Abstract
In recent years, special attention has been focused on the development of gel polymer electrolytes consisting of host polymers such as poly (acrylonitrile), PVC, poly (methyl methacrylate), poly (vinylidene fluoride) (PVdF), etc., as they may be found to have unique applications in consumer electronic and electric vehicle products. In the present study, blend-based polymer electrolytes composed of PVC, PVdF, NaClO4, and propylene carbonate is prepared using the solvent casting technique. The thermal behaviors of PVC/PVdF polyblend films have been examined using differential scanning calorimetry and scanning electron microscopy. Miscibility studies were performed using X-ray diffraction and Fourier transform infrared. The role of interaction between polymer hosts on conductivity is discussed using the results of AC impedance studies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most suitable battery type for customers, a battery that contains a large amount of energy in a small package, is light in weight and safe to use. The characterization of ionically conducting polymeric membranes has provided the interesting possibility of developing new types of lithium batteries. Today, lithium batteries are a valid candidate for the consumer electronic market and for electronic vehicles as a replacement for nickel-cadmium and lead-acid batteries. Gel polymer electrolytes (GPE), such as poly (acrylonitrile), poly (methyl methacrylate) (PMMA), and poly (vinylidene fluoride) (PVdF), are considered as promising candidates for high-energy electrochemical devices [1–7]. The modification of PVdF-based polymer electrolytes has been explored by blending with PMMA and poly (vinyl alcohol-co-vinyl acetate) [8–11]. A large number of studies have been carried out on polymer electrolytes based on PMMA containing the alkali metal salts. However, these materials have a major drawback—the ionic conductivity of 10−4 S/cm, which is necessary for high-power applications, can only be reached at about 100 °C. In this respect, most research works have been directed to the synthesis and characterization of new polymer electrolytes that exhibit higher ionic conductivity at ambient temperature via various approaches, such as using blends [12, 13], copolymers [14], comb branch polymers [15], and cross-linked networks [16, 17]. All these enhancements have been achieved either by reducing the crystallinity of polymers or by lowering the glass transition temperature (TG). However, it is generally observed that high conductivity is achieved at the expense of good dimensional stability.
For the present work, we have adopted one of the above techniques, namely, blending because of the ease of preparation and easy control of physical properties within the compositional regime, which thus reduces development costs. The inherent merits of using blend-based polymer electrolytes have been exemplified by several research groups [12, 13, 18]. Detailed studies of the blend-based polymer electrolytes can furnish valuable information on the relative importance of various factors, which affect the electrical, thermal, and mechanical properties of the polymer electrolytes.
The conductivity of solid polymer electrolytes (SPEs) can be enhanced by using polymer hosts with low TGs (T g). A variety of PVC-based gels consisting of solutions of lithium salts in propylene carbonate (PC)–ethylene carbonate mixtures was reported by Scrosati [19]. Due to its good optical properties, PVC becomes a good candidate for nonlinear optical applications in communication technology when it is blended with semicrystalline PVdF. PVdF-based SPEs are expected to have high anodic stabilities [20] due to the strongly electron-withdrawing functional groups. PVdF has high permittivity, a relatively low dissipation factor, and a high dielectric constant, which should assist in greater ionization of sodium salts, providing a high concentration of charge carriers. Choe et al. [21] reported two-phase polymer blend GPE, which includes a first phase—including one polymer for absorbing the electrolyte solution—and a second phase—including one polymer for enhancing the mechanical integrity of the polymer blend. Oliver [22] reported the kinetics, structural transitions, and dielectric behavior of P (VdF–TrFE)/PMMA blends. Stephan et al. [23] reported the ionic conductivity behavior of plasticized PVC/PMMA blends containing LiBF4 and LiClO4 as electrolyte salts.
In the present study, the preparation, ionic conductivity, and mechanism of ionic conduction of hybrid films composed of sodium per chlorate (NaClO4) and PVC/PVdF intermacromolecular complexes are discussed.
Experimental
High-purity PVC with an average molecular weight of 1×105 and PVdF with an average molecular weight 5.3×105 (both obtained from Aldrich) were used after drying. NaClO4 was used after drying in a vacuum oven at 373 K for 5 h. The PC (Aldrich) used in the preparation of the polymer electrolyte was of the highest quality and was used as received. The solvent, tetrahydrofuran (THF) from E-Merck, was used after distillation. Appropriate amounts of PVC and PVdF were dissolved in anhydrous THF. After complete dissolution, the sodium salt NaClO4 and the plasticizer PC were added to the mixed polymer solution. The slurry was stirred vigorously for 12 h. The blend GPE was prepared by casting the slurry onto a glass plate, then left to evaporate the solvent slowly at ambient temperature. The resulting films were then dried in a vacuum at 60 °C for 2 h. The samples were obtained in the thin-film form. The composition details of the samples are shown in Table 1.
The X-ray diffraction (XRD) patterns of the films were made with a HZG4/B-PC X-ray diffractrometer with CoKα radiation and graphite monochrometer. Fourier transform infrared (FTIR) absorption spectra of the films were recorded using a 60-SXB IR spectrometer with a resolution of 4 cm−1. The measurements were taken over a wave number range of 400–4,000 cm−1. For the differential scanning calorimetry (DSC) measurements, a Netzsch STA 409 PC, operating in dynamic mode (heating rate=10 K/min), was employed. Samples with weights of ≈5 mg were placed in sealed aluminum pans. Prior to use, the calorimeter was calibrated with metal standards, with an empty aluminum pan being used as a reference. The morphology of the samples was characterized by a JSM-5610LV scanning electron microscope (SEM). The AC impedance measurements of the polymer electrolytes were performed using an Agilent 4294A precision impedance analyzer in the range from 40 Hz to 100 kHz and in the temperature range 298–373 K. The bulk resistance (R p) determined from the equivalent circuit analysis by using frequency response analyzer software. The conductivity values (σ) were calculated from the equation σ=(1/R p)(t/A), where t is the thickness and A is the area of the sample.
Results and discussion
XRD studies
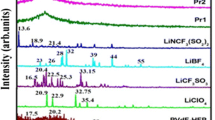
Figure 1 shows the XRD patterns of NaClO4 salt, (PVC+PC+NaClO4), (PVdF+PC+NaClO4), and (PVC+PVdF+PC+NaClO4) polymer electrolytes of different compositions. The XRD patterns of (PVC+PC+NaClO4) and (PVC+PVdF+PC+NaClO4) systems show (Fig. 1a,b) low crystallinity. In contrast, NaClO4 is found to be crystalline (Fig. 1e). The peaks observed at 2θ=18°, 20° for pure PVdF, disappear in the complex (Fig. 1c,d). This may be due to the effect of the addition of PVC, which reduces the long-range order in PVdF. The new peaks are observed at 2θ=40° and 55°; this may be due NaClO4 salt effect. The absence of strong crystallinity peaks of NaClO4 salt on XRD patters of complexed polyblend films confirmed the miscibility of NaClO4 salt in the complexed polyblend electrolyte systems.
FTIR studies
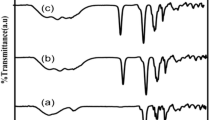
IR spectra of polymer complexes are shown in Fig. 2. The vibrational peaks of PVC (2,918, 1,793, 1,641, 1,090, and 970 cm−1), PVdF (1,638, 1,405, and 877 cm−1), and NaClO4 (3,552, 3,415, 1,638, 1,144, 1,089, and 941 cm−1) are shifted to 2,911, 1,791, 1,634, 1,088, and 976 cm−1; 1,634, 1,403, and 874 cm−1; and 3,593, 3,414, 1,634, 1,183, 1,088, and 976 cm−1, respectively. The absorption peaks of PVC (3,582, 2,956, 1,457, and 1,254 cm−1), PVdF (3,550, 3,026, 1,727, 1,618, and 1,278 cm−1), and NaClO4 (3,481, 3,237, and 2,025 cm−1) are found absent in the complexes. In addition to this, some new peaks at 2,866 and 763 cm−1 are found in the complexes. The frequencies 1,893 and 1,457 cm−1 are assigned to CH3 asymmetric stretching and bending vibrations of PVC. The absorption peaks at 1,727 and 1,278 cm−1 are assigned to the C=C and C—F stretching vibrations of PVdF. The frequency at 877 cm−1 is assigned to the vinylidene group. These results suggest that homogeneous polymer electrolytes are formed over all the blend compositions.
Thermal stability studies
DSC results show that there is a single TG (T g) in the region of 289–310 °C (Fig. 3), evidencing the blend’s miscibility. The single T g values of blend polymer films are higher than the T g values of both mother polymers. This positive T g deviation indicates that a strong interaction exists between the two polymers [24].
Thermal stability was evaluated on the basis of TG data. It has been found that all the blends under investigation degrade thermally through a similar three-step route (Fig. 4). The initial decomposition temperature was reached (255 °C). The maximum rate of decomposition was ascertained on the basis of first derivative of the TG curve and lay in the range 250–310 °C; it seems that conditions of diffusion of gaseous products through the polymer matrix are decisive for better stability of this polymer blend. When blending the two polymers, the T g value decreased due to the reducing of the particle sizes; the small particles show better stability.
Thermal stability parameters of (PVC+NaClO4+PC) and (PVdF+NaClO4+PC) have higher values than those of the blends. Lower thermal stability of the PVC/PVdF blends shows that the blend preparation technique (homogenization) plays an important role; this observation should be taken into account by processing of these novel materials.
It is, however, essential to note that samples C and D thermally stable up to 250 °C, which is usually a high enough temperature for processing and storage. However, in special thermoplastic processing under high temperature and shear stresses, and in reaction injection molding during which the temperature in the center of the mold may exceed 250 °C owing to the high reaction exotherm, thermal degradation may occur. This thermal dissociation may not be totally disadvantageous from the processing point because it allows molecular rearrangements by breaking the already-made vinylidene bonds.
Morphology studies
The electronic properties of PVC/PVdF films are intricately linked to the morphology; a smoother, more dense film will generally be more conductive, as it is likely to be less porous and more ordered, which can facilitate charge transport through the film [24]. In trying to understand the role that the ionic conductivity plays in producing such electrochemically active polymer films, electron microscopy was used to investigate the surface morphology. Conducting polymers typically show a nodular surface morphology, the origin of which is not well understood [25]. Electron micrographs of a film (PVC+NaClO4+PC) are given in Fig. 5a. The larger craters, which have formed on the surface, are due to the rapid evaporation of THF used in the preparation of the film. The fractured surface of the (PVdF+NaClO4+PC) film has morphology like interpenetrating networks (Fig. 5b). Figure 5c shows the typical nodular, “bright moon” appearance, with large variations in surface structure across the film. The film in Fig. 5d appears reasonably homogeneous, with small spherical structures, whereas the film in Fig. 5c appears very rough and pitted. The difference in morphology of the two films is consistent with the significant differences in the ionic conductivities of these two films.
Ionic conductivity studies
The ionic conductivity has been determined from AC impedance analysis using the cell with blocking electrodes as described in the “Experimental” section. The typical impedance plot of PVC (7.5%)+PVdF (17.5%)+NaClO4 (8%)+PC (67%) at ambient temperature is shown in Fig. 6. The bulk resistance was measured from the high-frequency intercept on the real axis. The conductivity of the polymer electrolyte was calculated from the measured resistance, area, and thickness of the polymer film.
If the impedance plots show typical impedance behavior as being a semicircular portion at high frequencies followed by a spike (residual tail) at low frequencies, then results suggest that the migration of ions may occur through the free volume of matrix polymer, which can be represented by a resistor. The immobile polymer chains, on the other hand, become polarized in the alternating field, and can therefore be represented by a capacitor. The ionic migration and bulk polarization are physically in parallel, and therefore, the portion of the semicircle can be observed at high frequencies. It is noted that the semicircle observed at high frequencies completely disappears in the polymer with 67 wt.% of PC, as shown in Fig. 6. This result suggests that only the restive component of the polymer electrolyte prevails when the amount of plasticizing solution is high. Hence, as the plasticizer content increases, a local effective pathway is constructed in the liquid phase for ionic conduction; as a result, ions can transport quickly in the liquid phase as the electric potential alternates between the positive and negative electrodes in an AC field.
Figure 7 presents the variation of ionic conductivity as a function of PVdF weight fraction into (PVC+NaClO4) complex. The room temperature conductivity of the PVC+NaClO4+PC complex increases to about 1.5×10−4 S/cm after the addition of PVdF and showed a maximum at some intermediate value of PVdF composition, beyond which it demonstrates the inverse effect. The initial increase in conductivity with PVdF concentration is explained due to the availability of mobile species and reaches a maximum value for certain concentrations (17.5 wt.%). Variations in conductivity, along with the concentration of the charge carriers, are also controlled by the mobility of the charge carriers and the predominant charge species. Higher weight ratio of PVdF in the electrolyte leads to higher viscosity, which, in turn, lowers the mobility of charge carriers and, hence, lowers the conductivity [26].
The ionic conductivity of PVC+PVdF+NaClO4+PC complexes is summarized in Table 2. It can be seen that the conductivity of the electrolyte is mainly determined by the weight ratio of PVdF. The addition of PVdF in PVC+NaClO4+PC electrolytes appears to enhance the conductivity by two orders of magnitude compared to the electrolyte containing only PVC at ambient temperature. This is most likely from the following sources:
-
1.
The high dielectric constant of PVdF, which helps to enhance ionic dissociation in the electrolyte, resulting in a higher concentration of ionic charges.
-
2.
Weaker coupling of ions with the polymer chain, which enhances ionic conductivity.
-
3.
The higher conductivity observed in the polymer electrolyte [PVC (7.5%)+PVdF (17.5%)+NaClO4 (8%)+PC (67%)] is believed to result from the lower degree of crystallinity that facilitates the fast sodium ion motion in the polymer network.
The temperature-dependent conductivities are plotted in Fig. 8. In the employed temperature range (up to 100 °C) in this study, it is observed that, as temperature increases, the conductivity increases in all the compositions. This behavior is in agreement with the theory of Armand et al. [27]. The curvature shown in this plot indicates that the ionic conduction obeys the voltage transfer function relation, which describes the transport properties in a viscous matrix. The curvature supports the idea that the ion moves through the plasticizer-rich phase; because the conducting medium, i.e., the plasticizer-rich phase, involves the plasticizer, the salt, and the polymers, the characteristics of the viscous matrix are brought out.
Conclusions
The GPEs comprising PVC/PVdF blend polymers were studied. The complexation has been confirmed from FTIR spectral studies. Thermal characteristics of PVC/PVdF blend polymers have been assessed by thermal analysis method. The decomposition route was found to consist of a two-step process. The maximum value of ionic conductivity for a PVC (7.5%)+PVdF (17.5%)+NaClO4 (8%)+PC (67%) system is 9.24×10−4 S/cm at 30 °C. The higher conductivities observed in the polymer electrolytes are believed to result from the lower degree of crystallinity and uniform morphology, which is confirmed from XRD and SEM studies.
References
Abraham KM (1997) In: International conference on applications of conducting polymers, book of abstracts. Rome, 13–16 April 1997, p 128
Peramunage D, Pasquariello DM, Abraham KM (1995) J Electrochem Soc 42:1798
Hong H, Liqquan C, Xujie H, Rongian X (1992) Electrochim Acta 37:1671
Bohke O, Frand C, Razrazi M, Rousselt C, Truche C (1993) Solid State Ionics 66:97
Boudin F, Andrieu X, Jehoulet C, Olsen II (1999) J Power Sources 81–82:804
Saito Y, Kataoka H, Quartarone E, Mustarelli P (2002) J Phys Chem B 106:7200
Saito Y, Kataoka H, Stephan AM (2001) Macromolecules 34:6995
Moussaif N, Jerome R (1999) Ploymer 40:3919
Jin Z, Pramoda KP, Goh SH, Xu G (2002) Mater Res Bull 37:271
Bauduin G, Boutevin B, Garmain P, Malinova A (1999) Eur Polym J 35:285
Rajendra S, Kannan R, Mahendran O (2002) Fuel 81:1077
Rajendra S, Kannan R, Mahendran O (2001) Mater Lett 49:172
Kim DW, Park JK, Rhee HW (1996) Solid State Ionics 83:46
Wieckzorek W, Stevens JR (1997) J Phys Chem B 101:1529
Booth C, Nicholas CV, Wilson DJ (1989) In: MacCallum JR, Vincent CA (eds) Polymer electrolyte reviews—2. Elsevier, London, p 229
Watanabe M, Nagano S, Sanvi K, Ogata N (1987) J Power Sources 20:327
MacCallum JR, Smith MJ, Vincent CA (1981) Solid State Ionics 11:307
Marco GD, Lanza M, Pierceccini M (1996) Solid State Ionics 89:117
Scrosati B (1992) J Electrochem Soc 139:2776
Tsutsumi N, Davis GT, Dereggi AS (1991) Macromolecules 24:6392
Choe HS, Giaccai J, Alamgir M, Abraham KM (1995) Electrochim Acta 40:2289
Oliver M (1997) Motorola. US patent 5,658,685, 19 Aug 1997
Stephan AM, Kumar TP, Muniyandi NGRN (2000) J Power Sources 89:80
Pielichowski K, Hamerton I (2000) Eur Polym J 36:171
Faria LO, Moreira RL (2000) J Polym Sci B Polym Phys 38:34
Periasamy P, Tatsumi K, Shikano M, Fujieda T, Sakai T, Saito Y, Mizuhata M, Kajinami A, Deki S (1999) Solid State Ionics 126:285
Armand MB, Chabagno JM, Duclot MJ (1979) In: Vashishta P, Murdy JN, Shenoy G (eds) Fast-ion transport in solids. North Holland, Amsterdam
Acknowledgements
One of the authors (Ch.V.S. Reddy) wishes to thank the Wuhan University of Technology Management for financial support to carry out the above work in the form of a postdoctoral fellowship. Opening Foundation of Hubei Ferroelectric and Piezoelectric Materials and Devices Key Laboratory supported this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reddy, C.V.S., Zhu, QY., Mai, LQ. et al. Electrochemical studies on PVC/PVdF blend-based polymer electrolytes. J Solid State Electrochem 11, 543–548 (2007). https://doi.org/10.1007/s10008-006-0192-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0192-1