Abstract
Nanometer dimension of citrate-capped gold nanoparticles can be firmly bound with various functionalized polymer-modified glass plate and indium tin oxide (ITO) substrates. Herein we report 3-aminopropyltriethoxysilane, polyvinyl pyridine, polyethylene imines, etc. as binding agents to modify these substrates to stabilize the charged colloidal gold nanoparticles through electrostatic stabilization of gold nanoparticles. When gold nanoparticles pretreated substrate are exposed into the seeding growth solution, the preadsorbed gold nanoparticles grow further and then form nanoislands of gold on glass and ITO substrates. The formation of nanoislands on microscope glass slide and ITO was monitored with UV-visible spectroscopy, cyclic voltammetry, and atomic force microscopy methods. The gold nanoislands and gold nanoparticles pretreated substrates can be used as platform to study the self-assembling behavior of long chain alkanethiols such as C12SH, C16SH, and C18SH. The binding, coverage, and electron transfer characteristics of monolayer assembly on modified gold nanoisland and nanoparticles modified substrates are studied using electrochemical studies. The gold substrates can be prepared by this method, which is simple and reproducible and can be applied to various sensor and electrocatalytic applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
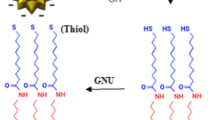
There is a tremendous interest in recent days to study the microscopic behavior of self-assembling of long chain alkanethiols on gold substrate. When gold-coated substrates are exposed into the ethanol solution of long chain alkanethiols, they spontaneously form a well-organized monolayer assembly on gold surface [1]. The monolayer assembly acts as a passive layer against oxidative damage including H2S atmosphere [2] and is relatively stable. Thermally evaporated gold film (10- to 100-nm-thick) on silicon wafer and glass substrate was used to study self-assembling behavior of long chain thiols. The ordering and packing density of thiol monolayer mainly depends on alkyl chain length, morphology, and pretreatment of the gold substrates [3–5]. It was reported that the flame-annealed gold substrate at 300°C shows an excellent quality of monolayer assembly [6]. Ultrathin gold film can generally be prepared by various methods including thermal evaporation [3], electrochemical deposition [7], and electroless deposition [8]. Thermal evaporation method was mostly utilized for the deposition of gold substrate on precleaned silicon, microscope glass slide, and freshly cleaved mica. Finot and McDermott [7] reported that the electrochemically deposited gold film on glassy carbon is a suitable substrate for the characterization of self-assembling of long chain thiols. Zei et al. [9] reported that a flat surface of Au(111)-oriented grains could be prepared on a glass surface by using evaporation method. This was useful in many applications but not suitable for electrochemical studies because it was hard to obtain electrical contact and the gold layer was easily peeled off from the substrate. To improve the adhesion between the gold film and the substrate, 3-mercaptopropyltrimethoxy silane (MPTMS) pretreated silicon substrate was used [10]. Alternatively, 3-aminopropyltriethoxysilane (APTMS) [11], amine terminal dentrimers (polyamidoamine) [12], and Ti or Cr as primer [3] was also used as adhesive layer for the deposition of gold by thermal evaporation method on silicon substrates. Though this adhesive layer was used as primer for the deposition of gold, it is less attractive and has some limitations for certain applications.
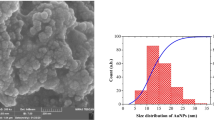
Metal colloids with nanometer dimension show an unusual optical and electronic property due to the collective vibration of gaseous electron and columbic charge separation within atomic scale. These noble metal nanoparticles can be prepared by means of chemical, electrochemical, and photochemical methods in the presence of suitable stabilizing agent [13–15]. The citrate-capped gold nanoparticles with 2–10 nm show strong plasmon vibration band at 520 nm. Because of their natural brilliant color and high extinction coefficient, they can be used as colorimetric sensor for the determination of biologically important molecules, drugs, metal ions, etc [16–18]. A thin layer of colloidal gold nanoparticles can be stabilized on optically transparent glass plates and conducting indium tin oxide (ITO) glasses using a bifunctional organic linker molecules like APTMS, MPTMS, etc., or polymers like polyethylene imine (PEI), polydiallylamine, and polyvinylpyridine (PVP).
Recently, Jin et al. [19] and Brown and Natan [20] reported the preparation of ultrathin gold film on glass plates by means of seeding growth method using gold nanoparticles as seed for the further growth of gold nanoislands. Such substrate can be utilized for the preparation of gold substrates for the surface-enhanced spectral studies because of its greater enhancement factor than the roughened gold surface [21] and can also be used as substrate for the surface plasmon resonance spectroscopy where binding of biomolecules with receptor can be monitored by recording the change in refractive index with respect to the unbound molecules [22]. Hou et al. [8] demonstrated the electroless deposition of gold on various substrates to study the self-assembling behavior of alkanethiols and characterized them by Fourier transform infrared and electrochemical methods. However, this method is not very popular because the sample preparation involved many steps and need extensive cleaning procedure and pretreatment. Hence, there is an urgent need for preparation of gold substrate by a simple method for various applications.
In the present investigation, we have chosen three different binding agents like APTMES, PEI, and PVP for the stabilization of colloidal gold nanoparticles through electrostatic stabilization. Bifunctional linker molecules like APTMS or linear polymers are mostly used as binding agent for the stabilization of colloidal gold nanoparticles and are also easy to modify in the microscope slide and ITO glass plates. It is important to note that some of the experimental parameters may control growth, morphology, and film thickness. The film thickness and morphology of the gold film depend on the coverage of seed colloidal nanoparticles, the composition of seeding growth solution, and the exposure time. This method is considered to be the easiest way of fabricating gold substrate in an ordinary wet chemical laboratory.
Experimental section
Chemicals
Hydrogen chloroauric acid, HAuCl4.3H2O, PVP, PEI, and trisodium citrate dihydride were obtained from Aldrich, USA. Hydroxylamine HCl and APTMS were purchased from Lancaster, UK. Dodecanethiol (C12SH), Hexadecanethiol (C16SH), and Octadecanethiol (C18SH) were purchased from Aldrich, USA. All other chemicals were used as received from commercial sources. Ultra pure water with 18 MΩ resistivity (MilliQ Water, Millipore System) was used for all the studies.
Preparation of gold nanoparticles (citrate method)
All glassware used was cleaned in the following procedure. They were cleaned in a bath of freshly prepared 3:1 molar ratio of HCl:HNO3 (aqua regia) and rinsed thoroughly in deionized (DI) water before use. Preparation of gold “seed colloid” solution of 10 nm diameter particles was performed as follows. In brief, 200 ml of aqueous 0.01% (w/v) HAuC14 was brought to boil and then 7 ml of aqueous 1% (w/v) sodium citrate was added (23). The color of the solution initially changed to a grayish black and then turned into a wine red within a few minutes. The solution was allowed to cool at room temperature and was filtered through 0.2 μm pore size nylon filter system.
Substrate preparation
Substrate preparation and cleaning are critically important in the experiments. Microscope glass slides were cleaned before use by soaking in a K2Cr2O7: conc.H2SO4 (chromic acid) solution to remove particulate material from the surface of the substrate. After rinsing with DI water, the slides were placed in a freshly prepared piranha solution (7:3 v/v) molar ratio of con.H2SO4:30% H2O2 at 70°C for 20 min to remove organic impurities (Caution: Piranha solution is a powerful oxidizing agent and reacts violently with organic compounds. It should be handled with extreme care). The slides were then rinsed thoroughly with DI water. ITO plates were cleaned thoroughly by ultrasonicating in different cleaning steps in soap water, water, acetone, methanol, and 0.5 M NaOH for 10 min.
Surface modification with APTMS, PEI, and PVP
ITO and glass slides were rinsed in ethanol and were then placed in a dilute solution of APTMS (0.3 ml of APTMS in 3 ml of ethanol) for 12 h. After which they were washed thoroughly with ethanol and then heated to 110°C for 10 min. Finally, the silylated glass plates were washed with ethanol [23]. The glass substrates were also modified with PEI. This was done by immersing the slides into a 2.8-ml aqueous solution of PEI and 0.5 M of sodium chloride for 20 min and then dried under nitrogen stream [24]. The PVP modified substrate was prepared by immersing into an alcohol solution of 1% PVP for 1 h, rinsed in alcohol, and dried under a stream of nitrogen [25, 26].
The APTMS, PVP, PEI, and modified substrates were immersed in a colloidal gold solution (∼10 nm) for 12–18 h to achieve uniform coverage of a monolayer assemble of gold nanoparticles. The monolayers of gold nanoparticles were then rinsed with water and dried under a nitrogen stream.
Seeding growth of gold nanoparticles
Substrates with a monolayer of nanosized gold particles were immersed in 6 ml of aqueous 0.4 mM hydroxylamine hydrochloride and 0.1% HAuCl4 [27]. The solution was agitated to ensure the formation of homogeneous gold nanoisland. The substrates changed color from pink to purple to blue and finally to reflective gold over 10 min. The gold plate substrates were rinsed thoroughly with water and dried under nitrogen.
Self-assembled monolayer formation
The gold plated substrates were subsequently immersed in 1 mM of chloroform solution of C12SH, C16SH, and C18SH for about 12 h. The substrates were then rinsed with chloroform and dried under nitrogen stream.
Instrumentation
UV-visible spectrophotometer
Optical spectra were taken using Shimadzu 1601 UV-visible spectrophotometer. All spectra were recorded with the path length of 1×1 cm2. The binding and coverage of gold nanoparticles on polymer-modified glass plates and ITO was investigated by directly inserting the respective plates in the sample holders. The background spectrum was corrected against either the plain glass or ITO plates.
Cyclic voltammetry
A scanning potentiostat CHI Electrochemical Analyser, Model 601 (CHI Instruments, USA) was used for all the electrochemical studies. A three-electrode cell setup was used and a platinum foil (1 cm2) and Ag/AgCl (3M NaCl) were used as counter and reference electrodes, respectively. The APTMS-modified ITO acts as working electrode with exposed area of 0.5×0.5 cm2. Potentials were measured against the reference electrode. Before starting the experiments, all the solutions were purged with purified N2 for 10 min.
AFM method
The Au nanoparticles and Au island films modified glass substrates were characterized by atomic force microscopy (AFM; Molecular Imaging, USA) using gold-coated Si3N4 cantilevers with a force constant of 3 N/W.
Results and discussion
Preparation of gold film on glass and ITO plates
Freeman et al. [28] and Dorun et al. [29] investigated the various applications of monolayer coverage of colloidal gold nanoparticles on optically transparent glass and ITO surfaces. Gold nanoparticles with 5–50 nm show a brilliant color with plasmon absorption at 520 nm where the absorption spectrum blue shifted for surface-bound colloidal gold nanoparticles. Such a surface-bound nanoparticles assembly in both monolayer and multilayer assembly of charged species is a tool for binding of biological molecules and electrochemical sensor applications [30]. Similar strategy can be utilized for the stabilization of colloidal gold particles and can be used for the development of ultrathin gold film through a seeding growth method. These gold nanoislands show similar characteristics of vacuum-evaporated gold film. Preparation of large-sized colloidal gold nanoparticles by citrate reduction method becomes difficult because citrate ion is unable to stabilize such large gold nanoparticles, which makes the nanoparticles coalesce. Alternatively, seeding growth method can be adopted for the preparation of large-sized gold nanoparticles in both solution- and surface-bound colloidal gold surfaces. The addition of a reducing agent like hydroxylamine in the presence of HAuCl4 initiate the growth over the surface of the seed gold nanoparticles and the particles size can be controlled by the concentration of the seeding solution, size of the seed, and exposure time. A similar analogy can also be applied for the surface-bound colloidal gold nanoparticles. It is possible to uniformly generate a monolayer of gold nanoparticles on functionalized polymer-modified microscope glass slide and ITO surfaces. By the application of seeding growth method, nanoislands of thin gold film can be generated on both microscope slide and ITO surfaces. In the present investigation, we have chosen three different functionalized polymer-modified surfaces for the stabilization of colloidal gold because they are widely used on silicon and ITO surfaces. The monolayer coverage of gold nanoparticles was monitored by UV-visible spectral studies because the gold nanoparticles exhibit a strong plasmon band at 520 nm. The increase in absorbance (plasmon band) was noted with increase in coverage of gold nanoparticles. The UV-visible spectral characteristics of three different polymer-stabilized gold nanoparticles on microscope glass plates are shown in Fig. 1. The plasmon band become broad and the peak position shifted to blue, when compared with free colloidal gold nanoparticles, due to the surface confinement of gold nanoparticles. The monolayer assembly of gold nanoparticles can be used as seed for the fabrication of nanoisland. These gold nanoparticles preadsorbed substrates were exposed into the seeding solution at different time intervals. It was found that the plasmon band becomes dampened for the thin film of nanoisland surfaces and the reaction was allowed for 10 min to achieve uniform coating of ultrathin gold film. If the reaction was further allowed to achieve uniform coating (10 min more), the substrate would have a golden luster due to the formation of dense gold film (opaque gold mirror). It is inferred that the growth rate is faster in gold nanoparticles adsorbed on APTMS-modified surface than the PEI- and PVP-modified surfaces. Dorun et al. [29] also pointed out that APTMS shows an excellent stabilizing activity toward gold nanoparticles than the MPTMSs though strong affinity of mercaptan with gold nanoparticle through covalent bonding nature. We tested the stabilizing ability of amine and pyridine functional linear polymers for instant PVP [25] and PEI [24] toward gold nanoparticles and found out that these polymers exhibit similar binding character like APTMS; these two polymers are used widely for the stabilization of gold nanoparticles on solid substrate for various applications. According to UV-visible spectral studies, it is concluded that the coverage of colloidal gold is found to be in the following order: APTMS>PEI>PVP. These polymer-modified microscope glass plate and ITO glass can be used for the seeding plating at different intervals to get uniform coating of nanoislands of thin gold film (Fig. 1). The surface morphology of monolayer assembly of gold nanoparticles and nanoislands were characterized by AFM studies. It was clearly observed that APTMS-modified surface are smoother and the grain sizes are more uniform, whereas the grains are larger and has less uniform coverage on the nanolands of gold of the other two polymer-modified glass surfaces. Figure 2 shows the AFM images of nanoislands of gold deposited by seeding growth of APTMS and PEI pretreated glass substrates. We conclude that APTMS-modified surface is an excellent binding agent for the stabilization of colloidal gold nanoparticles because it has a more uniform coverage of gold film obtained by the seeding growth method.
Electrochemical characterization of gold islands
Thermally evaporated gold substrates primed with 50 Å of chromium or 3-mercaptopropyl trimethoxysilane on silicon or glass slide were used for the electrochemical characterization of the self-assembling behavior of long chain thiols. These adhesive layers prevent the delamination of gold film during contact with an aqueous and organic solvent medium and cause the gold film to be delaminated from the substrate. Hence, it is difficult to use for electrochemical studies. For the electrochemical studies, polymer pretreated ITO substrates were used for the plating of gold film instead of the microscope glass plate. The cyclic voltammograms (CVs) were recorded for the surface-bound colloidal gold nanoparticles and the gold was plated by seeding growth method in 0.1 H2SO4 medium scanning between −0.2 and 1.8 V vs Ag/AgCl. An anodic peak appeared at 1.0 V vs Ag/AgCl followed by a broad peak in the cathodic side at 0.8 V vs Ag/AgCl, as shown in Fig. 3. These voltammetric curves are associated with the oxidation and reduction of gold film. The reduction wave at 1.0 V disappeared when the gold substrate was exposed to hexadecanethiol for 2 h. It is conjectured that the gold surface blocks the oxidation of gold film in 0.1 M H2SO4 medium. A similar observation was noted for the electrochemically deposited gold-plated glassy carbon electrode [7]. We examined the cyclic voltammetry of the ferrocyanide/ferricyanide redox couple (1 mM in 0.1 M KCl) at an ITO surface modified by a monolayer of gold nanoparticles array. The reversible voltammogram was observed in the case of bare ITO while the wider peak separation was observed for APTMS-modified ITO surface exposed into the citrate-capped gold colloids before and after gold plating when the seeding growth method was employed (Fig. 4). The widening of the peak separation in CV indicates quasireversiblity due to slow electron transfer reaction between the ITO electrode and gold nanoparticles while the peak separation of 105 mV refers to the slow heterogeneous electron transfer reaction. On the other hand, the peak separation between cathodic and anodic peak of 70 mV for the APTMS-modified ITO after gold plating by seeding growth method leads to a faster electron transfer reaction. The observed CV exhibits a single electron transfer reversible reaction. The cyclic voltammetric response for the redox probe exhibits different peak separation values when the monolayer of gold nanoparticle pretreated APTMS-modified ITO is exposed into dodecanethiol solution (1 mM in ethanol). A similar voltammetric response was observed for other long chain thiols such as C12SH, C16SH, and C18SH. The effective coverage of long chain thiol on gold nanoparticles and nanoisland films was investigated employing the redox probe, 1 mM K3Fe(CN)6 in 0.1 M KCl. The CVs are quasireversible in nature and peak separation is found to be greater than 140 mV, thus indicating the slow electron transfer reaction (Table 1). We have measured the peak current for the bare gold electrode and compared the electron transfer rate constant for the prepared gold substrates in 1 mM K3Fe(CN)6 and 0.1 M KCl. The peak current increases linearly while increasing the concentration of redox probe (in the millimolar concentration ranges of redox probe).
Conclusion
The types of electrode assembly described above can be used for electrocatalytic applications and for the selective determination of biologically important catechol in the presence of a large concentration of interfering analytes such as ascorbic acid [31]. It is deduced that these simple methods can be extended for studies involving various monolayer assemblies and also for diverse system applications. These optically transparent gold-plated and gold nanoparticle-modified substrate can be further derivatized with bifunctional thiols for effective stabilization of metal porphyrin and phenathiazine dyes. These chromophore-modified substrates are useful for colorimetric determination of poisonous gases such as HCN, H2S, and NO and are also effective for determining oxygen content using the above method.
References
Ulmann A (1991) An Introduction to ultrathin organic films from Langumir Blodgett to self assembly. Academic, San Diego, CA
Tao YT, Pandian K, Lee WC (2000) J Am Chem Soc 122:7072
Porter MD, Bright TB, Allara DL, Chidsey CED (1987) J Am Chem Soc 109:3559
Walczak MM, Alves CA, Lamp BD, Porter MD (1995) J Electroanal Chem 396:103
Lee MTH, Sueh CC, Freund MS, Ferguson GS (1998) Langmuir 14:6419
Widrig CA, Chung C, Porter D (1991) J Electroanal Chem 310:335
Finot MO, McDermott MT (2000) J Electroanal Chem 488:125
Hou Z, Abbott NL, Stroeve P (1998) Langmuir 14:3287
Zei MS, Nakai Y, Lehmfuhl G, Kolb DM (1983) J Electroanal Chem 150:201
Goss CA, Charych DH, Majda M (1991) Anal Chem 63:85
Dunaway DJ, Mc Carley RL (1994) Langmuir 10:3598
Baker LA, Zamborini FD, Sun L, Crooks RM (1999) Anal Chem 71:4403
Brust M, Walker M, Bethell D, Schiffrin DC, Whyman R (1994) Chem Commun 801
Yu YY, Chang SS, Lee CL, Wang CPC (1997) J Phys Chem B 101:6661
Esumi K, Matsuhisa K, Torigoe K (1995) Langmuir 17:3285
Reynolds RA, Mirkin CA, Letsinger RL (2000) J Am Chem Soc 122:3795
Joseph Y, Guse B, Yasoda A, Vossmeyer I (2004) Sens Actuators B Chem 98:188
Kusi Y, Johnson RC, Hupp JT (2001) Nano Lett 1:165
Jin Y, Kang X, Song Y, Zhang B, Cheng G, Dong S (2001) Anal Chem 73:2843
Brown KR, Natan MJ (1998) Langmuir 14:726
Menzel H, Mowery MD, Cai M, Evans CE (1999) Adv Mater 11:131
Fredrix F, Friedt JM, Choi KH, Laureyn W, Campitelli A, Mondelaers D, Maea G, Borghs G (2003) Anal Chem 75:6894
Natan MJ, Keating D (1999) J Chem Educ 76:949
Yu A, Liang Z, Cho J, Caurso F (2003) Nano Lett 3:1203
Malynych S, Luzinov I, Chumanov G (2002) J Phys Chem B 106:1280
Jackson JB, Halas NJ (2004) Proc Natl Acad Sci USA 101:17930
Jin Y, Dong S (2002) Chem Commun 1780
Freeman GR, Grabar KC, Allison KJ, Bright RM, Davis JA, Guthrie AP, Hommer MB, Jackson MA, Smith PC, Walter DG, Natan MJ (1995) Science 11:1313
Dorun A, Katz E, Willner I (1995) Langmuir 11:1313
Xia Y, Patoisky F, Katz E, Hainfeld JF, Willner I (2003) Science 299:1877
Senthil Kumar S, Mathiyarasu J, Phani, KLN (2005) J Electroanal Chem 578:95
Acknowledgements
One of the authors gratefully acknowledges the funding agents, UGC, New Delhi and DST, New Delhi, for providing financial support for this research program. One of the authors (K.P.) greatly acknowledges Prof. M.V. Sangaranarayanan, Department of Chemistry IIT Madras, Chennai-600 036, India for the scientific discussion and is grateful for the referee’s suggestions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shakila, V., Pandian, K. Preparation of gold nanoislands on various functionalized polymer-modified glass and ITO for electrochemical characterization of monolayer assembly of alkanethiols. J Solid State Electrochem 11, 296–302 (2007). https://doi.org/10.1007/s10008-006-0107-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10008-006-0107-1