano-flakes. The aforementioned product formation mechanism is simply presented in the schematic drawing, as shown in Fig. 5.
Electrochemical measurements
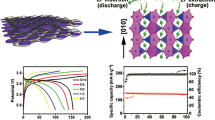
Figure 6 shows the initial charge/discharge curves at 0.1 C in the voltage range of 3.0∼4.5 V and cycling performance for sample A and sample B at different discharge rates. As shown in Fig. 6a, the curves present three pairs of charge/discharge plateaus around 3.60/3.55, 3.68/3.65, and 4.10/4.05 V, respectively, which correspond to a series of phase transformations of Li3V2(PO4)3. The first two pairs of charge/discharge plateaus correspond to the reactions from Li3V2(PO4)3 to Li2V2(PO4)3 that an intermediate phase Li2.5V2(PO4)3 exists in the reaction. The intercalation/deintercalation of the second lithium ion takes place on the last pair of charge/discharge plateau with V3+ completely oxidized to V4+. The potential differences of each pair of charge/discharge plateaus are very small that can even be neglected, indicating small electrode polarizations; in other words, the transportation of electrons and the diffusion of lithium ions in the electrodes goes through easily. As shown in Fig. 6b, it can be seen fairly clear that the two samples possess large discharge capacity and little capacity fading at low discharge rate. For sample B, it delivers a discharge capacity of 129 mAh g−1 with the coulomb efficiency of 98.8 % at 0.1 C; however, for sample A, it is only 126 mAh g−1 with the coulomb efficiency of 97.3 % at the same rate. In Fig. 6c, the middle and high-rate capability of sample A and sample B are further compared in the voltage range of 3.0∼4.5 V. The sample B shows a discharge capacity of 120, 116, 110, 108, and 102 mAh g−1 at 1 C, 10 C, 20 C, 30 C, and 40 C, respectively; however, sample A has a lower discharge capacity of 119, 93, 84, 75, and 57 mAh g−1 to the corresponding rate. At the rate of 50 C, the sample B delivers a discharge capacity of 96 mAh g−1, which keeps 73.28 % of the theoretical capacity. Relatively, sample A delivers a lower discharge capacity of 34 mAh g−1 and about 25.95 % of the theoretical capacity than the PVA-assisted sample. What is more, sample A and sample B deliver a discharge capacity of 113 and 119 mAh g−1, respectively, when the rate returns to 1 C again after cycles at 50 C, demonstrating that high-rate cycling does not destroy the crystal structure, and the composites have the excellent reversibility. The further study on rate performance for the two samples at C-rate varying from 0.1 C to 50 C is displayed in Fig. 6d. Clearly, along with increasing discharge rate, sample B delivers a better discharge capacity and less attenuation ratio than sample A. The sample using PVA as a template presents a flake-like morphology and provides short diffusion lengths (about 100 to 300 nm) for electrons and lithium ions, which are believed to be helpful to the electrochemical performance.
a Initial charge/discharge curves for sample A and sample B at 0.1 C in the voltage range of 3.0∼4.5 V. b Cycling performance at low discharge rate of sample A and sample B. c Cycling performance at middle and high discharge rate of sample A and sample B. d Rate performance for sample A and sample B at C-rate varying from 0.1 C to 50 C
Figure 7 shows the CV curves of sample A and sample B under the voltage range of 3.0∼4.5 V at the scanning rate of 0.1 mV s−1. It can be clearly seen that the CV curves of sample A and sample B are so similar. They both have three pairs of sharp redox peaks; corresponding to the first two lithium ions are inserted/extracted reversibility from the lattice of monoclinic Li3V2(PO4)3, which agree well with the charge–discharge curves in Fig. 6a. However, there are some differences between the two samples. Sample B has smaller potential distance between the cathodic peaks and the corresponding anodic peaks and higher current peak intensity than sample A, which demonstrate the better cycle reversibility and more wonderful kinetics properties.
Electrochemical impedance spectroscopy (EIS) measurements are carried out to discuss the effect of adding PVA or not. What is more, EIS is considered to be a powerful technique to evaluate the diffusion coefficient of lithium ion [29], for the reason that the Warburg impedance in the low frequency is directly related to the lithium ion diffusion process in an electrode material [30]. Figure 8 shows the Nyquist plots of sample A and sample B, and the equivalent circuit used for imitating the experimental impedance data is shown in the inset. On the basis of the view of Barsoukov et al. [31], an intercept of the Z′-axis in the ultra-high-frequency region accords with the ohmic resistance (R 1), combining with the resistance of lithium ions and electrons getting through the electrolyte. The semicircle in the high-frequency region is the resistance that lithium ions get through the insulating layer of active material, R 2 is the resistance of solid electrolyte interface (SEI), and CPE1 is the capacitance between SEI films. The semicircle in the middle-frequency region represents charge transfer resistance (R 3), the so-called electrochemical reaction resistance is in this zone as well, and CPE2 is the electric double-layer capacity. The sloping line in the low-frequency region indicates the Warburg resistance (W 1), which is related to the diffusion of lithium ion in material particle [32].
In Fig. 8a, it can be seen clearly that R 3 of sample B is smaller than that in sample A, which indicates the contact resistance of Li3V2(PO4)3 particles is smaller; it may be the reason that sample B has higher rate capability. The fitting impedance parameters according to the equivalent circuit and the diffusion coefficient of lithium ions are presented in Table 2.
The diffusion coefficients of lithium ions can be calculated by using the formula [29, 33] as follows:
In this formula, T is the absolute temperature, R is the gas constant, n is the number of charge transfer, A is the surface area of cathode, F is the Faraday constant, C is the concentration of lithium ions, and σ is the Warburg constant which can be calculated by the relation of frequency and impedance, obeying the following formula:
The ω is the angle frequency. According to this formula, the slope σ is obtained. The lithium ion diffusion coefficients of the two samples can be calculated by utilizing this σ in the first formula. The lithium ion diffusion coefficients of sample A and sample B are 4.19 × 10−9 and 4.99 × 10−8 cm2 s−1, respectively. It is well known that the larger lithium ion diffusion coefficient values, the easier they lead to electrochemical kinetics with Li+ extraction. In other words, sample B has better lithium ion diffusion property, leading to faster Li+ diffusion and better electrochemical performance.
Conclusions
The PVA-assisted Li3V2(PO4)3/C was obtained by rheological phase method, and Li3V2(PO4)3/C without PVA assistance was prepared for comparison. The SEM results show that the sample using PVA as a template displays a flake-like morphology with a thickness of 100∼300 nm, which can provide short diffusion lengths and benefit for the transformation of electrons and lithium ions. A uniform surface carbon layer is coated on the surface of Li3V2(PO4)3 particles according to the TEM images. And it also can be clearly seen from the HRTEM image that the lattice fringes with a spacing of 0.428 nm corresponding to the (1 2 1) plane. It is demonstrated that the PVA-assisted sample achieves an outstanding rate capability, which delivers a discharge capacity of 120, 110, and 96 mAh g−1 in the voltage range of 3.0∼4.5 V at 1 C, 20 C, and 50 C rates, respectively. However, the sample without PVA owning a regular granular shape exhibits a lower discharge capacity. The EIS results show that the PVA-assisted sample has smaller charge transfer resistance and bigger lithium ion diffusion coefficient compared with the sample without PVA. In summary, the sample using PVA as a template can effectively shorten diffusion lengths for electrons and lithium ions by controlling sizes and morphologies and make better the comprehensive electrochemical properties.
References
Liang GC, Wang L, Ou XQ, Zhao X, Xu SZ (2008) J Power Sources 184:538–542
Tang H, Guo XD, Tang Y, Zhong BH, Li LY (2012) Chin J Inorg Chem 28:809–814
Damen L, Giorgio FD, Monaco S, Veronesi F, Mastragostino M (2012) J Power Sources 218:250–253
Zhong SK, Chen W, Li YH, Zou ZG, Liu CJ (2010) Trans Nonferrous Metal Soc 20:s275–s278
Wang LJ, Liu HB, Tang ZY, Ma L, Zhang XH (2012) J Power Sources 204:197–199
Yan J, Yuan W, Xie H, Tang ZY, Mao WF, Ma L (2012) Mater Lett 71:1–3
Wei JW, Zhang XF, Liu J, Yang GL, Ge YC, Yu ZJ, Wang RS, Pan XM (2010) Electrochim Acta 55:6879–6884
Li YJ, Hong L, Sun JQ, Wu F, Chen S (2012) Electrochim Acta 85:110–115
Jian ZL, Zhao L, Wang R, Hu YS, Li H, Chen W, Chen LQ (2012) RSC Adv 2:1751–1754
Ding ZJ, Zhao L, Suo LM, Jiao Y, Meng S, Hu YS, Wang ZX, Chen LQ (2011) Phys Chem Chem Phys 13:15127–15133
Yao JH, Wei SS, Zhang PJ, Shen CQ, Aguey-Zinsou KF, Wang LB (2012) J Alloys Compd 532:49–54
Hao WJ, Zhan HH, Yu J (2012) Mater Lett 83:121–123
Rui XH, Li C, Chen CH (2009) Electrochim Acta 54:3374–3380
Fu P, Zhao YM, An XN, Dong YZ, Hou XM (2007) Electrochim Acta 52:5281–5285
Zhang LL, Liang G, Peng G, Zou F, Huang YH, Croft MC, Ignatov A (2012) J Phys Chem 116:12401–12408
Chen QQ, Wang JM, Tang Z, He WC, Shao HB, Zhang JQ (2007) Electrochim Acta 52:5251–5257
Pan AQ, Liu J, Zhang JG, Xu W, Cao GZ, Nie ZM, Arey BW, Liang SQ (2010) Electrochem Commun 12:1674–1677
Wang L, Zhang LC, Lieberwirth I, Xu HW, Chen CH (2010) Electrochem Commun 12:52–55
Jiang T, Du F, Zhang KJ, Wei YJ, Li Z, Wang CZ, Chen G (2010) Solid State Sci 12:1672–1676
Qiao YQ, Wang XL, Xiang JY, Zhang D, Liu WL, Tu JP (2011) Electrochim Acta 56:2269–2275
Huang JS, Yang L, Liu KY (2011) Mater Chem Phys 128:470–474
Jian ZL, Zhao L, Pan HL, Hu YS, Li H, Chen W, Chen LQ (2012) Electrochem Commun 14:86–89
Chang CX, Xiang JF, Shi XX, Han XY, Yuan LJ, Sun JT (2008) Electrochim Acta 53:2232–2237
Nakajima C, Saito T, Yamaya T, Shimoda M (1998) Fuel 77:321–326
Wang LJ, Tang ZY, Ma L, Zhang XH (2011) Electrochem Commun 13:1233–1235
Pan AQ, Choi D, Zhang JG, Liang SQ, Cao GZ, Nie ZM, Arey BW, Liu J (2011) J Power Sources 196:3646–3649
Xie RM, Xiao DB, Yao JN (2002) Acta Phys Chim Sin 18:34–38
Jiu JT, Li LP, Ge Y, Zhang SR, Tu F, Hua ZR, Nie L (2001) Chin J Inorg Chin J Inorg Chem 17:361–365
Du XY, He W, Zhang XD, Yue YZ, Liu H, Zhang XG, Min DD, Ge XX, Du Y (2012) J Mater Chem 22:5960–5969
Zeng RH, Li WS, Lu DS, Huang QM (2007) J Power Sources 174:592–597
Zhuang QC, Xu SD, Qiu XY, Cui YL, Fang L, Sun SG (2010) Process Chem 22:1044–1057
Barsoukov E, Kim JH, Yoon CO, Lee H (1998) J Electrochem Soc 145:2711–2717
Cui Y, Zhao XL, Guo RS (2010) Electrochim Acta 55:922–926
Acknowledgments
This work was supported by the Sichuan University Funds for Young Scientists (no. 2011SCU11081) and the Research Fund for the Doctoral Program of Higher Education, the Ministry of Education (no. 20120181120103).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Tang, Y., Zhong, B. et al. Facile synthesis of Li3V2(Po4)3/C nano-flakes with high-rate performance as cathode material for Li-ion battery. J Solid State Electrochem 18, 215–221 (2014). https://doi.org/10.1007/s10008-013-2249-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-013-2249-2